Abstract
Our aim in this study was to evaluate the use of diffusion-weighted imaging (DWI) for acute renal parenchymal changes occurring as a result of unilateral ureteral obstruction due to stones. Twenty four patients with obstructed and opposite unobstructed kidney were enrolled in this prospective study. DWI was used at two different b values (b = 0 s/mm2 and b = 1000 s/mm2). Apparent diffusion coefficient (ADC) measurements were completed on the upper pole, central section and lower pole parenchyma of both kidneys. ADC values were calculated. The unpaired t test was used to assess differences between the groups. The results of measurements identified a reduction in ADC values in obstructed renal parenchyma compared to unobstructed opposite renal parenchyma. The reduction in ADC values was greater in the upper and lower pole parenchyma and was statistically significant (p < 0.001, for both). Diffusion changes in renal parenchyma due to acute unilateral ureteral obstruction linked to stone may be quantitatively shown with DWI. The reduction in ADC values was more pronounced in the upper and lower pole parenchyma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
If acute ureteral obstruction due to stones is not treated in the early period, interstitial fibrosis and irreversible renal parenchymal damage may occur as a result of reduced renal blood flow and glomerular infiltration [1]. In daily practice for the diagnosis of ureteral stones causing renal ectasia, ultrasonography (US), intravenous pyelography (IVP) and computed tomography (CT) are used. Both unenhanced and enhanced imaging methods have certain limitations. Contrast-enhanced ultrasound or CT studies can provide useful functional information. However, these enhanced studies are either elaborate or involve the risk of significantly increased ionizing radiation. On the other hand, an MRI may not be available quickly and in spite of their limitations, contrast-enhanced ultrasound or CT scan may be obtained fast. Moreover, there may be controversy on the selection of the optimal imaging modality for the urologist and radiologist, since these disciplines come across patients under different settings and circumstances.
Diffusion-weighted imaging (DWI) is a method that reveals differences in biological tissue based on the in vivo diffusion characteristics of water molecules. However, as this method is sensitive to cardiac, respiratory and peristaltic movements, its use was initially limited to brain imaging. With the development of echo-planar imaging sequences and strong diffusion gradients and technological developments in MR devices, there was a reduction in artifacts caused by physiological movement, and MR devices began to be used for abdominal and pelvic organs. The hypomotility of water molecules, high blood flow and fluid-carrying functions of renal parenchyma make it an appropriate environment for diffusion studies. Studies on renal DWI have focused more on differential diagnosis of renal masses. As a result, there are a limited number of studies on the role of DWI in evaluating renal functions in parenchymal diseases [2–4].
In this study, our aim was to evaluate the use of DWI to quantitatively examine acute renal parenchymal changes occurring as a result of unilateral ureteral obstruction due to stones.
Materials and methods
Patients
This prospective study entitled “The utility of diffusion-weighted imaging to assess acute renal parenchymal changes due to unilateral ureteral stone obstruction” was completed after receiving permission from the local clinical research ethics committee of Inonu University. From March 2013 to September 2013, 24 cases (17 male, 7 female, age interval 18–71, mean age 41.5 years) with a unilateral ureter stone diagnosed by previous US, IVP or CT had DW-MRI tests performed. The mean duration between initial symptoms and DWI was 28.3 h (13–70 h). To exclude pharmaceutical effects, cases were not given any medication before tests. As DWI images were sufficient to assess all cases, no case was excluded from the study. Those with a previous treatment for kidney stones, acute or chronic parenchymal or infectious renal disease and those with contraindications for MRI were excluded from the study.
DW-MRI technique
Cases had DW-MRI investigation performed in a 1.5 Tesla (T) MRI device (Magnetom Avanto Siemens, Erlangen, Germany) with a six phase array body coil. DWI was obtained in the transverse plane, with a single shot echo-planar sequence, at two different b values (b0, b1000 sn/mm2), across diffusion sensitive gradients (TR/TE: 2673/60 ms (b0), TR/TE: 3656/89 ms (b1000), matrix 128 × 256, FOV of 35–40 cm, slice thickness of 5 mm, and a gap of 1 mm). To avoid severe chemical shift artifacts, fat suppression pulses were used. Isotropic images from ADC maps were created automatically by the device.
Image evaluation
The apparent diffusion coefficient (ADC) calculated from the DWI is based on the impacts of capillary perfusion and water diffusion [5]. Quantitative ADC mapping can be accomplished via recording a series of DW MR images with different diffusion-sensitizing gradient factors [5]. Since ADC directly indicates the microenvironment and capillary perfusion of the diffusing water molecules in tissue, it may provide information on the normal and abnormal structure of tissues including kidneys [5]. An ADC of a tissue is expressed in units of mm2/s.
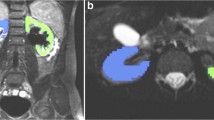
Renal parenchymal ADC measurements were completed on ADC maps uploaded to a work station (Leonardo console, software version 2.0; Siemens AG Medical Solutions, Forchheim, Germany). Measurements located a region of interest (ROI) in the upper and lower pole and central section parenchyma of the obstructed and opposite unobstructed kidney. Mean ADC values were calculated. Quantitative evaluations were completed by two researchers (GE and KD with 10 and 2 years of experience in urinary radiology, respectively) together.
Statistical analysis
Statistical evaluations used the “SPSS 17.0 for Windows” program. The Shapiro–Wilk normality test was used to determine whether data had normal distribution. Measurable (quantitative) data are given as x ± sd. The differences between the two groups were examined using the unpaired t test. Countable data (qualitative) are given as percentages. Sensitivity and specificity of diagnostic criteria were calculated. For analysis, statistical significance was taken as p < 0.05.
Results
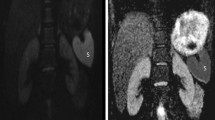
The ADC values of obstructed and opposite unobstructed renal parenchyma are summarized in Table 1. The minimum and maximum ADC values in obstructed renal parenchyma were 0.19–3.61 × 10−3 mm2/s in the upper pole, 0.54–3.36 × 10−3 mm2/s in the central section and 0.57–3.23 × 10−3 mm2/s in the lower pole. In unobstructed opposite renal parenchyma, these values were 0.54–3.86 × 10−3 mm2/s in the upper pole, 0.42–3.78 × 10−3 mm2/s in the central section and 0.74–3.56 × 10−3 mm2/s in the lower pole (Fig. 1). The difference between the upper and lower pole parenchyma ADC values in the obstructed kidney was significant compared to the upper and lower pole parenchyma ADC values in the unobstructed opposite kidney (p < 0.001, upper and lower pole). Though there was a difference between the ADC values in the central section, it was not statistically significant (p > 0.05).
a Axial CT slice: two stone densities observed at the vesicoureteral junction in the distal left ureter (white arrow). b–d MRI sequences showing ADC values in obstructed left and unobstructed right kidney; upper pole left 1.44 × 10−3 mm2/s and right 1.68 × 10−3 mm2/s, central section left 1.50 × 10−3 mm2/s and right 1.70 × 10−3 mm2/s, and lower pole left 1.45 × 10−3 mm2/s and right 1.64 × 10−3 mm2/s, respectively
Discussion
This study used DWI with a high ‘b’ value to assess acute renal parenchymal diffusion changes resulting from unilateral obstruction with a stone and found two important results. The first was that DWI quantitatively shows acute renal parenchymal diffusion changes with ADC values. The second was that the reduction in ADC values was more defined in the upper and lower pole parenchyma compared to the central section.
The movement of water molecules in biological tissue due to heat effects is called diffusion or Brownian motion. In biological tissues, the microscopic movement of water molecules is linked to diffusion and microcirculation (perfusion) in blood and capillary areas. DWI is a non-invasive MRI technique to measure the diffusion of water molecules in biological tissues. ADC is a parameter quantitatively measuring the water diffusion and capillary perfusion in extracellular and extravascular cavities. Evaluation with DWI may be qualitative or quantitative. On qualitative evaluation, hyperintensity results from both diffusion limitations and ‘T2 shine through’ effects of high T2 weight. To avoid this effect, ADC maps are used. ADC shows both true diffusion and perfusion (pseudo diffusion). The effect of perfusion on ADC is more pronounced at low b values (b = 50–400 s/mm2). At high b values, the effect of diffusion is more defined [5]. As a result, we used the high b value in our study.
In normal renal parenchyma, there are differences in ADC values in the cortex and medulla. When ADC measurements are separately performed in the cortex and medulla, different studies have found different ADC values. When the ADC values for the cortex and medulla are compared, they were reported by Namimoto et al. [3], (2.55–2.84 × 10−3 mm2/s), Siegel et al. [6], (1.79 ± 0.39 × 10−3 to 2.95 ± 0.58 × 10−3 mm2/s) and Kılıçkesmez et al. [7] (2.08 ± 0.22 × 10−3 to 1.9 ± 0.18 × 10−3 mm2/s). Without separating the cortex and medulla, measurements at the corticomedullary level have obtained low ADC values. This result is linked to the choice of a high b value [5, 8]. A study with a b value of 500 reported an ADC value of 2.19 ± 0.17 × 10−3 mm2/s for normal kidney. This value is between the separately calculated values for the cortex and medulla and has lower standard deviation [9]. Additionally, due to limited spatial resolution on echo-planar images and movement artifacts, it is difficult to separately define ROI for both cortex and medulla. As a result, in our study, ADC measurements were completed to include parenchyma from cortex and medulla. In our study, the ADC values for unobstructed opposite renal parenchyma were similar to values from previous studies. Numerical differences between ADC values may have been due to the device used and parameters, gradient changes, shooting technique and different b values chosen.
There are a limited number of DWI studies evaluating acute renal parenchymal changes resulting from unilateral ureteral obstruction linked to stones. In one of two different studies on unilateral ureteral occlusion in pig models, there was a reduction in ADC values identified in the obstructed kidney [10]. The second study reported a clear reduction in ADC values in both areas, with increased chronic partial obstruction on separate measurements of the cortex and medulla 24 h after acute ureter occlusion [11]. A study using low and high b values to evaluate changes in perfusion and diffusion in unilateral ureteral obstruction identified no differences in the ADC values in obstructed and unobstructed kidney after separate measurements of the cortex and medulla [12]. A study by Bozgeyik et al. [13] used different b values and measured only the parenchyma from the central section and found that though low ADC values were obtained in the obstructed kidney, there was no significant difference compared to the unobstructed kidney. All cases in the study had an obstruction, which lasted less than 1 week, so the lack of difference may have been due to a short period of stenosis. Similarly, in our study, there was a difference in ADC measurements made in the central portion of the obstructed and unobstructed kidney, but this was not statistically significant. A study by Soylu Boy et al. [14] found low ADC values in the cortex of the obstructed kidney as a result of separate measurements in the cortex and medulla and different portions of the kidney at low and high b values. There was no difference in the medulla ADC values. It was not clearly determined which section of the kidney decreased the ADC values.
Different sections of the kidney have different ADC values due to differences in degree of perfusion. A study using low b values found that the upper pole of the kidney had higher ADC values compared to the central section. However, this difference was not identified at high b values. This situation is explained as the high ADC value at the poles being due to increased perfusion and the effect of perfusion being revealed at low b values. Additionally, at the poles, the amount and blood supply to the cortex is higher compared to the medulla [5]. Increased intraluminal pressure as a result of mechanical obstruction of urine flow causes dilatation of the proximal collection system and increased interstitial pressure in the cortex and medulla. The resulting reduction in renal blood flow causes ischemia and a reduction in ADC values [2]. In our study, to determine the diffusion effect in the acute period, measurements were made in the early period (the mean duration between symptoms and DWI was 28.3 h) using high b values. Due to the reduction in spatial resolution at high b values, measurements were made in the corticomedullary region. Due to differences in the degree of perfusion, separate ROI were located in the central section and poles and changes in ADC values were calculated. Changes were greater at the poles, with a reduction in ADC values for all zones. This result shows that the poles of the kidney are more sensitive to ischemia resulting from acute obstruction.
Various studies have been performed on ADC measurements in the human kidney [6, 10, 15]. Siegel et al. [6] demonstrated that the diffusion coefficient in normal volunteers to be 2.00 × 10−3 mm2/s for the cortex and 1.79 × 10−3 mm2/s for the medulla. Nevertheless, artifacts may occur attributed to the bulk tissue motion during respiration. Muller et al. suggested that renal ADC was 3.54 × 10−3 mm2/s [10]. Another study by Muller indicated that the mean ADC value for dehydrated subjects was 2.9 × 10−3 mm2/s; while it was 3.6 × 10−3 mm2/s [15]. Impact of slice misregistrations and need for multiple acquisitions with different b values are important. The apparent diffusional anisotropy in the kidney linked with the highly vascular and anisotropic structure that contains many vessels and tubules with a non-random flow pattern must be taken into account.
Results of the present study imply that changes in ADC may alert the clinician for alterations in renal parenchyma associated with acute obstruction due to urolithiasis. Utilization of DWI for early diagnosis and follow-up of changes in renal tissue necessitate implementation of further controlled trials on larger series.
There are some limitations to our study. The first is that quantitative measurements were not completed after treatment. The second is that there was no control group. Additionally, low b values were not used to assess perfusion, and there were a relatively low number of cases analyzed. Notably, the wide range of time between first symptoms and measurements may minimize effects that could be seen in a smaller interval.
In conclusion, DWI is a non-invasive method showing changes occurring in acute renal parenchymal diffusion due to unilateral ureteral stone obstruction quantitatively with ADC values. The reduction in ADC values was more pronounced in the upper and lower pole parenchyma.
References
Vaughan ED Jr, Marion D, Poppas DP, Felsen D (2004) Pathophysiology of unilateral ureteral obstruction: studies from Charlottesville to New York. J Urol 172:2563–2569
Thoeny HC, De Keyzer F, Oyen RH, Peeters RR (2005) Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 235:911–917
Namimoto T, Yamashita Y, Mitsuzaki K, Na-kayama Y, Tang Y, Takahashi M (1999) Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging 9:832–837
Xu Y, Wang X, Jiang X (2007) Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging 26:678–681
Fukuda Y, Ohashi I, Hanafusa K, Nakagawa T, Ohtani S, An-naka Y, Hayashi T, Shibuya H (2000) Anisotropic diffusion in kidney: apparent diffusion coefficient measurements for clinical use. J Magn Reson Imaging 11:156–160
Siegel CL, Aisen AM, Ellis JH, Londy F, Chenevert TL (1995) Feasibility of MR diffusion studies in the kidney. J Magn Reson Imaging 5:617–620
Kiliçkesmez O, Yirik G, Bayramoglu S, Cimilli T, Aydin S (2008) Non-breath-hold high b-value diffusion-weighted MRI with parallel imaging technique: apparent diffusion coefficient determination in normal abdominal organs. Diagn Interv Radiol 14:83–87
Toyoshima S, Noguchi K, Seto H, Shimizu M, Watanabe N (2000) Functional evaluation of hydronephrosis by diffusion-weighted MR imaging. Relationship between apparent diffusion coefficient and split glomerular filtration rate. Acta Radiol 4:642–646
Cova M, Squillaci E, Stacul F et al (2004) Diffusion-weighted MRI in the evaluation of renal lsions: preliminary results. Br J Radiol 77:851–857
Muller MF, Prasad PV, Bimmler D, Kaiser A, Edelman RR (1994) Functional imaging of the kidney by means of measurement of the apparent diffusion coefficient. Radiology 193:711–715
Pedersen M, Wen JG, Shi Y et al (2003) The effect of unilateral ureteral obstruction on renal function in pigs measured by diffusion-weighted MRI. APMIS 109:29–34
Thoeny HC, Binser T, Roth B, Kessler TM, Vermathen P (2009) Noninvasive assessment of acute ureteral obstruction with diffusion-weighted MR imaging: a prospective study. Radiology 252:721–728
Bozgeyik Z, Kocakoc E, Sonmezgoz F (2009) Diffusion-weighted MR imaging findings of kidneys in patients with early phase of obstruction. Eur J Radiol 70:138–141
Soylu Boy FN, Kayhan A, Karakas HM, Alp T, Verit A (2015) Diffusion-weighted MR imaging and Doppler ultrasonography in the evaluation of renal parenchyma in acute ureteral obstruction. Int J Clin Exp Med 8:2719–2726
Müller MF, Prasad P, Siewert B, Nissenbaum MA, Raptopoulos V, Edelman RR (1994) Abdominal diffusion mapping with use of a whole-body echo-planar system. Radiology 190:475–478
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Düzenli, K., Öztürk, M., Yıldırım, İ.O. et al. The utility of diffusion-weighted imaging to assess acute renal parenchymal changes due to unilateral ureteral stone obstruction. Urolithiasis 45, 401–405 (2017). https://doi.org/10.1007/s00240-016-0924-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-016-0924-3