Abstract
Brinjal or eggplant (Solanum melongena L.) is an important solanaceous edible crop, and salt stress adversely affects its growth, development, and overall productivity. To cope with excess salinity, vacuolar Na+/H+ antiporters provide the best mechanism for ionic homeostasis in plants under salt stress. We generated transgenic eggplants by introducing wheat TaNHX2 gene that encodes a vacuolar Na+/H+ antiporter in to the eggplant genome via Agrobacterium-mediated transformation using pBin438 vector that harbors double35S:TaNHX2 to confer salinity tolerance. Polymerase chain reaction and southern hybridization confirmed the presence and integration of TaNHX2 gene in T1 transgenic plants. Southern positive transgenic eggplants showed varied levels of TaNHX2 transcripts as evident by RT-PCR and qRT-PCR. Stress-inducible expression of TaNHX2 significantly improved growth performance and Na+ and K+ contents from leaf and roots tissues of T2 transgenic eggplants under salt stress, compared to non-transformed plants. Furthermore, T2 transgenic eggplants displayed the stable leaf relative water content and chlorophyll content, proline accumulation, improved photosynthetic efficiency, transpiration rate, and stomatal conductivity than the non-transformed plants under salinity stress (200 mM NaCl). Data showed that the T2 transgenic lines revealed that reduction in MDA content, hydrogen peroxide, and oxygen radical production associated with the significant increase of antioxidant enzyme activity in transgenic eggplants than non-transformed plants under salt stress (200 mM NaCl). This study suggested that the TaNHX2 gene plays an important regulatory role in conferring salinity tolerance of transgenic eggplant and thus may serve as a useful candidate gene for improving salinity tolerance in other vegetable crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global agricultural productivity is subject to increasing environmental constraints, particularly to salinity due to their high magnitude of impact and wide distribution. Traditional breeding programs trying to improve abiotic stress tolerance have had some success but are limited by the multigenic nature of the trait. The yield and productivity of many crops including vegetables are hampered by the enormous amounts of soluble salts in soil in many parts of the world (FAO 2002; AVRDC 2006). Salt stress affects each phase of vegetable crop development including morphology, physiological function, yield, and nutritional value (Zhuang et al. 2014; Shahbaz et al. 2012; Prasad et al. 2014). To meet the food supply, it is an essential to produce salt-tolerant crops, which can be sustained on salt-affected lands. Among crops, vegetables play vital role in the human diet because of their nutritional importance in providing vitamins, carbohydrates, proteins, and mineral nutrients. Plants have developed a specialized network of cation channels across the cellular and vacuolar membranes to regulate the movement of Na+, K+ and their balanced availability for cellular functions (Almeida et al. 2017; Blumwald 2000; Shi et al. 2002). The membrane and vacuolar Na+/H+ antiporters afford the best mechanism for ionic homeostasis in plants under salt stress. A vacuole Na+/H+ antiporter actively moves Na+ into the vacuole by H+-ATPase and H+-PPase coupled with the vacuolar H+-translocating enzymes, with the H+-ATPase and the H+-PPase producing electrochemical H+ gradients (Liang et al. 2018; Jiang et al. 2010; Bassil and Blumwald 2014).
Physiological and biochemical data had suggested that Na+/H+ antiporters are involved in intracellular ion (Na+), pH regulation, and K+ homeostasis in plants (McCubbin et al. 2014; Leidi et al. 2010; Gaxiola et al. 1999; Blumwald 2000). The significant role of NHX genes has been highlighted with the generation of salt-tolerant transgenic plants through the overexpression of NHX genes in a wide variety of species (Zeng et al. 2017; Li et al. 2017; Kumar et al. 2017; Sahoo et al. 2016; Fan et al. 2015; Bhaskaran and Savithramma 2011; Tang et al. 2010; Gaxiola et al. 1999; Fukuda et al. 1999; Xia et al. 2002; Wu et al. 2004; Chen et al. 2007; Li et al. 2007). The three vacuolar Na+/H+ antiporter genes, namely, TaNHX1, TaNHX2, and TaNHX3, have been functionally characterized in wheat. All these genes complement the growth of salt sensitive yeast mutants under salt stress conditions (Brini et al. 2005; Yu et al. 2007; Lu et al. 2014). The ectopic expression of TaNHX1 (Brini et al. 2007) and TaNHX3 (Lu et al. 2014) in tobacco conferred salt stress tolerance. The class I NHX gene TaNHX2 also played critical role to confer salt stress tolerance to salt sensitive yeast mutants (Yu et al. 2007). Significant progress has been reported to enhance salinity stress tolerance by expressing TaNHX2 gene in higher plants such as soybean, alfalfa, and rice (Cao et al. 2011; Zhang et al. 2015; Wu et al. 2012) and including vegetables, i.e., tomato (Yarra et al. 2012) and chili pepper plants (Bulle et al. 2016).
Eggplant or brinjal or aubergine (Solanum melongena L.) and tomato belong to Solanaceae family (Daunay 2008) native to India and China. Eggplant is a commonly grown vegetable plant including potatoes and tomatoes (Doganlar et al. 2002). Increasing demand for vegetables globally boosted the vegetable production. The substantial rise in production has been particularly essential in key vegetable crops such as eggplant, tomato, onion, cucumber, cauliflower, pepper, lettuce, carrot, and spinach (Koike et al. 2007). Eggplant is considered to be a salt-sensitive vegetable (Bresler et al. 1982). However, tolerance varies among eggplant varieties (Unlukara et al. 2010). Shalhevet et al. (1983) observed that 50% yield loss of eggplant at irrigation water salinity, having electrical conductivity of 8.5 dS m−1. Salinity stress in eggplant severely affects the growth and development at the germination and seedling stages (Akinci et al. 2004). It has been observed that salinity stress in eggplant markedly diminishes both fruit weight and number of fruits per plant (Abbas et al. 2010). Improving the salinity stress tolerance of eggplant has become a primary objective in most eggplant growing zones. Although ample improvement has been made in eggplant genetic transformation, achievement in developing transgenic eggplants with high salt tolerance has been limited. Until now, very few studies have been reported to enhance the salinity tolerance of eggplant by expressing bacterial mannitol-1-phosphodehydrogenase (mtlD) (Prabhavathi et al. 2002), oat arginine decarboxylase, adc gene (Prabhavathi and Rajam 2007), and yeast halo tolerance gene HAL1 (Kumar et al. 2014).
To date, no vacuolar Na+/H+ antiporter genes were introduced in eggplant genome to enhance salt stress tolerance. In our study, TaNHX2 expression in eggplant significantly improves the plant growth under salt stress, strictly associated with the improvement of related physiological processes, including the increased antioxidant enzymatic activities and enhanced contents of photosynthetic parameters. These findings clearly demonstrated that TaNHX2 acts as an important regulator in salinity tolerance of plants and can be used as a gene resource for molecular breeding of salt-tolerant crop cultivars.
Materials and methods
Plant material
Seeds of elite cultivars of eggplant (Solanum melongena L.) PPL variety were procured from National Seeds Corporation Ltd., Secunderabad, India. Seeds were imbibed for 6 h in distilled water and then surface sterilized with 0.1% mercuric chloride (HgCl2) for 3–5 min, washed three times with sterile distilled water, and germinated on the surface of MS (Murashige and Skoog 1962) basal medium with 15 g l−1 sucrose and 4 g l−1 agar. The pH was adjusted to 5.8 before autoclaving. The cultures were maintained under 16 h photoperiod, 25 °C, relative humidity of 60–65%, with fluorescence light (60 μmol m−2 s−1). Young and fully expanded leaves of 3–4 cm in length and 2–3 cm in width were excised from all parts of the shoot grown for a month on MS medium and used as explants for transformation experiments.
Binary plasmid and Agrobacterium strain
Agrobacterium tumefaciens strain LBA4404 harboring a binary vector pBin438-TaNHX2 was used for transformation of eggplant. The binary vector pBin438 containing wheat Na+/H+ antiporter (TaNHX2) gene driven by a double Cauliflower Mosaic Virus (CaMV) 35S promoter (Supplementary Fig. S1) was generously provided by Professor Shouyi Chen and Jinsong Zhang, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, P. R. China.
Generation of transgenic eggplants
Leaf segments derived from in vitro-regenerated 1-month-old plants were used as explants for transformation and were pre-cultured for 2 days on MS medium augmented with 11.10 μM 6-Benzylaminopurine (BAP) and 2.85 μM Indole-3-acetic acid(IAA). Pre-cultured leaf explants were used to infect with Agrobacterium suspension harboring pBin438-TaNHX2 plasmid for 10 min. The infected explants were blotted on a sterile filter paper and transferred onto co-cultivation medium for 2 days. During co-cultivation, explants were placed on solid MS medium with 30 g l−1 sucrose, 4 g l−1 agar, 11.10 μM BAP + 2.85 μM IAA, and 100 μM acetosyringone for 2 days maintained in the dark at 25 ± 2 °C. After co-cultivation, leaf explants were transferred onto selection medium containing MS medium with 11.10 μM BAP + 2.85 μM IAA, 100 mg l−1 kanamycin, and 250 mg l−1 cefotaxime and cultured under 16 h photoperiod at 25 ± 2 °C for 4 weeks. Explants were transferred to a fresh medium once every 2 weeks until kanamycin-resistant buds differentiated and shoots developed. The cultures were transferred onto fresh shoot elongation medium supplemented with 2.22 μM BAP, 100 mg l−1 kanamycin, and 250 mg l−1 cefotaxime for two sequences of subculture until the shoots achieved a height of 2–4 mm. After 3–4 weeks, the elongated shoots (15–20 mm) were transferred onto half-strength MS medium containing 7.35 μM IBA, 50 mg l−1 kanamycin, and 250 mg l−1 cefotaxime for rooting. The putative transformed plantlets with well-developed shoots and roots were shifted to pots containing organic substrate and vermiculite (3:1) mixtures, maintained in a greenhouse at 25 ± 2 °C under a 16/8-h light/dark photoperiod and a light intensity of 60 μmol m−2 s−1.
Molecular analysis of transgenic eggplants
PCR confirmation of transgenic plants
Genomic DNA was isolated from kanamycin-resistant plants (T0) as well as from non-transformed plants by CTAB method (Dellaporta et al. 1983) to test for the presence of TaNHX2 gene in the putative eggplant transgenics using gene-specific primers (TaNHX2-1-F and TaNHX2-1-R; Supplementary Table S1), which was expected to produce 800 bp, corresponding to the TaNHX2 gene. PCR amplifications were conducted with initial denaturation at 94 °C for 30 s, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 58 °C for 30 s, extension at 72 °C for 50 s, and final extension at 72 °C for 10 min. Similar PCR conditions were also used for detection of TaNHX2 in the T1 and T2 generations. The amplified PCR products were separated on a 1.0% (w/v) agarose gel and visualized using a gel documentation system.
Southern hybridization analysis
Southern blot analysis was performed to verify the TaNHX2 gene integration and copy number. Genomic DNA (~ 15 μg) isolated from PCR positive plants and non-transformed plants was digested with restriction enzyme, HindIII. The digested DNA was separated by electrophoresis on a 0.8% agarose gel and then blotted onto Hybond N+ nylon membrane (GE Bioscieces, Hong Kong) according to the manufacturer’s instructions. A 0.80-kb PCR product of the TaNHX2 gene fragment was used as a probe, and its radiolabelling was carried out using BioPrime DNA Labeling System (Fischer Scientific, India). After transfer to nylon membrane and hybridizing with probe, the insertion copy number of the transgene was observed on autoradiography film.
RNA isolation and semi-quantitative RT-PCR
Total RNA was extracted using RNAiso Plus reagent (Takara, India) from the T2 southern positive transgenic and non-transformed plants for TaNHX2 expression analysis. DNase-treated total RNA samples (2 μg) were used for the synthesis of first strand cDNA using MaximaÆ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, India). Semi-quantitative RT-PCR was performed using primers (TaNHX2-2-F and TaNHX2-2-R; Supplementary Table S1) for amplifying a 376-bp fragment of TaNHX2. Eggplant adenine phosphoribosyl transferase (APRT, accession JX448345) used as reference gene (Gantasala et al. 2013) to check the expression levels of transgenes using primer pair (APRT-1-F and APRT-1-R; Supplementary Table S1), which give a 163-bp product with cDNA. The PCR products were analyzed on 1% agarose gel and stained with ethidium bromide.
Real-time PCR
To investigate the TaNHX2 expression level of transgenic eggplants, quantitative real-time PCR (qRT-PCR) assay was conducted using TaNHX2 gene-specific primers (TaNHX2-3-F and TaNHX2-3-R; Supplementary Table S1) and eggplant adenine phosphoribosyl transferase gene (APRT, accession JX448345) (APRT-2-F and APRT-2-R; Supplementary Table S1) as an internal control. Leaves were harvested from T2 transgenic plants (B1, B2, B4, B5, B8, and B11) and the non-transformed plants when subjected to 200 mM salt stress. Three biological replicates and three technical replications were performed. All experiments were done in triplicate for cDNA synthesis from three batches of plants.
Evaluation of transgenic eggplants under salt stress
Salt stress of transgenic egg plants was assessed by growing T1 transgenic and control plants in hydroponics and 1-month-old T2-independent transgenic lines and control plants in pots and exposing them to salt stress under greenhouse conditions. T1 transgenic lines and control plants were exposed to NaCl stress (150 mM) in hydroponic method for 2 days followed by recovery in water without NaCl for 5 days. The fresh weight and dry weight were recorded after 2 days of NaCl stress. T2 transgenic eggplant and control plants grown in pots were irrigated with water once in 2 days with gradually increasing concentrations of NaCl (50, 100, 150, and 200 mM), and the 200 mM NaCl treatment continued till 3 weeks. The growth and phenotype were observed after salt stress.
Physiological and biochemical analysis of transgenic and WT eggplants exposed to salt stress
Physiological and biochemical experiments were performed when plants exposed to 200 mM NaCl stress. The leaves and roots of untreated controls (UC, wild-type plants without salt stress), salt-treated control (SC, wild-type plants exposed to 200 mM NaCl), and salt treated (200 mM NaCl) T2 transgenic lines (B1, B2, B4, B5, B8, and B11) were collected and used for determination of cellular Na+ and K+ concentrations, relative water content (RWC), total chlorophyll, proline, ascorbate and malondialdehyde (MDA) content. Quantification of H2O2 and O2− and antioxidant enzymes assay were carried out in the leaves of materials (UC, SC, T2 lines). For Na+ and K+ concentration measurements, the harvested roots and leaves described above were dried before digesting with concentrated HNO3 at 90 °C for 30 min and centrifuged at 12,000 rpm for 10 min. The digested samples were diluted with sterile Milli-Q water and analyzed for Na+ and K+ content in flame photometer. All measurements were conducted in triplicate. To investigate effect of salt stress on chlorophyll fluorescence of transgenic and control plants, chlorophyll fluorescence (Fv/Fm) was measured with MINI-PAM-II, photosynthesis yield analyzer (Heinz Walz GmbH, Germany). Apart from that, stomatal conductance and transpiration rate of above described plants were also measured by leaf porometer (DECAGON Devices, USA). The photosynthetic and chlorophyll fluorescence estimations were taken in the vicinity of 10:00 and 12:00 h when the encompassing light force was 1000–1200 μmol m−2 s−1. After growth in greenhouse for 4 weeks, fully expanded leaves were harvested for estimation of RWC according to Yarra et al. (2012). The total chlorophyll content of the leaves was estimated according to the method described by Hiscox and Israelstam (1979). Antioxidant enzyme assays, ascorbate peroxidase, APX (Chen and Asada 1989); superoxide dismutase, SOD (Wang et al. 2012); guaiacol peroxidase, GPX (Chance and Maehly 1955); glutathione reductase, GR (Smith et al. 1988); free proline (Bates et al. 1973); malondialdehyde, MDA (Heath and Packer 1968); hydrogen peroxide, H2O2 (Sagisaka 1976); and super oxide, O2− (Elstner and Heupel 1976) were carried out by collecting the leaf materials from the salt stressed (200 mM NaCl) plants as well as from control plants.
Statistical analysis
All experiments were comprised of three samples and performed three times. All data were presented as mean ± SD. Statistical significance was determined by Student’s t test. Significance was defined as P ≤ 0.05 and indicated by asterisks.
Results
Eggplant transformation and selection of transformants
The binary vector pBin438-TaNHX2 was introduced in to eggplant genome via A. tumefaciens (LBA4404)-mediated genetic transformation approach. About 70% pre-cultured leaf explants, which had been co-cultivated with Agrobacterium, formed shoot buds on MS medium augmented with 11.10 μM BAP + 2.85 μM IAA, 100 mg l−1 kanamycin, and 250 mg l−1 cefotaxime. Shoots developed from the leaf explants on media of similar composition after four successive weeks of culture (Supplementary Fig. S2b), whereas no shoots were observed from untransformed leaf explants on MS medium containing 100 mg l−1 kanamycin (Supplementary Fig. S2a). The elongation of shoots was achieved on MS media containing 2.22 μM BAP, 100 mg l−1 kanamycin, and 250 mg l−1 cefotaxime after 3 weeks of culture (Supplementary Fig. S2c). The elongated shoots were multiplied subsequently on the same media to recover putative transgenic plants (Supplementary Fig. S2d). The recovered putative transgenic lines were rooted on half-strength MS medium containing 7.35 μM IBA, 50 mg l−1 kanamycin, and 250 mg l−1 cefotaxime (Supplementary Fig. S2e). Subsequently, they were transferred to the greenhouse for acclimatization (Supplementary Fig. S2f). The T0 transgenic plants were self-pollinated to produce T1 seeds. These transgenic eggplants from each line were phenotypically identical and indistinguishable from control plants.
Transgene integration and expression
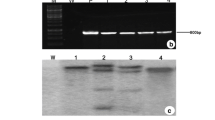
PCR analysis for genomic integration of the TaNHX2 gene was carried out on T0 plants randomly with one non-transgenic line as a negative control. The amplification reaction was carried out using TaNHX2 gene-specific primers revealed that all the kanamycin-resistant plants became acclimatized and exhibited positive amplification. An 800-bp fragment was observed with the TaNHX2 gene-specific primers (Fig. 1a), whereas no corresponding band was detected in the untransformed plant (Fig. 1a). The integration of the TaNHX2 gene into the eggplant genome was further analyzed by southern hybridization for revealing the copy number of transgene. The genomic DNA of TaNHX2 eggplant transgenics (T2) and non-transformed plants was digested with HindIII and probed with TaNHX2 gene. The transformed plants demonstrated single to three copy integration events, whereas no hybridization signal was noticed in non-transformed plants (Fig. 1b). The expression of double CaMV35S promoter–driven TaNHX2 gene in T2 plants was confirmed by RT-PCR using TaNHX2 primers specific to 376-bp fragment. As expected, 376-bp band was observed and confirmed the expression of TaNHX2 in transgenic T2 plants, whereas no expression was observed in control plants (Fig. 2a). Further verification by real-time PCR analysis also showed the significant expression of TaNHX2 transcript in all tested T2 transgenic eggplants under 200 mM NaCl stress (Fig. 2b). As expected, no expression was observed in non-transformed plants (Fig. 2b).
Molecular analysis of transgenic lines overexpressing TaNHX2.a PCR confirmation of 0.8 kb product of TaNHX2 in transgenic lines (B1, B2, B4, B5, B8, and B11). M marker, NT non-transformed. b Southern blot hybridization of HindIII digested genomic DNA of the six independent T2 transgenic and NT plants using TaNHX2 probe
Expression analysis of TaNHX2 gene in T2 transgenic eggplants. aTaNHX2 expression analysis by semi-quantitative RT-PCR in southern positive T2 transgenic lines (B1, B2, B4, B5, B8, and B11) using TaNHX2 gene-specific primers. Eggplant adenine phosphoribosyl transferase gene (APRT) as internal control. b Real-time RT-PCR analysis used to further precisely quantified the expression levels of TaNHX2 in transgenic eggplants (B1, B2, B4, B5, B8, and B11). APRT gene was used as an internal control
Enhanced tolerance of transgenic plants to high salinity stress (150 mM and 200 mM NaCl)
To further understand the expression of TaNHX2 improves salinity tolerance of transgenic egg plants, the performance of transgenic plants against NaCl-induced salinity stress was verified. All plants (NT and T1 transgenic (B2, B4, B5) were watered with 150 mM NaCl in hydroponic method for 2 days in a greenhouse and allowed recovery for up to 5 days. Remarkably, transgenic plants exhibited an increased growth with more leaves, compared with non-transformed plants (Fig. 3). In addition, the transgenic plants exhibited higher fresh weights and biomass than control plants under salt treatment (Fig. 3b, c). The seedlings of three T1 transgenic lines (B2, B4, and B5) showed 2.07, 2.2, and 1.99 times more fresh weight (Fig. 3b), and 1.52, 1.58, and 1.43 times more dry weight (Fig. 3c) respectively, as compared to seedlings of non-transformed plants.
a Phenotypic performance of non-transformed (NT) and transgenic T1 seedlings (B2, B4, B5) after salt stress (150 mM) for 2 days. b Fresh weight. c Dry weight in TaNHX2 transgenic and NT seedlings after salt stress (150 mM). Values are mean ± SD (n = 3). Asterisks indicate significant difference compared with NT at P ≤ 0.05
One-month-old non-transformed plants and southern positive T2 lines (B1, B2, B4, B5, B8, and B11) were assessed for salinity stress test grown in pots in greenhouse. These plants were irrigated with increasing concentrations of NaCl (0, 50, 100, and 150 mM) for 7 days followed by 200 mM NaCl for a period of 3 weeks (Fig. 4). The transgenic and non-transformed plants were phenotypically indistinguishable under normal conditions (0 mM NaCl) (data not shown). However, after treatment with increased salinity stress conditions, the transgenic plants appeared phenotypically close to normal and increased growth even at 200 mM NaCl (Fig. 4), whereas non-transformed plants were chlorotic with retarded growth and ultimate death (Fig. 4).
Enhanced salt tolerance (200 mM NaCl) of TaNHX2 overexpressing transgenic eggplants. One-month-old non-transformed plants and southern positive T2 lines (B1, B2, B4, B5, B8, and B11) were subjected salinity stress test grown in pots in greenhouse. These plants were irrigated with increasing concentrations of NaCl (0, 50, 100, and 150 mM) for 7 days followed by 200 mM NaCl for a period of 3 weeks. NT plants show severe chlorosis caused by Na+ toxicity, whereas the transgenic eggplants show normal phenotype
Increased ion content (Na+ and K+) in transgenic plants
To determine whether overexpression of TaNHX2 enhances the concentration of ions in transgenic plants, Na+ and K+ in leaf and root tissues from transgenic and non-transformed were measured before and after salt treatment. Na+ content of leaves and roots increased in transgenic plants than non-transformed plants at 200 mM NaCl (Fig. 5a, d). Similarly, treatment with 200 mM NaCl led to significantly increase of K+ contents in both leaf and root tissues of transgenic plants compared to non-transformed plants (Fig. 5b, e). The similar results were observed that the K+/Na+ ratio of leaves and roots of transgenic plants was also higher than that of non-transformed plants (Fig. 5c, f). The transgenic plants accumulated more Na+ and K+ in both leaves and roots than non-transformed plants which were treated with NaCl, indicating that the overexpression of TaNHX2 induced the accumulation of Na+ and K+ under salt stress conditions.
Sodium (Na+), potassium (K+) ion contents, and K+/Na+ ratios of leaves and roots from T2 transgenic eggplants and non-transformed plants (UC, SC) under salt stress (200 mM NaCl). a Na+ content in leaves. b K+ content in leaves. c K+/Na+ in leaves. d Na+ content in roots. e K+ content in roots. f K+/Na+ in roots. Ion content measurements are expressed in “μmol g−1 DW.” UC untreated control, SC salt-treated control. B1, B2, B4, B5, B8, and B11: salt-treated transgenic plants. Values are mean ± SD (n = 3). Asterisks indicate significant difference compared with SC at P ≤ 0.05
Physiological responses in transgenic eggplants under salt stress
To explore the consequence of TaNHX2 overexpression on salinity tolerance of eggplants, we examined the following physiological parameters when transgenic and non-transformed plants subjected to 200 mM NaCl stress: relative water content, chlorophyll content, proline, ascorbate, and MDA contents. In the absence of salinity stress, no significant differences were found for these physiological parameters tested between non-transgenic and transgenic lines (data not shown). Relative water content and chlorophyll content of transgenic plants was higher than that of non-transformed plants under salt stress (Fig. 6a, b). The percentage of relative water content in transgenic lines was ranging from 77 to 79%, while it was only 45% in salt treated non-transformed plants (Fig. 6a). Interestingly, chlorophyll content of transgenic eggplants was significantly higher when subjected to 200 mM NaCl compared to non-transformed plants and 1.68–1.60-fold higher (Fig. 6b). TaNHX2-overexpressing plants had less MDA content (− 2.5- to − 2.2-fold) (Fig. 7a) than non-transformed plants under salinity stress, indicating salinity stress damage due to reactive oxygen species in non-transformed plants (Fig.7a). When subjected to salinity stress (200 mM NaCl), an obvious increase in proline content was observed in transgenic lines relative to the non-transformed plants (Fig.7b) and found to be 2.0- to 2.25-fold higher accumulation in transgenic plants (Fig. 7b). Quantification of H2O2 and O2− in both transgenic and non-transformed plants suggested that the transgenic lines displayed a significant inhibition in H2O2 and O2− accumulation compared to non-transformed plants under salinity stress (Fig. 7c, d).
Stabilized relative water content (RWC) and chlorophyll content in TaNHX2 overexpressed T2 transgenic eggplants under salt stress (200 mM NaCl). a Relative water content (%). b Chlorophyll content (mg g−1 FW). UC untreated control, SC salt-treated control. B1, B2, B4, B5, B8, and B11: salt-treated transgenic plants. Values are mean ± SD (n = 3). Asterisks indicate significant difference compared with SC at P ≤ 0.05
Analysis of lipid peroxidation, proline accumulation, and ROS scavenging capacity in T2 transgenic eggplants and non-transformed plants (UC, SC) under salt stress (200 mM NaCl). a Levels of lipid peroxidation expressed in terms of MDA content (nmol g−1 FW). b Changes in the level of proline accumulation (μmol g−1 FW). c H2O2 content (μmol g−1 FW). d O2− content (μmol g−1 FW). UC untreated control, SC salt-treated control. B1, B2, B4, B5, B8, and B11: salt-treated transgenic plants. Values are mean ± SD (n = 3). Asterisks indicate significant difference compared with SC at P ≤ 0.05
Examining the consequence of salt stress on chlorophyll fluorescence of transgenic and non-transformed eggplants subjected to salinity stress (200 mM NaCl), it showed that the maximum efficiency of photosystem II (Fv/Fm) was found be increased significantly (1.60–2.00-fold) in transgenic compared to non-transformed plants (Fig. 8a). Similarly, the transpiration rate and stomatal conductance were higher in transgenic eggplants compared to non-transformed plants (Fig. 8b, c). Transgenic plants maintained 1.45–1.75-fold higher transpiration rate (Fig. 8b) and 1.35–1.75-fold higher stomatal conductance (Fig. 8c) than non-transformed plants.
Photosynthetic performance in the leaves of T2 transgenic eggplants and non-transformed plants (UC, SC) under salt stress (200 mM NaCl). a PSII photosynthetic efficiency (Fv/Fm). b Transpiration rate (mM (H2O) m−2x s−1). c Stomatal conductance (μM(H2O) m−2x s−1). UC untreated control, SC salt-treated control. B1, B2, B4, B5, B8, and B11: salt-treated transgenic plants. Values are mean ± SD (n = 3). Asterisks indicate significant difference compared with SC at P ≤ 0.05
Expression of TaNHX2 improves ROS scavenging capacity in transgenic eggplants
Antioxidant enzymes are responsible for scavenging of ROS in plants. Subsequent assays were carried out to investigate superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX) and glutathione reductase (GR) activities in transgenic and non-transformed plants subjected to 200 mM NaCl stress. In non-transformed plants, the SOD, APX, GPX, and GR activities were lower under salinity stress. However, in transgenic lines, the salinity stress effects on SOD, APX, GPX, and GR activities were significantly alleviated than the non-transformed plants (Fig. 9a–d). The T2 transgenic eggplants exhibited 2.42–2.43-fold SOD (Fig. 9a), 1.61–1.75-fold APX (Fig. 9b), 1.35–1.42-fold GPX activity (Fig. 9c), and 1.46–1.73-fold GR activity (Fig. 9d) higher than non-transformed plants.
Effect of salt stress (200 mM NaCl) on antioxidant responses of T2 transgenic eggplants and non-transformed plants (UC, SC). a Changes in superoxide dismutase enzyme activity (SOD, unit g−1 FW). b Changes in ascorbate peroxidase enzyme activity (APX, unit g−1 FW). c Changes in guaiacol peroxidase enzyme activity (GPX, unit g−1 FW). d Changes in glutathione reductase enzyme activity (GR, unit g−1 FW). UC untreated control, SC salt-treated control. B1, B2, B4, B5, B8, and B11: salt-treated transgenic plants. Values are mean ± SD (n = 3). Asterisks indicate significant difference compared with SC at P ≤ 0.05
Discussion
Salinity is one of the main threats to sustainable agriculture worldwide (Yadav et al. 2011; Hasegawa 2013). Excessive salinity in soil and irrigation water significantly disturbs the growth, development, and productivity of vegetable crops (FAO 2002; AVRDC 2006). The TaNHX2 gene discussed in this study belongs to class I NHX family localized in vacuolar membrane which is the unique feature of this class. Previous studies have shown that TaNHX2 gene can enhance the salinity tolerance in few species (Cao et al. 2011; Zhang et al. 2015; Wu et al. 2012; Yarra et al. 2012; Bulle et al. 2016). Therefore, to improve salinity tolerance of plants through overexpression of TaNHX2 gene by transgenic technology has attracted ample attention of plant researchers. Eggplant is an essential vegetable for diet consumption worldwide, and the growth and development of eggplants have been affected by salinity stress, leading to the substantial drop of eggplant productivity. In this work, we intended to improve salinity stress tolerance of eggplants by overexpression of TaNHX2 gene through Agrobacterium mediated transformation.
Salinity-induced fresh weight reduction is a common phenomenon for most of the crop plants and studied (Mozafariyan et al. 2013). Plant dry matter content is a worthwhile consideration to evaluate the plant strategy for the use and procurement of resource. Dadkhah and Grrifiths (2006) reported that plant dry matter is significantly reduced under salinity stress conditions. The effect of salt stress (150 mM NaCl) on biomass of transgenic eggplants overexpressing TaNHX2 was evaluated at seedling stage, and the T1 lines exhibited significantly higher fresh and dry weight compared with non-transformed plants. This significant increase in biomass under salinity stress clearly indicated that the transgenic eggplants withstand to salinity stress conditions.
Compared with the non-transformed plants, the T2 eggplants exhibited improved salinity tolerance. After exposure to high salinity stress (200 mM NaCl), the non-transformed plants displayed growth inhibition, chlorosis, and even death, whereas the T2 plants maintained their normal growth and survival. These results indicate that overexpression of TaNHX2 gene under salt stress is likely responsible for the increased compartmentalization of Na+ into vacuoles confers the improved salt tolerance (Gaxiola et al. 2001, 2007; Yamaguchi et al. 2013; Yarra et al. 2012, Bulle et al. 2016, Sahoo et al. 2016).
Ion homeostasis with low Na+ and high K+ concentrations in the cytoplasm is vital for maintaining normal metabolic and physiological processes, e.g., the activity of many cytosolic enzymes (Zhu 2003). A commensurate increase was found in the Na+/K+ contents in leaves and roots of TaNHX2 transgenic compared with non-transformed plants under salt stress conditions, indicating that Na+/H+ antiporter gene TaNHX2 improved the salt tolerance by increasing Na+ accumulation and retained K+/Na+ equilibrium. However, accumulation of Na+ content in roots is significantly higher than the leaves in transgenic plants. This is possibly advantageous for the normal growth and development of transgenic plants under slat stress conditions. The occurrence of higher Na+ and K+ contents in leaves and roots of transgenic plants overexpressing NHX genes was also reported in mungbean (Sahoo et al. 2016), sweet potato (Fan et al. 2015), and alfalfa (Li et al. 2011). Our results strongly supported that NHX proteins largely functioned as Na+/K+(H+) antiporter and played significant role in Na+/K+ homeostasis by regulating their uptake, transport, and compartmentalization.
Measurement of relative water content (RWC) is necessary to know the plant water status under salinity stress to ascertain that up to what extent cellular water content is retained, as all metabolic activities within the cell are dependent on the availability of adequate amount of water (Haripriya et al. 2010; Ashraf et al. 2011). Shaheen et al. (2013) reported that there is a significant decrease in relative water content of eggplants under salinity stress. In contrast, TaNHX2 overexpressing transgenic eggplants displayed significant increase in relative water content compared to non-transformed plants under high salinity conditions (200 mM NaCl). Our results are in agreement with the previous reports, where relative water content of the TaNHX2 expressing transgenic plants is significantly higher compared to non-transformed plants under salt stress conditions (Bulle et al. 2016; Yarra et al. 2012).
At 200 mM NaCl salt stress, the decrease in chlorophyll contents in the transgenic plants was lesser than the non-transformed plants, indicating a positive association between the expression of TaNHX2 and salinity stress tolerance in leaf tissues. These finding are consistent with the previous reports of TaNHX2 overexpression in tomato (Yarra et al. 2012) and chili pepper plants (Bulle et al. 2016). Photosynthetic apparatus is sensitive and easily damaged under salt stress conditions (Sixto et al. 2006), which leads to reduction of the complete plant growth and development. The transgenic eggplants expressing TaNHX2 gene were found able to retain higher chlorophyll fluorescence ratio (Fv/Fm), higher transpiration rate, and higher stomatal conductivity under 200 mM NaCl stress compared to non-transformed plants. These observations indicated that the overexpression of TaNHX2 in eggplant alleviated the inhibition of photosynthesis and PSII photoinhibition under salt stress conditions. This is consistent with previous reports, which suggest that NHX gene expression in transgenic plants protects the damage of photosynthetic apparatus under salt stress conditions (Kumar et al. 2017).
It has been known that variation in the levels of free proline content is a common phenomenon in plants response to salinity stress (Liu and Zhu 1997; Armengaud et al. 2004). We also found the increased amounts of proline contents in transgenic plants than non-transformed plants under high saline conditions (200 Mm NaCl), suggesting that TaNHX2 may induce the proline synthesis genes to confer salt tolerance in eggplants. This is consistent with the results obtained from overexpressing TaNHX2 in chili pepper plants (Bulle et al. 2016), RtNHX1 in Arabidopsis (Li et al. 2017), and AtNHX1 in sweet potato (Fan et al. 2015).
Oxidative stress is an indication, evidenced by the accumulation of reactive oxygen species ROS in plants when subjected to salinity stress (Verslues et al. 2007). However, plants have adopted a complex antioxidant system to detoxify stress-induced ROS, in which various enzymes play vital roles, in order to scavenging ROS and protecting the cells against oxidative stress (Jaleel et al. 2009; Miller et al. 2010). In salt-stressed eggplants, increased oxidative stress was observed with improved H2O2 and O2− contents, however significantly less H2O2 and O2− and increased SOD, APX, GPX, and GR activities in TaNHX2 transgenic eggplants in comparison with non-transformed plants. Lipid peroxidation generally happens through accumulation of MDA content when plants subjected to abiotic stresses (Verslues et al. 2007). Indeed, TaNHX2 transgenic plants displayed less accumulation of MDA content in leaves compared to non-transformed plants, indicating effectively improved cell membrane homeostasis leads to the salinity tolerance of transgenic eggplants. Previous studies indicated that transgenic plants overexpressing NHXs were more effective in scavenging reactive oxygen species because they had improved antioxidant enzyme activity and decreased MDA content (Li et al. 2017; Sahoo et al. 2016; Bulle et al. 2016; Gouiaa et al. 2012; Wei et al. 2011; Wang et al. 2016). Taken together, all these results indicated that TaNHX2 gene was able to confer salt tolerance in transgenic eggplants and might facilitate the transgenic plants to make the indispensable osmotic and antioxidant adjustments.
In conclusion, our results confirmed that heterologous expression of TaNHX2 gene explicitly improved salt tolerance and growth of transgenic eggplants, through enhanced sequestration of ions into the vacuoles, unhampered photosynthesis, altering the activation of ROS scavenging system, and the levels of protective compounds, such as MDA and proline. However, the fact that overexpression of TaNHX2 dramatically enhances salinity tolerance of eggplants suggests that this gene will have the potential to prominently improve stress tolerance in other vegetable crops like eggplant. Further field trials of transgenic eggplants under severe environmental conditions such as salinity remain to be carried out in order to grow them in salt-affected regions. This data laid a concrete foundation for engineering NHX genes like TaNHX2 for developing salinity stress-tolerant edible crop plants without any growth defects.
Abbreviations
- RT-PCR:
-
Reverse transcription PCR
- qRT-PCR:
-
Quantitative real-time PCR
- SOD:
-
Superoxide dismutase
- APX:
-
Ascorbate peroxidase
- GPX:
-
Guaiacol peroxidase
- GR:
-
Glutathione reductase
- MDA:
-
Malondialdehyde
References
Abbas W, Ashraf M, Akram NA (2010) Alleviation of salt-induced adverse effects in eggplant (Solanum melongena L.) by glycinebetaine and sugarbeet extracts. Sci Hortic 125:188–195
Akinci IE, Akinci S, Yilmaz K, Dikici H (2004) Response of eggplant varieties (Solanum melongena) to salinity in germination and seedling stages. New Zealand J Crop and Hort Sci 32:193–200
Almeida DM, Oliveira MM, Saibo NJM (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt tolerance in crop plants. Genet Mol Biol 40:326–345
Armengaud P, Thiery L, Buhot N, Grenier-De March G, Savoure A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120:442–450
Ashraf M, Akram NA, Al-Qurainy F, Foolad MR (2011) Drought tolerance: roles of organic osmolytes, growth regulators and mineral nutrients. Adv Agron 111:24996
AVRDC (2006) Proceedings of the 2006 APSA-AVRDC workshop. AVRDC-The world vegetable center, Shanhua, Tainan, Taiwan. AVRDC Publication, p 06–677
Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol 22:1–6
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bhaskaran S, Savithramma DL (2011) Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J Exp Bot 62:5561–5570
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434
Bresler E, McNeal BL, Carter DL (1982) Saline and sodic soils. Springer-Verlag, Berlin
Brini F, Gaxiola RA, Berkowitz GA, Masmoudi K (2005) Cloning and characterization of a wheat vacuolar cation/proton antiporter and pyrophosphatase proton pump. Plant Physiol Biochem 43:347–354
Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter TNHX1 and Hþ-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58:301–308
Bulle M, Yarra R, Abbagani S (2016) Enhanced salinity stress tolerance in transgenic chilli pepper (Capsicum annuum L.) plants overexpressing the wheat antiporter (TaNHX2) gene. Mol Breed 36:36. https://doi.org/10.1007/s11032-016-0451-5
Cao D, Hou W, Liu W, Yao WW, Wu C, Liu X, Han T (2011) Overexpression of TaNHX2 enhances salt tolerance of ‘composite’ and whole transgenic soybean plants. Plant Cell Tissue Organ Cult 107:541–552
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30(7):987–998
Chen H, An R, Tang JH, Cui XH, Hao FS, Chen J, Wang XC (2007) Over-expression of a vacuolar Na+/H+antiporter gene improves salt tolerance in an upland rice. Mol Breed 19:215–225
Dadkhah AR, Grrifiths H (2006) The effect of salinity on growth, inorganic ions and dry matter partitioning in sugar beet cultivars. J Agric Sci Technol 8:199–210
Daunay M (2008) Eggplant. In: Vegetables II, Prohens J, Nuez F (eds) Handbook of plant breeding. Springer, New York, pp 163–220
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Report 1:19–21
Doganlar S, Frary A, Daunay MC, Lester RN, Tanksley SD (2002) A comparative genetic linkage map of eggplant (Solanum melongena) and its implications for genome evolution in the solanaceae. Genetics 161:1697–1711
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Fan W, Deng G, Wang H, Zhang H, Zhang P (2015) Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas). Physiol Plant 154:560–571
FAO (2002) Working with local institutions to support sustainable livelihoods. Food and Agriculture Organization, Rome
Fukuda A, Nakamura A, Tanaka Y (1999) Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. Biochim Biophys Acta 1446:149–155
Gantasala NP, Papolu PK, Thakur PK, Kamaraju D, Sreevathsa R, Rao U (2013) Selection and validation of reference genes for quantitative gene expression studies by real-time PCR in eggplant (Solanum melongena L). BMC Res Notes 6:312
Gaxiola RA, Rao R, Sherman A, Grisafi F, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNHX1 and AVP1, can function in cation detoxification in yeast. Proc Natl Acad Sci U S A 96:1480–1485
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001) Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci U S A 98:11444–11449
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Gouiaa S, Khoudi H, Leidi EO, Pardo JM, Masmoudi K (2012) Expression of wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Mol Biol 79(1):137–155
Haripriya D, Selvan N, Jeyakumar N, Periasamy R, Marimuthu J, Irudayaraj V (2010) The effect of extracts of Selaginella involvens and Selaginella inaequalifolia leaves on poultry pathogens. Asian Pac J Trop Med 3:67881
Hasegawa PM (2013) Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot 92:19–31
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi HJ et al (2009) Antioxidant defense response: physiological plasticity in higher plants under abiotic constrains. Acta Physiol Plant 31:427–436
Jiang X, Leidi EO, Pardo JM (2010) How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal Behav 55:792–795
Koike M, Sugimoto M, Aiuchi D, Nagao H, Shinya R, Tani M, Kuramochi K (2007) Reclassification of Japanese isolate of Verticillium lecanii to Lecanicillium spp. Jpn J Appl Entomol Zool 51:234–237
Kumar SK, Sivanesan I, Murugesan K, Jeong BR, Hwang SJ et al (2014) Enhancing salt tolerance in eggplant by introduction of foreign halotolerance gene, HAL1isolated from yeast. Hortic Environ Biotechnol 55:222–229. https://doi.org/10.1007/s13580-014-0141-3
Kumar S, Kalita A, Srivastava R, Sahoo L (2017) Co-expression of Arabidopsis NHX1 and bar improves the tolerance to salinity, oxidative stress, and herbicide in transgenic mungbean. Front Plant Sci 8:1896
Leidi EO, Barragan V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B, Fernandez JA, Bressan RA, Hasegawa PM, Quintero FJ, Pardo JM (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61:495–450
Li J, Jiang G, Huang P, Ma J, Zhang F (2007) Overexpression of the Na+/H+ antiporter gene from Suaeda salsa confers cold and salt tolerance to transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cult 90:41–48
Li W, Wang D, Jin T, Chang Q, Yin D, Xu S, Liu B, Liu L (2011) The vacuolar Na+/H+ antiporter gene SsNHX1 from the halophyte Salsola soda confers salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Mol Biol Report 29:278–290
Li N, Wang X, Ma B, Du C, Zheng L, Wang Y (2017) Expression of a Na+/H+ antiporter RtNHX1 from a recretohalophyte Reaumuria trigyna improved salt tolerance of transgenic Arabidopsis thaliana. J Plant Physiol 218:109–120
Liang W, Ma X, Wan P, Liu L (2018) Plant salt-tolerance mechanism: a review. Biochem Biophys Res Commun 495:286–291
Liu J, Zhu JK (1997) Proline accumulation and salt–stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114:591–596
Lu W, Guo C, Li X, Duan W, Ma C, Zhao M, Gu J, Du X, Liu Z, Xiao K (2014) Overexpression of TaNHX3, a vacuolar Na+/H+ antiporter gene in wheat, enhances salt stress tolerance in tobacco by improving related physiological processes. Plant Physiol Biochem 76:17–28
McCubbin T, Bassil E, Zhang S, Blumwald E (2014) Vacuolar Na+/H+ NHX-type antiporters are required for cellular K+ homeostasis, microtubule organization and directional root growth. Plants 3:409–426
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mozafariyan M, Bayat KSAE, Bakhtiari S (2013) The effects of different sodium chloride concentrations on the growth and photosynthesis parameters of tomato (Lycopersicum esculentum cv. Foria). Int J Agri Crop Sci 6(4):203–207
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays of tobacco tissue cultures. Physiol Plant 15:473–497
Prabhavathi VR, Rajam MV (2007) Polyamine accumulation in transgenic eggplant enhances tolerance to multiple abiotic stresses and fungal resistance. Plant Biotechnol 24:273–282
Prabhavathi V, Yadav JS, Kumar PA, Rajam MV (2002) Abiotic stress tolerance in transgenic eggplant (Solanum melongena L.) by introduction of bacterial mannitol phosphodehydrogenase gene. Mol Breed 9:137–147
Prasad SM, Parihar P, Singh VP (2014) Effect of salt stress on nutritional value of vegetables. Biochem Pharmacol 3:e160. https://doi.org/10.4172/2167-0501.1000e160
Sagisaka S (1976) The occurrence of peroxide in a perennial plant, Populus gelrica. Plant Physiol 57:308–309
Sahoo DB, Kumar S, Mishra S, Kobayashi Y, Panda SK, Sahoo L (2016) Enhanced salinity tolerance in transgenic mungbean overexpressing Arabidopsis antiporter (NHX1) gene. Mol Breed 36:144. https://doi.org/10.1007/s11032-016-0564-x
Shahbaz M, Ashraf M, Al-Qurainy F, Harris PJC (2012) Salt tolerance in selected vegetable crops. Crit Rev Plant Sci 31(4):303–320. https://doi.org/10.1080/07352689.2012.656496
Shaheen S, Naseer S, Ashraf M, Akram NA (2013) Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J Plant Interact 8:85–96
Shalhevet J, Heuer B, Meiri A (1983) Irrigation interval as a factor in the salt tolerance of eggplant. Irrig Sci 4:83–93
Shi H, Lee BH, Wu SJ et al (2002) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Sixto H, Aranda I, Grau JM (2006) Assessment of salt tolerance in Populus alba clones using chlorophyll fluorescence. Photosynthetica 44:169–173
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,50-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Tang R, Li C, Xu K, Du Y, Xia T (2010) Isolation, functional characterization, and expression pattern of a vacuolar Na(+)/H(+) antiporter gene TrNHX1 from Trifolium repens L. Plant Mol Biol Report 28:102–111
Unlukara A, Kurunc A, Kesmez GD, Yurtseven E, Suarez DL (2010) Effects of salinity on eggplant (Solanum melongena L.) growth and evapotranspiration. Irrig Drain 59:203–214
Verslues PE, Batelli G, Grillo S, Agius F, Kim YS, Zhu JH, Agarwal M, Katiyar-Agarwal S, Zhu JK (2007) Interaction of SOS2 with nucleoside diphosphate kinase 2 and catalases reveals a point of connection between salt stress and H2O2 signalling in Arabidopsis thaliana. Mol Cell Biol 27:7771–7780
Wang N, Hua H, EgrinyaEneji A, Li Z, Duan L, Tian X (2012) Genotypic variations in photosynthetic and physiological adjustment to potassium deficiency in cotton (Gossypium hirsutum L.). J. Photochem Photobiol 110:1–8
Wang B, Zhai H, He S, Zhang H, Ren Z, Zhang D, Liu Q (2016) A vacuolar Na+/H+, antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweetpotato. Sci Hortic 201:153–166
Wei Q, Guo YJ, Cao HM, Kuai BK (2011) Cloning and characterization of an AtNHX2-like Na+/H+ antiporter gene from Ammopiptanthus mongolicus (Leguminosae) and its ectopic expression enhanced drought and salt tolerance in Arabidopsis thaliana. Plant Cell Tissue Organ Cult 105:309–316
Wu M, Chen W, Zhao Y, Feng SG, Ying QC, Liu JJ, Wang HZ (2012) Salt tolerance enhancement of transgenic rice with Na+/H+ antiporter gene driven by root specific promoter PmPgPR10. Chin J. Rice Sci 26:643–650
Wu CA, Yang GD, Meng QW, Zheng CC (2004) The Cotton GhNHX1 gene encoding a novel putative tonoplast Na+/H+ antiporter plays an important role in salt stress. Plant Cell Physiol 45:600–607
Xia T, Apse MP, Aharon GS, Blumwald E (2002) Identification and characterization of a NaCl-inducible vacuolar Na+/H+ antiporter in Beta vulgaris. Physiol Plant 116:206–212
Yadav S, Irfan M, Ahmad A, Hayat S (2011) Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol 32:667–685
Yamaguchi T, Hamamoto S, Uozumi N (2013) Sodium transport system in plant cells. Front Plant Sci 4:410
Yarra R, He SJ, Abbagani S, Ma B, Bulle M, Zhang WK (2012) Overexpression of a wheat Na+/H+ antiporter gene (TaNHX2) enhances tolerance to salt stress in transgenic tomato plants (Solanum lycopersicum L.). Plant Cell Tissue Organ Cult 111(1):49–57
Yu JN, Huang J, Wang ZN, Zhang JS, Chen SY (2007) An Na+/H+ antiporter gene from wheat plays an important role in stress tolerance. J Biosci 32:1153–1161
Zeng Y, Li Q, Wang H, Zhang J, Du J, Feng H, Blumwald E, Yu L, Xu G (2017) Two NHX-type transporters from Helianthus tuberosus improve the tolerance of rice to salinity and nutrient deficiency stress. Plant Biotechnol J 16:310–321. https://doi.org/10.1111/pbi.12773
Zhang YM, Zhang HM, Liu ZH, Li HC, Guo XL, Li GL (2015) The wheat NHX antiporter gene TaNHX2 confers salt tolerance in transgenic alfalfa by increasing the retention capacity of intracellular potassium. Plant Mol Biol 87:317–327
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Zhuang J, Zhang J, Hou XL, Wang F, Xiong AS (2014) Transcriptomic, proteomic, metabolomic and functional genomic approaches for the study of abiotic stress in vegetable crops. Crit Rev Plant Sci 33(2–3):225–237
Acknowledgements
Authors acknowledge the Head, Department of Plant Sciences for access to the research facilities provided by DST-FIST, DBT-CREBB, and UGC-SAP to the Department of Plant Sciences, University of Hyderabad. The authors are thankful to Prof. Shouyi Chen and Prof. Jinsong Zhang, Institute of Genetics and Developmental Biology, CAS, Beijing for generous offer of the plasmid used in this study. We thank the anonymous reviewers for their valuable comments in improving the manuscript.
Funding
The Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Govt. of India provided fund and fellowship under Young Scientist Scheme (SB/FT/LS-445/2012).
Author information
Authors and Affiliations
Contributions
RY and PBK conceived the experiment. RY performed the experiment. RY and PBK analyzed the data. RY and PBK wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 234 kb)
Rights and permissions
About this article
Cite this article
Yarra, R., Kirti, P.B. Expressing class I wheat NHX (TaNHX2) gene in eggplant (Solanum melongena L.) improves plant performance under saline condition. Funct Integr Genomics 19, 541–554 (2019). https://doi.org/10.1007/s10142-019-00656-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-019-00656-5