Abstract
Key message
Exogenous application of 24-epibrassinolide can alleviate oxidative damage, improve photosynthetic capacity, and regulate carbon and nitrogen assimilation, thus improving the tolerance of grapevine (Vitis vinifera L.) to drought stress.
Abstract
Brassinosteroids (BRs) are a group of plant steroid hormones in plants and are involved in regulating plant tolerance to drought stress. This study aimed to investigate the regulation effects of BRs on the carbon and nitrogen metabolism in grapevine under drought stress. The results indicated that drought stress led to the accumulation of superoxide radicals and hydrogen peroxide and an increase in lipid peroxidation. A reduction in oxidative damage was observed in EBR-pretreated plants, which was probably due to the improved antioxidant concentration. Moreover, exogenous EBR improved the photosynthetic capacity and sucrose phosphate synthase activity, and decreased the sucrose synthase, acid invertase, and neutral invertase, resulting in improved sucrose (190%) and starch (17%) concentrations. Furthermore, EBR pretreatment strengthened nitrate reduction and ammonium assimilation. A 57% increase in nitrate reductase activity and a 13% increase in glutamine synthetase activity were observed in EBR pretreated grapevines. Meanwhile, EBR pretreated plants accumulated a greater amount of proline, which contributed to osmotic adjustment and ROS scavenging. In summary, exogenous EBR enhanced drought tolerance in grapevines by alleviating oxidative damage and regulating carbon and nitrogen metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought is complex abiotic stress which has adverse effects on plant growth and production. Drought stress disturbs a series of morphological, physiological, biochemical, and molecular changes (Mukarram et al. 2021). For instance, photosynthesis was inhibited as a result of stomata closure and non-stomatal limitation under drought stress (Mukarram et al. 2021). Moreover, drought stress induced the massive accumulation of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide radicals (O2−), and hydroxyl radicals (HO·), which seriously damage macromolecules. Meanwhile, ROS-induced peroxidation of polyunsaturated fatty acids leads to ion leakage, which results in the disturbed structural and functional profile of cells (Ahanger et al. 2021; Mukarram et al. 2021).
Brassinosteroids (BRs) are a group of polyhydroxylated phytosterols that are widely found in higher plants. In 1979, brassinolide (BL), the most active BR, was first isolated and identified from Brassica napus pollen grains. Since then, extensive studies have been conducted to reveal the role of BRs in plants. Shreds of evidence have been found that BRs regulate multiple biological processes in plants, such as seed germination, cell elongation, photomorphogenesis, vascular differentiation, and root development (Li et al. 2018; Manghwar et al. 2022). Moreover, BRs are involved in a wide spectrum of abiotic and biotic stress responses of plants, such as drought, heat, chilling, salt, heavy metal toxicity, and nutrient deficiency (Manghwar et al. 2022; Yao et al. 2023). It has been demonstrated that exogenous BR can enhance plant drought resistance by improving photosynthetic capacity, stimulating ROS clearance, promoting the accumulation of osmotic substances, and modulating phytohormones metabolism (Avalbaev et al. 2020; Lone et al. 2022; Zeng et al. 2022; Zhang et al. 2022).
Carbon and nitrogen metabolism are fundamental processes to perform routine and primary cellular activities during plant growth and development (Baslam et al. 2021). The processes of carbon metabolism include photosynthetic carbon assimilation, sucrose and starch metabolism, and carbohydrate transport and utilization. CO2 is fixed in leaf chloroplasts by photosynthesis. A portion of photosynthates is exported from the chloroplast to satisfy immediate respiratory or sucrose export requirements, and a proportion is transiently stored as starch in anticipation of the night, as well as during periods of carbon excess (MacNeill et al. 2017). Nitrogen is a necessary nutrient to maintain the growth and development of plants since it is a crucial structural component of nucleic acids, amino acids, proteins, chlorophylls, phytohormones, and secondary metabolites (O'Brien et al. 2016). Thus, the continuity of many central metabolic pathways, such as photosynthesis and amino acid biosynthesis, depends on the availability of nitrogen in plants (Erdal 2019). Nitrate (NO3−) is the most abundant source of nitrogen in nature and is absorbed through the nitrate transporters located in the plasma membranes of the cells (O'Brien et al. 2016). It is firstly reduced into nitrite (NO2−) with the action of nitrate reductase (NR) in the cytoplasm, then NO2− is reduced into ammonium (NH4+) by nitrite reductase (NiR) in plastids. NH4+ assimilation is catalyzed by glutamine synthetase (GS) and glutamate oxoglutarate aminotransferase (GOGAT) or glutamate dehydrogenase (GDH). In this process, inorganic nitrogen (NH4+) is converted into organic nitrogen (glutamate). Subsequently, glutamate acts as a donor of the amino group that distributes nitrogen to almost all nitrogenous compounds (Liu et al. 2022a, b; Baslam et al. 2021). This assimilation process is essential for plant growth and productivity. Besides, nitrogen assimilation and amino acid biosynthesis require a large amount of reducing agents, carbon skeletons, and cellular energy, which are provided by photosynthesis and mitochondrial respiration (Baslam et al. 2021). It has been confirmed that maintaining the balance between carbon and nitrogen assimilation is crucial for plant growth and response to environmental stress (Ren et al. 2021; Pandey et al. 2022).
Grape is one of the most important fruit crops and is cultivated in more than 90 countries for the production of wine, distilled liquors, juice, raisins, and table grapes. Most wine regions are located in temperate zones and many have a Mediterranean climate with warm and dry summers. In these regions, grapevines are regularly exposed to periods of drought, which can negatively affect the growth, and productivity of vines (Gambetta et al. 2020). Previous studies have focused on the role of BRs in regulating photosynthetic capacity, cellular redox, and osmotic pressure (Manghwar et al. 2022; Yao et al. 2023). Here, we hypothesized that BR could mitigate the inhibitory effect of drought stress on carbon and nitrogen metabolism, thereby enhancing the drought tolerance of grapevines. Photosynthetic activity, and enzyme activity, metabolite contents, and gene expression levels involved in carbon and nitrogen were investigated in grapevine leaves under drought stress with or without an EBR supplement.
Materials and methods
Plant materials and treatments
Brawny one-year-old Cabernet Sauvignon (Vitis vinifera L.) grape canes with fullness buds were collected, cut into approximately 10 cm long (containing two buds each), and rooted in plastic pots (28 × 18 cm) filled with a mixture of garden soil, perlite, and humus (2:1:1, v/v/v). They were placed in greenhouse for about 8 weeks at 24/18◦C day/night cycle under 16/8 h light/ dark photoperiod. A total of 135 healthy young grapevines with 8–10 fully expanded leaves were selected for the experiment. All grapevines were well-watered before treatment. Afterward, grapevines were divided into three groups: (1) ample water combined with distilled water pretreatment (Control, CK); (2) drought stress combined with distilled water pretreatment (Drought stress, DS); (3) drought stress combined with 0.2 μM 24-epibrassinolide (EBR) pretreatment (DS + 0.2 μM EBR). Fifteen grapevines were chosen as one replication and three biological replications were performed. The EBR concentration was based on a previous study (Zeng et al. 2022). EBR was dissolved with distilled water containing 0.1% Tween 80 and sprayed on the grapevine leaves for three successive days. At 15 days of drought treatment, the grapevine leaves displayed foliar wilting. Leaf samples were collected, frozen in liquid nitrogen, and stored at − 80 °C.
Determination of relative water content and relative electrolyte leakage
The relative water content (RWC) was measured based on the method of Gao (2006). The leaf samples were immediately weighed to obtain the fresh weight (FW) and soaked in deionized water for 12 h to obtain the turgid weight (TW). Afterward, the samples were dried at 105 °C for 30 min and 80 °C for 12 h to record the dry weight (DW). RWC was computed according to the following formula: RWC (%) = (FW-DW)/(TW-DW) × 100%. The relative electrolyte leakage (REL) was measured using a DDS-307 electrical conductivity meter (Leici, Shanghai, China). Nine leaf discs (1 cm) derived from three leaves were immersed in deionized water and shaken at 150 rpm for 3 h to obtain initial electrolyte leakage (E1). Then, samples were boiled for 30 min to determine the total electrolyte leakage (E2). REL was calculated with the following formula: EL (%) = E1/E2 × 100%.
Determination of ROS accumulation and antioxidant concentration
The accumulation of O2− and H2O2 was detected according to the methods of Wang and Luo (1990) and Patterson (1984), respectively. The malondialdehyde (MDA) content was measured using the thiobarbituric acid reaction, as previously described by Heath and Packer (1968). The concentrations of ascorbic acid (AsA) and glutathione (GSH) were determined following the methods of Kampfenkel et al. (1995) and Anderson (1985), respectively.
Scanning electron microscopy observation
After 15 days of drought treatment, young leaves were sampled, cut into small segments (5 mm × 5 mm), and fixed in 4% glutaraldehyde solution at 4 °C for 12 h. Then, the leaf segments were washed four times with 0.1 M sodium phosphate buffer (pH 6.8) for 30 min each time, dehydrated in a graded series of ethanol for 15 min each time, and dried in an Emitech K850 critical-point drying machine (Quorum, UK). After metal spraying, leaf surface was observed with a Nano 450 scanning electron microscopy (FEI, USA).
Determination of photosynthetic pigment and gas exchange parameters
The photosynthetic pigment was extracted in 80% (v/v) acetone for 12 h. The absorbance of supernatant was measured at 663, 645, and 470 nm, respectively. The contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid were calculated according to the formula of Arnon (1949). The gas exchange parameters were measured with a GSF 3000 portable photosynthesis system (Zeal Quest, Shanghai, China) between 9:00 a.m. and 11:00 a.m.
Determination of carbohydrates
Glucose, fructose, and sucrose separation and quantification were performed by a high-performance liquid chromatography system (LC-2030CD; Shimadzu, Tokyo, Japan) fitted with a ZORBAX Carbohydrate column (150 × 4.6 mm, 5.0 μm) (Agilent, USA). Briefly, Leaf samples (0.5 g) were extracted with 5 mL of 80% (v/v) ethanol, incubated at 80 °C for 10 min, and centrifuged at 10,000 × g for 10 min. After three extraction processes, the supernatant was combined, dried with a water bath at 90 °C, dissolved to 5 mL with distilled water, and filtered with a 0.45 μm membrane. The mobile phase was acetonitrile solution (70%). Starch content was measured with the method of Hansen and Moller (1975). The residue obtained in the above extraction process was resuspended in 2 mL of distilled water and boiled for 20 min. Then, starch was extracted with 2.0 mL of 9.2 M and 4.6 M perchloric acid separately for 20 min and centrifuged at 10,000 × g for 10 min. 1.0 mL of supernatant was mixed with 2.5 mL of anthrone reagent and incubated at boiling water for 15 min. After cooling to room temperature, the absorbance was assessed at 620 nm.
Determination of enzymes involved in sucrose metabolism
The activity of sucrose synthase (SS, EC 2.4.1.13), sucrose phosphate synthase (SPS, EC 2.4.1.14), acid invertase (AI, EC 3.2.1.26), and neutral invertase (NI, EC 3.2.1.26) was quantified as described by Gao (2006). Approximately 0.5 g of leaf powder was extracted with 5 mL of 100 mM Tris–HCl buffer (pH 7.0) containing 5 mM MgCl2, 2 mM ethylenediaminetetraacetic acid disodium salt, 2% glycol, 0.2% bovine serum albumin, 2% polyvinylpolypyrrolidone (PVP) and 5 mM dithiothreitol (DTT), centrifuged at 10,000 × g for 10 min, and the supernatant was collected. The mixture of 0.05 mL of dialyzed enzyme solution, 0.4 mL of enzyme reaction buffer (100 mM Tris–MES containing 10 mM fructose, 5 mM magnesium acetate, and 5 mM DTT), and 0.1 mL of uridine diphosphate glucose were diluted to 1 mL, incubated at 30 °C for 10 min and boiled for 3 min. The SS activity was measured at 480 nm. The SPS activity was assayed with a similar method, except that replaced the fructose with fructose 6-phosphate. The activities of SS and SPS were calculated as the amount of fructose (mg per h per g) obtained from the fresh leaves. For AI detection, 0.05 mL of crude enzyme solution was mixed with 0.95 mL of AI reaction buffer (80 mM acetic acid-K3PO4 buffer containing 50 mM sucrose), incubated at 30 °C for 10 min, and boiled for 3 min. The reading was performed at 540 nm. NI activity was measured with a similar method, except by replacing the AI reaction buffer with phosphate buffer (pH 7.0). The activities of AI and NI were expressed as the amount of glucose (mg per h per g) obtained from the fresh leaves.
Determination of NO 3 − , NH 4 + and soluble protein
Approximately 0.5 g of leaf sample was ground in 10 mL of deionized water, boiled for 60 min, and centrifuged for 15 min at 8000 × g. NO3− content was measured according to the method of Cataldo et al. (1975). A 0.1 mL aliquot of the supernatant was mixed with 0.4 mL of 5% salicylic-H2SO4 to and incubated at room temperature for 20 min. Then, 9.5 mL of 8% NaOH was added and the absorbance was read at 410 nm. The NH4+ content was quantified as described by Hao et al. (2004). The reaction was prepared with 2 mL of supernatant, 3 mL of ninhydrin hydrate, and 0.1 mL of 1% ascorbic acid. The mixture was incubated in boiling water for 20 min and the absorbance was recorded at 580 nm. The soluble protein was quantified using Coomassie brilliant blue G-250 reagent according to Bradford (1976).
Determination of free amino acids
Free amino acid quantification was performed by liquid chromatography-mass spectrometry system (QTRAP5500; AB SCIEX, Washington, USA) fitted with an Inertsil ODS-4 C18 column (150 × 3.0 mm, 3.5 μm; Shimadzu, Tokyo, Japan). Approximately 0.1 g of leaf tissue was homogenized in 1 mL of 50% ethanol (including 0.1 M HCl), shaken for 20 min, and centrifuged for 10 min at 8000 × g at 4◦C. A 50 μL aliquot of the supernatant was collected, diluted to 1 mL, and filtered with a 0.22 μm microporous membrane. The mobile phases were 0.5% (vol/vol) methanoic acid in H2O (A) and methanol (B). The flow rate was 0.5 mL/min and the injection volume was 10 μL. The gradient elution was as follows: 0–1.0 min, 25% B; 1.0–5.0 min, 25–95% B; 5.0–6.5 min, 95% B; 6.5–6.6 min, 95%-25% B; and 6.6–10 min, 25% B. The MS conditions were as follows: the spray voltage was 5500 V; the pressure of nebulizer and aux gas was 60 and 35 psi, respectively; and the atomizing temperature was 600 °C. Data were quantified by the comparison of the peak surface areas with commercial standards. The major parameters of 19 free amino acids standards (Sigma-Aldrich, St. Louis, MO, USA) are shown in Table S1.
Determination of NR and GS
NR (EC 1.6.6.1) activity was determined according to Gao (2006). Leaf powder (0.5 g) was homogenized with 5 mL of 25 mM phosphate buffer (pH 8.7) and centrifuged at 8000 × g for 10 min. Enzyme solution (0.2 mL) was mixed with 0.1 mL of 0.1 M KNO3, and 0.5 mL of 2 mg/mL nicotinamide adenine dinucleotide and incubated at 25 °C for 30 min. The reaction was terminated by adding 1 mL of 30% trichloroacetic acid. Then, the mixture was mixed with 2 mL of 1% sulfanilamide and 2 mL of 14 mM α-naphthylamine and incubated at room temperature for 15 min. The absorbance was recorded at 520 nm. NR activity was calculated as the amount of NO2− (μg per h per g) obtained from the fresh plant material. GS (EC 6.3.1.2) activity was measured using a Micro Glutaminase (GS) Assay kit (Comin Biotechnology Co., Ltd., Suzhou, China) in accordance with the manufacturer’s instructions at an absorbance of 540 nm. GS activity was expressed as the amount of ϒ-glutamyl hydroxamic acid (μmol per h per g) obtained from the fresh plant material.
Gene expression analysis
Approximately 100 mg of leaf sample was ground into powder in liquid nitrogen, and the total RNA was extracted using an RNAout kit (Bioteke, Beijing, China). Then, 500 ng of total RNA was reverse transcribed according to the instructions of the PrimeScript®RT reagent kit with gDNA eraser (TransGen, Beijing, China). Quantitative Real-Time polymerase chain reaction (qRT-PCR) was performed using an iQ6 real-time PCR detection system (Life Technology Co., Ltd., USA) with SYBR green qPCR mix kit (Bioteke, Beijing, China). The specific primers are listed in Table S2. The relative gene expression level was calculated according to the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
SPSS 22.0 software was used for statistical data analysis. One-way analysis of variance and Duncan’s multiple range tests (P < 0.05) were performed to evaluate the differences between means. The values are represented as means ± standard deviations of three replicates.
Results
Effects of EBR on plant growth and ROS levels under drought stress
After 15 days of drought treatment, drought-stressed grape seedlings displayed typical drought injury, including drooping shoots and wilted leaves, while the damage in EBR pretreated plants was lessened (Fig. 1a). The RWC level was 28% reduced due to drought stress, while it increased by 24% in EBR pretreatment (Fig. 1b). Drought stress significantly enhanced the generation of H2O2 (75%) and O2− (13%), resulting in an 86% increase in MDA and a 266% increase in REL (Fig. 1c–f). EBR pretreatment diminished the levels of H2O2, O2−, MDA, and REL by 13%, 5%, 13%, and 17%, respectively. In addition, EBR pretreatment increased the accumulation of AsA and GSH. The concentrations of AsA and GSH in EBR pretreatment were 2% and 10% higher than those in drought stress (Fig. 1g and h).
Effect of EBR on plant growth and ROS levels under CK (normal control), DS (drought stress), and DS + 0.2 μM EBR (drought stress combined with 0.2 μM EBR). a The phenotype of grapevines; b relative water content; c H2O2 content; d O2− content; e MDA content; f relative electrolyte leakage g AsA content, and h GSH content. Data represent means ± SD of three replicates. Different letters indicate significant differences according to Duncan’s multiple range tests (P < 0.05)
Effect of exogenous EBR on photosynthesis under drought stress
Stomata are the important portal to control the exchanges of carbon and water between leaves and the atmosphere in plants. Drought stress induced the stomatal closure; the stomatal aperture in drought stress exhibited a 29% decrease in comparison with the control (Fig. 2a and b). Meanwhile, drought stress resulted in a 21% decrease in stomatal density (Fig. 2c). Compared with drought stress, the stomatal aperture and stomatal density in EBR pretreatment increased by 22% and 26% respectively.
Effect of EBR on photosynthesis under drought stress. a Scanning electron microscopy images of stoma, b stomatal aperture, and c stomatal density. Data represent means ± SD of ten replicates. Different letters indicate significant different (P < 0.05, Duncan’s multiple range tests). d Chlorophyll a content, e chlorophyll b content, f total chlorophyll content, g carotenoid content; h photosynthetic rate, i stomatal conductance, and j transpiration rates. Data represent means ± SD of three replicates. Different letters indicate significant differences according to Duncan’s multiple range tests (P < 0.05)
Due to drought stress, the concentrations of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid decreased by 31%, 39%, 33%, and 29%, respectively, while they were 19%, 25%, 20%, and 24% improved in EBR pretreatment, respectively (Fig. 2d–g). Simultaneously, the photosynthetic rate (Pn), stomatal conductance (Gs), and transpiration rates (Tr) were reduced by 50%, 48%, and 56% under drought stress, respectively (Fig. 2h–j). However, EBR pretreatment alleviated the decrease; the Pn, Gs, and Tr values in EBR pretreatment were 26%, 25%, and 30% higher than those in drought stress, respectively.
To further assess the changes in photosynthesis, the transcription levels of several genes were detected (Fig. 3a). Under drought stress, the gene encoding light-harvesting protein (LHC) and photosynthetic reaction center proteins (PSB) were downregulated in drought-stressed grapevines, while this effect was partially alleviated in EBR pretreated plants. Similarly, genes encoding rubisco activase (RCA), glyceraldehyde phosphate dehydrogenase (GAPDH), and phosphoribulokinase (PRK) were also significantly upregulated in EBR pretreatment (Fig. 3a).
Effect of EBR on the expression of genes involved in photosynthesis (a), sucrose and starch metabolism (b), and nitrogen assimilation (c) under drought stress. Data represent means ± SD of three replicates. Different letters indicate significant differences according to Duncan’s multiple range tests (P < 0.05). LHC light-harvesting chlorophyll a/b-binding protein, PSB photosystem II subunit; PRK phosphoribulokinase, RCA rubisco activase, GAPDH glyceraldehyde-3-phosphate dehydrogenase, SBE starch branching enzyme, AMY α-amylase, BAM β-amylase, cwInv cell wall invertase, AMT ammonium transporter, NRT nitrate transporter
Effect of EBR on starch and sucrose metabolism under drought stress
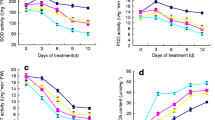
Drought stress resulted in a reduction of 37% in the starch and 38% in sucrose concentration, while an induced increase of 125% in fructose and 123% in glucose (Fig. 4a–d). Compared with drought stress, starch and sucrose concentration in EBR pretreatment increased by 17% and 190% respectively, while fructose and glucose decreased by 10% and 9% respectively. The SPS activity significantly declined under drought stress, and it was 44% lower than that in control (Fig. 4e). Relative to drought stress, the SPS activity increased by 65% in EBR pretreatment. In contrast, drought stress increased the activities of SS, AI, and NI by 51%, 50%, and 53%, respectively (Fig. 4f–h), while in EBR pretreatment, they decreased by 32%, 6%, and 13%, respectively.
Effect of EBR on carbohydrate contents and related enzyme activity under drought stress. a starch content, b sucrose content, c glucose content, d fructose content e SPS activity, f SS activity, g AI activity, and h NI activity. Data represent means ± SD of three replicates. Different letters indicate significant differences according to Duncan’s multiple range tests (P < 0.05)
As shown in Fig. 3b, gene encoding starch branching enzyme (SBE) and sucrose phosphate synthase (SPS) were downregulated due to drought stress, while in EBR pretreatment, they were 148% and 209% increased. On the contrary, drought up-regulated the genes encoded α-amylase (AMY) and β-amylase (BAM). Compared with drought stress, EBR pretreatment significantly decreased the VvAMY2 (79%), VvBAM2 (60%), and VvBAM3 (69%), suggesting that EBR might contribute to alleviating the starch hydrolysis activated by drought. Similarly, the gene encoding cell wall invertase (cwInv) decreased by 77% in EBR pretreatment. These observations indicated that the EBR application might positively regulate the accumulation of starch and sucrose.
Effect of EBR on nitrogen assimilation under drought stress
Drought stress significantly enhanced the accumulation of NO3− and NH4+, they were 65% and 97% higher than those in control respectively, while EBR pretreatment slowed the increase (Fig. 5a and b). Compared with drought stress, they decreased by 26% and 33% in EBR pretreatment, respectively. Conversely, the soluble protein content decreased by 29% due to drought stress, while it was increased by 18% in EBR pretreatment (Fig. 5c). The activities of NR and GS significantly diminished under drought stress, and they were 58% and 28% lower than those in control, respectively (Fig. 5d and e). EBR pretreatment improved the activities of NR and GS by 57% and 13%, respectively.
The nitrate transporter (NRT) and ammonium transporter (AMT) encode enzymes that catalyze the transportation of NO3− and NH4+, respectively. As shown in Fig. 3c, drought stress inhibited the transcription of VvNRT2.4, VvNRT2.5, and VvNRT5.1, while EBR pretreatment partially alleviated the inhibition. The expression of these genes in EBR pretreatment was 92%, 103%, and 84% higher than those in drought stress, respectively. On the contrary, VvAMT3.1 and VvAMT3.3 were up-regulated due to drought stress, while in EBR pretreatment, they decreased by 55% and 59% compared with drought stress. Moreover, EBR pretreatment application significantly the expression of VvNR (41%) and VvGS (98%) under drought stress.
Effect of EBR on free amino acids under drought stress
The steady state level of individual amino acids was quantified in grapevine leaves. We observed that total free amino acid concentration improved by 39% due to drought stress, while in EBR pretreatment (Fig. 6a), it declined by 21%. EBR pretreatment further promoted the proline accumulation; it was 33% higher than that in drought stress (Fig. 6b). The branched-chain amino acids isoleucine, leucine, and valine; the aromatic amino acids phenylalanine and tyrosine; together with γ-aminobutyric acid, glycine, histidine, threonine, asparagine, alanine, and glutamine, significantly increased by 1–10 times under drought conditions (Fig. 6c–n). Interestingly, the levels of these amino acids were markedly reduced when exogenous EBR was applied. Similarly, the lysine and arginine also declined in EBR pretreatment (Fig. 6p and q). Drought stress significantly improved the methionine concentration, but no significant difference was observed between drought and EBR pretreatment (Fig. 6o). In contrast, the levels of serine, aspartate, and glutamate significantly decreased upon drought, while EBR pretreatment partially alleviated the decrease (Fig. 6r–t). These results indicated that EBR pretreatment alters the amino acid metabolism under drought conditions.
Effect of EBR on the contents of free amino acids under drought stress. a total amino acid, b proline, c isoleucine, d valine, e leucine, f phenylalanine, g tyrosine, h γ-aminobutyric acid, i glycine, j histidine, k threonine, l aspartate, m alanine, n glutamine, o methionine, p lysine, q arginine, r serine, s asparagine, and t glutamate. Data represent means ± SD of three replicates. Different letters indicate significant differences according to Duncan’s multiple range tests (P < 0.05)
Discussion
Drought stress interferes with many physiological and metabolic processes, leading to a reduction in plant growth and productivity (Mukarram et al. 2021). BRs are a class of ubiquitous phytohormones, which are involved in regulating plant resistance to drought stress (Yao et al. 2023). In this study, we explored the effects of exogenous EBR on carbon and nitrogen metabolism in grapevine under drought stress.
EBR mitigated oxidative stress in grapevines under drought stress
Drought stress induces the over-accumulation of ROS that seriously damages various cellular components, leading to metabolic disturbance and cell death (Ahanger et al. 2021; Mukarram et al. 2021). The toxic ROS can be eliminated by antioxidases (e.g., superoxide dismutase, catalase) and antioxidants (e.g., AsA, GSH, proline) (Gill and Tuteja 2010). Previous studies have confirmed that exogenous EBR could diminish ROS accumulation triggered by drought stress (Lone et al. 2022; Xia et al. 2022). In the present study, EBR pretreated grapevines exhibited lower levels of H2O2 and O2− than drought-stressed plants. Meanwhile, EBR pretreatment decreased the concentration of MDA, which is a stable product of lipid peroxidation, indicating that EBR could protect both cellular and organelle membranes against drought-induced oxidative damage. AsA is the most abundant, powerful, and water-soluble antioxidant in plants. It can provide protection to membranes by directly scavenging the O2− and OH· and by regenerating α-tocopherol from tocopheroxyl radical (Gill and Tuteja 2010). GSH takes part in the scavenging of 1O2, H2O2, and OH· directly or indirectly (Gill and Tuteja 2010). It has been demonstrated that exogenous EBR could promote the AsA and GSH metabolism thereby improving stress resistance in plants (Zeng et al. 2022). Consistently, we also found that EBR pretreatment increased the concentrations of AsA and GSH. These results suggested that EBR could alleviate drought-induced oxidative damage.
EBR improved carbon metabolism in grapevines under drought stress
Photosynthesis is the principal process of capturing light energy to synthesize carbohydrates, which is closely related to plant growth and development. However, it is sensitive to drought stress. Drought stress severely affects photosynthetic efficiency, primarily due to stomatal closure and damage to the photosynthetic apparatus (Mukarram et al. 2021). According to our data, EBR pretreatment improved the stomatal aperture, photosynthetic pigment contents, and the transcription of related genes, ultimately partially alleviating the drought-induced photosynthetic inhibition in grapevines. Similar phenomena were observed in wheat (Zhao et al. 2017) and maize (Talaat 2020) under drought stress. The positive effects of EBR are probably associated with enhanced antioxidant capacity because chloroplast is the main target of oxidative damage in response to drought stress. Additionally, a recent study reported that BRs enhanced the photosynthetic capacity of tomato plants through key transcription factors of BR signaling, BRASSINAZOLE RESISTANT 1 (BZR1) mediated activation of Calvin cycle genes (Yin et al., 2023).
Carbohydrates are the structural components and the energy source for the production and maintenance of biomass. Starch is the main form of stored carbohydrate (Hennion et al. 2019). It has been reported that drought stress activates starch hydrolysis and promotes the conversion of starch to soluble sugars (Thalmann and Santelia 2017). In agreement with previous studies, decreased starch content was observed in drought stressed grapevine leaves. Moreover, EBR pretreatment improved the starch concentration, accompanied by up-regulated VvSEB and down-regulated VvAMY and VvBAM, indicating that EBR contributes to starch accumulation. The positive roles of BRs in modulating starch accumulation have been revealed in several plant species. BRs deficiency resulted in a strong reduction in starch concentration in leaves of cotton and Arabidopsis, which may be related to the changed photosynthetic efficiency and sugar metabolism (Chen et al. 2019; Schluter et al. 2002). In tomato, both exogenous BR application and overexpression of BR synthesis genes promoted starch accumulation through upregulating the expression of starch biosynthesis genes, thereby enhancing low light stress tolerance (Liu et al. 2022a, b). It appears that EBR could promote starch accumulation in grapevine by regulating photosynthesis, sugar metabolism, or by regulating starch biosynthesis genes. Moreover, the starch metabolism enzymes are also regulated by protein phosphorylation. Recent studies revealed that BR-induced sprouting in potato tubers was associated with the phosphorylation of proteins involved in starch and sucrose metabolism (Li et al. 2020).
Sugars are easily available organic osmolytes in the cell and play important roles in maintaining the cell turgor and protecting the structure of proteins and membranes (Mukarram et al. 2021). Sucrose is the predominant carbohydrate that is transported from the mature leaves (source) to the sink organ in higher plants (Baslam et al. 2021). Several key enzymes including SPS, SS, AI, and NI are involved in sucrose metabolism. In detail, SPS and SS reversibly catalyze the formation and degradation of sucrose, and AI and NI irreversibly hydrolyzed sucrose into glucose and fructose (Ruan 2012). According to our data, drought stress significantly induced the accumulation of glucose and fructose, which may be attributed to the improved SS, AI, and NI activity. This phenomenon partially explains why the sucrose content decreased in the leaves under drought stress. Meanwhile, drought stress inhibited the SPS activity, a rate-limiting enzyme of sucrose biosynthetic pathways. In addition, EBR pretreatment increased SPS activity, and decreased SS, AI, and NI activities, resulting in enhanced sucrose concentration. The result seems to be inconsistent with the result of Chen et al. (2023), which may be due to the difference in stress type, severity, and adaptation time. It is well known that different stress types provoke a different repertoire of plant response inherent to the different strategies that plants use for survival during these conditions. Lu et al. (2019) also found that exogenous EBR could decline the activities of AI, NI, and SS and the concentrations of glucose and fructose in kiwifruit during storage. Zhang et al. (2023) found that exogenous EBR induced the expression of PpBZR1, which directly binds to the PpVIN2 promoter to inhibit its expression, ultimately leading to increased sucrose concentration and clod tolerance in peach.
EBR improved nitrogen assimilation in grapevines under drought stress
Nitrogen is an essential constituent of amino acids, proteins, and nucleic acids. Therefore, nitrogen metabolism plays a crucial role in modulating plant growth and development. Numerous studies reported that drought stress inhibited root nitrogen uptake and assimilation (He et al. 2022; Huang et al. 2018). Moreover, drought tends to promote more nitrogen allocated in the root by reducing NO3− transport from root to shoot. In the shoot, drought stress decreased nitrogen accumulation and altered nitrogen metabolite concentration in leaves (Ren et al. 2021; Liang et al. 2018). Unexpectedly, we noted that the NO3− content increased markedly in drought stressed plants. It is speculated that this boost in NO3− accumulation by drought stress may be related to the role of NO3− in osmotic regulation (McIntyre 1997). Huang et al. (2018) also reported that drought stress induced the accumulation of NO3− in the leaves of apple plants. Meanwhile, drought stress triggered a marked diminution in the activities of NR and GS and an increase in NH4+ content. Previously, the role of EBR in promoting nitrogen assimilation in stressed plants has been documented (Gupta et al. 2017; Xia et al. 2022; Shu et al. 2016; Yadavet al. 2023). In agreement with previous studies, we found that EBR supplementation improved the NR and GS activity, while decreasing the NH4+ concentrations, suggesting that EBR could promote NO3− reduction and NH4+ assimilation. This is probably due to the fact that EBR pretreatment enhanced the photosynthesis, thereby promoting the synthesis of carbon skeleton, and providing sufficient substrate for nitrogen metabolism. Recent studies revealed that the BR signaling is also involved in nitrogen absorption. Exogenous application of BR up-regulated the expression of NRT2.1 and NRT2.2 in Arabidopsis thaliana. BRASSINOSTEROIDINSENSITIVE 1-EMS-SUPPRESSOR 1 (BES1), the closest homolog of BZR1, directly bound to the promoters of NRT2.1 and NRT2.2 to promote their expression, increasing NO3− uptake in response to nitrogen deficiency (Wang et al. 2023). Yang et al. (2021) also found that BR positively controls NH4+ uptake partially via BZR1-mediated activation of AMT1;2 in rice.
Amino acids are constituents of proteins and precursors of many secondary metabolites and nitrogen carriers in plants. Under drought stress, amino acids not only act as osmotic regulator but also as alternative substrates for mitochondrial respiration (Heinemann and Hildebrand 2021; Ozturk et al. 2020). It has been reported that drought stress leads to the accumulation of free amino acids (Hildebrandt 2018). Consistent with previous studies, we observed that drought stressed grapevines displayed higher level of total free amino acid than control plants. Interestingly, EBR pretreatment improved the soluble protein concentration, while decreasing the total free amino acid concentration. It is speculated that EBR could alleviate drought-induced protein degradation. Recent studies reported that the accumulation of branched-chain amino acids leucine, isoleucine, and valine, is primarily the result of protein degradation under drought conditions in Arabidopsis thaliana (Huang and Jander 2017). Similarly, Hildebrandt (2018) reported that most of the low-abundant amino acids, such as lysine, methionine, and branched-chain amino acids are not synthesized but they accumulate due to increased protein degradation under drought stress.
Proline acts as both osmolyte and ROS scavenger and plays a vital role in maintaining osmotic equilibrium and redox balance (Mukarram et al. 2021; Ozturk et al 2020). Also, proline can improve protein stability and protect membrane integrity by binding to hydrogen bonds (Ozturk et al. 2020). In addition, proline increases the formation of ROS in mitochondria via the electron transport chain and affects signal pathways. The resulting ROS causes a hypersensitive response in plants (Liang et al. 2013). It has been confirmed the close correlation between proline metabolism and plant drought tolerance. For example, drought-tolerant cultivars accumulated more proline than sensitive species under drought conditions (Zegaoui et al. 2017; Furlan et al. 2020). SIWRKY81-silenced tomato mutants possessed higher sensibility to drought stress because of attenuated proline biosynthesis (Ahammed et al. 2020). In this study, EBR pretreatment promoted the accumulation of proline under drought stress. Here, in addition to its function in osmotic adjustment, proline may also have acted as a ROS scavenger to protect the photosynthetic apparatus under drought stress in EBR pretreated plants. Xia et al. (2022) also revealed that EBR promoted the proline accumulation in kiwifruit seedlings under drought stress by modulating genes involved in proline biosynthesis and degradation.
Conclusion
In conclusion, exogenous EBR could improve the drought resistance of grapevines by alleviating oxidative damage and modulating carbon and nitrogen metabolism (Fig. 7). EBR pretreatment decreased the accumulation of H2O2 and O2− under drought stress. Moreover, EBR pretreatment positively regulated the accumulation of starch and sucrose by improving photosynthetic capacity and modulating key enzymes activity (SPS, SS, AI, and NI). In addition, EBR improved NR and GS activity, leading to promoted nitrogen assimilation. Meanwhile, EBR promoted proline accumulation, which is conducive to osmotic adjustment and ROS scavenging. This study provides new insights into EBR-induced drought tolerance in grape, with potential implications for crop production. However, whether BZR1/BES1 play a role in EBR mediated regulation of carbon and nitrogen metabolism and whether there is a direct regulatory link between BZR1/ BES1 and these key genes in grape remains unclearly, and the underlying molecular mechanism needs further studies.
Data availability
Data will be made available on request.
References
Ahammed GJ, Li X, Wang HJ, Zhou Z, Chen Y (2020) SlWRKY81 reduces drought tolerance by attenuating proline biosynthesis in tomato. Sci Hortic 170:109444. https://doi.org/10.1016/j.scienta.2020.109444
Ahanger MA, Siddique KHM, Ahmad P (2021) Understanding drought tolerance in plants. Physio Plantarum 172:286–288. https://doi.org/10.1111/ppl.13442
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymo 113:548–555. https://doi.org/10.1016/s0076-6879(85)13073-9
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Betavulgaris Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Avalbaev A, Bezrukova M, Allagulova C, Lubyanova A, Kudoyarova G, Fedorova K, Maslennikova D, Yuldashev R, Shakirova F (2020) Wheat germ agglutinin is involved in the protective action of 24-epibrassinolide on the roots of wheat seedlings under drought conditions. Plant Physiol Biochem 146:420–427. https://doi.org/10.1016/j.plaphy.2019.11.038
Baslam M, Mitsui T, Sueyoshi K, Ohyama T (2021) Recent advances in carbon and nitrogen metabolism in C3 plants. In J Mol Sci 22:318. https://doi.org/10.3390/ijms22010318
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ana Bioche 72:248–254. https://doi.org/10.1006/abio.1976.9999
Cataldo DA, Maroon M, Schrader E, Young L (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sc Plant Anal 6:71–80. https://doi.org/10.1080/00103627509366547
Chen EY, Zhang XY, Yang ZR, Zhang CJ, Wang XQ, Ge XY, Li FG (2019) BR deficiency causes increased sensitivity to drought and yield penalty in cotton. BMC Plant Bio 19:220. https://doi.org/10.1186/s12870-019-1832-9
Chen YH, Wang YL, Chen HZ, Xiang J, Zhang YK, Wang ZG, Zhu DF, Zhang YP (2023) Brassinosteroids mediate endogenous phytohormone metabolism to alleviate high temperature injury at panicle initiation stage in rice. Rice Sci 30(1):70–86. https://doi.org/10.1016/j.rsci.2022.05.005
Erdal S (2019) Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep 38:1001–1012. https://doi.org/10.1007/s00299-019-02423-z
Furlan AL, Bianucci E, Giordano W, Castro S, Becker D (2020) Proline metabolic dynamics and implications in drought tolerance of peanut. Plant Physiol Bioch 151:556–578. https://doi.org/10.1016/j.plaphy.2020.04.010
Gambetta GA, Herrera JC, Dayer S, Feng QS, Hochberg U, Castellarin SD (2020) The physiology of drought stress in grapevine: towards an integrative definition of drought tolerance. J Exp Bot 71:4658–4676. https://doi.org/10.1093/jxb/eraa245
Gao J (2006) Experimental Guidance for Plant Physiology. Higher education press, Beijing
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gupta P, Srivastava S, Seth CS (2017) 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 411:483–498. https://doi.org/10.1007/s11104-016-3043-6
Hansen J, Moller I (1975) Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem 68:87–94. https://doi.org/10.1016/0003-2697(75)90682-x
Hao Z, Cang J, Xu Z (2004) Plant Physiology Experiment. Harbin Industrial University Press, Harbin
He Q, Hu W, Li YX, Zhu HH, Zou J, Wang YH, Meng YL, Che B, Zhao WQ, Wang SS, Zhou ZG (2022) Prolonged drought affects the interaction of carbon and nitrogen metabolism in root and shoot of cotton. Environ Exp Bot 197:104839. https://doi.org/10.1016/j.envexpbot.2022.104839
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Heinemann B, Hildebrandt TM (2021) The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J Exp Bot 72:4634–4645. https://doi.org/10.1093/jxb/erab182
Hennion N, Durand M, Vriet C, Doidy J, Maurousset L, Lemoine R, Pourtau N (2019) Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere. Physiol Plant 165:44–57. https://doi.org/10.1111/ppl.12751
Hildebrandt TM (2018) Synthesis versus degradation: directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol Biol 98:121–135. https://doi.org/10.1007/s11103-018-0767-0
Huang T, Jander G (2017) Abscisic acid-regulated protein degradation causes osmotic stress-induced accumulation of branchedchain amino acids in Arabidopsis thaliana. Planta 246:737–747. https://doi.org/10.1007/s00425-017-2727-3
Huang LL, Li MJ, Zhou K, Sun TT, Hu LY, Li CY, Ma FW (2018) Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol Biochem 127:185–193. https://doi.org/10.1016/j.plaphy.2018.03.031
Kampfenkel K, Vanmontagu M, Inze D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167. https://doi.org/10.1006/abio.1995.1127
Li QF, Lu J, Yu JW, Zhang CQ, He JX, Liu QQ (2018) The brassinosteroid-regulated transcription factors BZR1/BES1 function as a coordinator in multisignal-regulated plant growth. BBA-Gene Regul Mech 1861:561–571. https://doi.org/10.1016/j.bbagrm.2018.04.003
Li LQ, Deng MS, Lyu CC, Zhang J, Peng J, Cai CC, Yang SM, Lu LM, Ni S, Liu F, Zheng SL, Yu LP, Wang XY (2020) Quantitative phosphoproteomics analysis reveals that protein modification and sugar metabolism contribute to sprouting in potato after BR treatment. Food Chem 325:126875. https://doi.org/10.1016/j.foodchem.2020.126875
Liang X, Zhang L, Natarajan SK, Becker DF (2013) Proline mechanisms of stress survival. Antioxid Redox Sign 19(9):998–1011. https://doi.org/10.1089/ars.2012.5074
Liang BW, Ma CQ, Zhang ZJ, Wei ZW, Gao TT, Zhao Q, Ma FW, Li C (2018) Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ Exp Bot 155:650–661. https://doi.org/10.1016/j.envexpbot.2018.08.016
Liu XJ, Hu B, Chu CC (2022a) Nitrogen assimilation in plants: current status and future prospects. J Genet Genomics 49:394–440. https://doi.org/10.1016/j.jgg.2021.12.006
Liu Y, Qi ZY, Wei JS, Yu JQ, Xia XJ (2022b) Brassinosteroids promote starch synthesis and the implication in low-light stress tolerance in Solanum lycopersicum. Environ Exp Bot 201:104990. https://doi.org/10.1016/j.envexpbot.2022.104990
Livak KJ, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-CT method. Methods 25:402–408
Lone WA, Majeed N, Yaqoob U, John R (2022) Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep 41:603–617. https://doi.org/10.1007/s00299-021-02763-9
Lu ZM, Wang XL, Cao MM, Li YY, Su JL, Gao H (2019) Effect of 24-epibrassinolide on sugar metabolism and delaying postharvest senescence of kiwifruit during ambient storage. Sci Hortic 253:1–7. https://doi.org/10.1016/j.scienta.2019.04.028
MacNeill GJ, Mehrpouyan S, Minow MAA, Patterson JA, Tetlow IJ, Emes MJ (2017) Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. J Exp Bot 68(16):4433–4453. https://doi.org/10.1093/jxb/erx291
Manghwar H, Hussain A, Ali Q, Liu F (2022) Brassinosteroids (BRs) role in plant development and coping with different stresses. Int J Mol Sci 23:1012. https://doi.org/10.3390/ijms23031012
McIntyre GI (1997) The role of nitrate in the osmotic and nutritional control of plant development. Aust J Plant Physiol 24:103–118. https://doi.org/10.1071/PP96064
Mukarram M, Choudhary S, Kurjak D, Kurjak A, Khan MMA (2021) Drought: sensing, signalling, effects and tolerance in higher plants. Physiol Plantarum 172:1291–1300. https://doi.org/10.1111/ppl.13423
O’Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, Gutiérrez R (2016) Nitrate transport, sensing, and responses in plants. Mol Plant 9:837–856. https://doi.org/10.1016/j.molp.2016.05.004
Ozturk M, Unal BT, Garcia-Caparros P, Khursheed A, Gul A, Hasanuzzaman M (2020) Osmoregulation and its actions during the drought stress in plants. Physiol Plantarum 172:1321–1335. https://doi.org/10.1111/ppl.13297
Pandey K, Kumar RS, Prasad P, Sushma PV, Trivedi PK, Shirke PA (2022) Synchronised interaction of carbon and nitrogen provides drought tolerance in Cyamopsis tetragonoloba. Environ Exp Bot 199:104899. https://doi.org/10.1016/j.envexpbot.2022.104899
Patterson BD, Macrae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492. https://doi.org/10.1016/0003-2697(84)90039-3
Ruan YL (2012) Signaling role of sucrose metabolism in development. Mol Plant 5:763–765. https://doi.org/10.1093/mp/sss046
Ren JH, Xie T, Wang YL, Li HB, Liu TT, Zhang SQ, Yin LN, Wang SW, Deng XP, Ke QB (2021) Coordinated regulation of carbon and nitrogen assimilation confers drought tolerance in maize (Zea mays L.). Environ Exp Bot 176:104086. https://doi.org/10.1016/j.envexpbot.2020.104086
Schluter U, Kopke D, Altmann T, Mussig C (2002) Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroid-deficient cbb1 mutant. Plant Cell Environ 25:783–791. https://doi.org/10.1046/j.1365-3040.2002.00860.x
Shu S, Tang YY, Yuan YH, Sun J, Zhong M, Guo SR (2016) The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol Biochem 107:344–353. https://doi.org/10.1016/j.plaphy.2016.06.021
Talaat NB (2020) 24-epibrassinolide and spermine combined treatment sustains maize (Zea mays L.) drought tolerance by improving photosynthetic efficiency and altering phytohormones profile. J Soil Sci Plant Nut 20:516–529. https://doi.org/10.1007/s42729-019-00138-4
Thalmann M, Santelia D (2017) Starch as a determinant of plant fitness under abiotic stress. New Phytol 214:943–951. https://doi.org/10.1111/nph.14491
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 6:55–57
Wang T, Li MJ, Yang JZ, Li M, Zhang ZQ, Gao HL, Wang C, Tian H (2023) Brassinosteroid transcription factor BES1 modulates nitrate deficiency by promoting NRT2.1 and NRT2.2 transcription in Arabidopsis. Plant J 114:1443–1457. https://doi.org/10.1111/tpj.16203
Xia H, Liu XL, Wang YM, Lin ZY, Dengn HH, Wang J, Lin LJ, Deng QX, Lv XL, XuLiang KF (2022) 24-Epibrassinolide and nitric oxide combined to improve the drought tolerance in kiwifruit seedlings by proline pathway and nitrogen metabolism. Sci Hortic 297:110929. https://doi.org/10.1016/j.scienta.2022.110929
Yadav RK, Analin B, Panda MK, Ranjan A, Singh AP (2023) Brassinosteroids-regulated nitrogen metabolism fine-tunes growth physiology and low nitrogen response in tomato. Environ Exp Bot 216:105528. https://doi.org/10.1016/j.envexpbot.2023.105528
Yang S, Yuan DP, Zhang Y, Sun Q, Xuan YH (2021) BZR1 regulates brassinosteroid-mediated activation of AMT1; 2 in rice. Front Plant Sci 12:665883. https://doi.org/10.3389/fpls.2021.665883
Yao TS, Xie RJ, Zhou CY, Wu XX, Li DD (2023) Roles of Brossinosteroids signaling in biotic and abiotic stresses. J Agric Food Chem 71:7947–7960. https://doi.org/10.1021/acs.jafc.2c07493
Yin XW, Tang MJ, Xia XJ, Yu JQ (2023) BRASSINAZOLE RESISTANT 1 mediates Brassinosteroid-induced Calvin cycle to promote photosynthesis in tomato. Front Plant Sci 12:811948. https://doi.org/10.3389/fpls.2021.811948
Zegaoui Z, Planchais S, Cabassa C, Djebbar R, Belbachir OA, Carol P (2017) Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J Plant Physiol 218:26–34. https://doi.org/10.1016/j.jplph.2017.07.009
Zeng GH, Gao FF, Li C, Li DD, Xi ZM (2022) Characterization of 24-epibrassinolide-mediated modulation of the drought stress responses: morphophysiology, antioxidant metabolism and hormones in grapevine (Vitis vinifera L.). Plant Physiol Bioch. https://doi.org/10.1016/j.plaphy.2022.05.019
Zhang YH, Xiao YZ, Zhang YG, Dong Y, Liu YQ, Liu L, Wan SQ, He JY, Yu YB (2022) Accumulation of galactinol and ABA is involved in exogenous EBR induced drought tolerance in tea plants. J Agric Food Chem 70:13391–13403. https://doi.org/10.1021/acs.jafc.2c04892
Zhang SY, Cao KF, Wei YY, Jiang S, Ye JF, Xu F Chen Y (2023) PpBZR1, a BES/BZR transcription factor, enhances cold stress tolerance by suppressing sucrose degradation in peach fruit. Plant Physiol Bioc 202:107972. https://doi.org/10.1016/j.plaphy.2023.107972
Zhao GW, Xu HL, Zhang PJ, Su XY, Zhao HJ (2017) Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul 81:377–384. https://doi.org/10.1007/s10725-016-0214-7
Funding
This work was supported by the National Key Research and Development Program of China (No. 2019YFD1000102-11) and China Agriculture Research System for Grape (No. CARS-29-zp-6).
Author information
Authors and Affiliations
Contributions
ZX and ZZ conceived and designed the experiments. GZ, ZW, and RX performed the experiment. GZ, BL, FG, and CL analyzed the data. All authors contributed to the writing and revision of the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
All authors have read and approved the final manuscript.
Additional information
Communicated by Sheng Ying.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, G., Wan, Z., Xie, R. et al. 24-epibrassinolide enhances drought tolerance in grapevine (Vitis vinifera L.) by regulating carbon and nitrogen metabolism. Plant Cell Rep 43, 219 (2024). https://doi.org/10.1007/s00299-024-03283-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00299-024-03283-y