Abstract
Acute splenic infarcts classically present with left upper quadrant pain, but may be discovered incidentally in many hospitalized patients with otherwise vague complaints. The purpose of our study was to document causes or predisposing conditions in patients found to have acute splenic infarctions on imaging. Following IRB approval, a retrospective review of an imaging database from May 2008 to May 2015 was performed for cases of acute splenic infarctions. The electronic medical record was then reviewed for potential predisposing factors or known causes. Specific note was made of cases with active malignancy, vascular disorders, or inflammatory conditions with an increased risk of vasculopathy. Echocardiogram and electrocardiogram results were reviewed when available. One hundred twenty-three patients with acute splenic infarcts were identified, 65 female and 58 male. The average age was 57 years (range of 22 to 88). Active malignancy was present in 40 patients or 33 %. The most common malignancy in patient with nontraumatic splenic infarctions was pancreatic cancer, present in 16 patients (13 %). In these patients, splenic infarction was due to direct invasion of vessels in the splenic hilum. Acute pancreatitis (severe) was directly responsible for splenic infarction in seven additional cases (6 %). Additional visceral infarcts were present in 18 patients (15 %), most commonly concomitant hepatic or renal infarcts. Documented atrial fibrillation was present in 12 patients, but only 2 cases of left-sided cardiac thrombi were seen on CT (1 atrial, and 1 ventricular thrombus). Eight cases of endocarditis with valvular vegetations were documented on echocardiography (7 %). Splenomegaly was present in 32 patients (26 %) with acute splenic infarction. In patients with nontraumatic splenic infarctions, there appears to be a relatively high association with active malignancy (up to a third of patients). Pancreatic disorders, malignant and inflammatory, also appear to be an important cause of splenic infarction, presumably due to the close proximity of the pancreas to the splenic vessels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with acute splenic infarction (ASI) usually present with left upper quadrant pain, nausea or vomiting, and an elevated LDH [1]. In some patients, particularly hospitalized patients, the symptoms may be vague or nonspecific and splenic infarction may only be detected on imaging. Although splenic infarction may be detected on ultrasound with the use of contrast-agents [2], contrast-enhanced CT is the most reliable means of detection [3]. Classically, acute splenic infarctions appear as well-defined, wedge-shaped, peripherally-located hypoattenuating lesions on contrast-enhanced CT [3]. In hospitalized patients, recent abdominal surgery is a known risk factor for ASI [4], but other causes include infective endocarditis, pancreatitis, and hemoglobinopathies [1]. The purpose of our study was to document causes or predisposing conditions in patients found to have acute splenic infarctions on imaging at our tertiary-care institution (Figs. 1 and 2).
A 59-year-old man with acute gallstone pancreatitis. The first image (a) is a contrast-enhanced CT showing a small splenic infarct with inflammatory stranding at the splenic hilum (blue arrow). The second image (b) shows poor definition of the pancreatic tail, with surround inflammatory changes (blue arrow). The third image (c) is a mesenteric angiogram showing a small pseudoaneurysm (blue arrow). Splenic vein thrombosis was also present (not shown)
A 59-year-old man with pancreatic cancer. The first image (a) is a contrast-enhanced CT of the abdomen showing a small splenic infarct (blue arrow). The second image (b) at a slightly more inferior level shows intrahepatic biliary ductal dilatation (blue arrow). The third image (c) shows a hypoattenuating mass at the head of the pancreas (blue arrow), with atrophy of the body and tail of the pancreas. Upstream pancreatic ductal dilatation is also present
Methods
Study design
An application for Institutional Review Board (IRB) approval was submitted. After IRB approval, a retrospective review of an imaging database from May 2008 to May 2015 was performed for cases of acute splenic infarctions. The terms “acute, splenic, infarction, defect, spleen, wedge, hypoattenuating” were entered into the database using multiple permutations until no new cases were found. The database search engine used was Primordial (Primordial Inc., San Mateo, CA).
Imaging protocol
CTs of the abdomen and pelvis were reviewed. The typical protocol performed in cases of confirmed acute splenic infarction was a CT of the abdomen and pelvis performed after the intravenous administration of 120 mL of iodinated contrast with scanning performed in the portal venous phase. All scans were performed on two 64-slice multidetector CTs located in the Emergency Department at our institution (Lightspeed, General Electric Medical Systems, Milwaukee, WI, USA and Brilliance, Phillips Healthcare, Amsterdam, Netherlands). Positive oral contrast was also almost always administered for bowel opacification. Axial 5 mm slices were obtained from the heart through the lesser trochanter. Sagittal and coronal reconstructions were reconstructed from 1.25 mm axial slices and reviewed. The appearance of the normal spleen in the portal venous phase was usually homogeneous, which was crucial for improving the detection of acute splenic infarction in this study. All imaging studies were interpreted by 10 board-certified, fellowship-trained abdominal radiologists with 5 to 22 years of experience (average and median years of experience of 14 and 8, respectively). Studies were reviewed by another fellowship-trained radiologist (S.D.) with 8 years of experience for inclusion in the study. The diagnosis of acute splenic infarction was made on CT by detecting a wedge-shaped hypoattenuating defect with the base extending to the periphery of the spleen. Acute cases of splenic infarction were distinguished from chronic cases by noting the absence of retraction of splenic capsule at the site of the wedge-shaped defect: capsular retraction was felt to be more indicative of a chronic process.
Clinical correlation
The electronic medical record was also reviewed for potential causes of a hypercoagulable state in the patient’s medical history. Specific attention was directed towards cases with active malignancy, vascular disorders, or inflammatory conditions with increased risk of vasculopathy. The results of echocardiograms and electrocardiograms were reviewed when available for the presence of endocarditis or cardiac thrombus (echocardiogram) and for the presence of atrial fibrillation (electrocardiogram).
Results
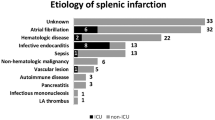
Overall, there were 123 patients identified in the database with acute splenic infarcts on CT. Sixty-five patients were female, and 58 were male. The average age of the patients at the time of diagnosis was 57 years (range of 22 to 88). Review of the chart revealed active malignancy in 40 patients or 33 %, and many of the solid malignancies were obvious on the current CT of the abdomen and pelvis (see Table 1). Most patients were receiving chemotherapy for their malignancy at the time that acute splenic infarction was diagnosed. The most common active malignancy in our patient population with nontraumatic splenic infarctions was pancreatic cancer, which was present in 16 patients (13 %). In the 16 patients with pancreatic cancer, 7 had direct evidence of malignant invasion of the vessels at the splenic hilum, with resultant infarction of the spleen. Acute pancreatitis (severe) was directly responsible for splenic infarction in seven additional cases (6 %). Two of the seven patients with acute pancreatitis had evidence of a pseudoaneurysm on CT. When the results were combined, benign and malignant pancreatic disorders were responsible for acute splenic infarction in 23 patients (19 %). Splenomegaly was present in 32 patients (26 %) with acute splenic infarction. We defined splenomegaly as splenic length greater than 12 cm in craniocaudal dimension and greater than 6 cm in width in equivocal cases.
Fourteen cases of acute splenic infarct occurred within two weeks of a major procedure (11 %). Four cases were discovered after solid organ transplantation (three liver transplants and one renal transplant). Eight cases occurred after a mesenteric angiogram, and two cases were diagnosed in patients on extracorporeal membrane oxygenation (ECMO). No splenic artery thrombus was seen or reported intraprocedurally during the mesenteric angiograms.
The electronic medical record was reviewed for any significant past medical history that could put patients at risk for hypercoagulability. Two patients had sickle cell anemia, two had systemic lupus erythematosus, and another two had vasculitis (biopsy-proven). In total, six cases of acute splenic infarction could be attributable to systemic disease (5 %).
Additional visceral infarcts were present in 18 patients (15 %), most commonly concomitant hepatic or renal infarcts (see Table 2). Documented atrial fibrillation was present in 12 patients, but only 2 cases of left-sided cardiac thrombi were seen on CT (1 atrial, and 1 ventricular thrombus). Eight cases of endocarditis with valvular vegetations were documented on echocardiography (7 %). Three cases of patent foramen ovale (PFO) and one case of transpulmonary shunting were also discovered during the work-up of this subgroup of patients.
Despite clear evidence of acute splenic infarction on CT, a filling defect within the splenic artery was only identified in 4 patients (3 %), one of whom was on ECMO. This may have been due to the phase of imaging: scanning in the portal venous is less sensitive for the detection of mesenteric artery thrombosis compared with a mesenteric angiogram.
Discussion
The most striking finding in our study was the high rate of active malignancy in patients with acute splenic infarction: approximately a third of patients with acute splenic infarction had active malignancy and was undergoing treatment at the time of imaging. While it is well documented that malignancy increases the risk of venous thromboembolism, the fact that malignancy also increases the risk of arterial thrombosis is only recently being recognized [5, 6]. Pancreatic cancer in particular, was the most common malignancy associated with acute splenic infarction in our study. Part of the reason may be due to the close proximity of the splenic vessels to the pancreas, which puts them at risk for direct invasion. Another reason for the high association with acute splenic infarction may be that patients with active pancreatic cancer (and other cancers) secrete humoral factors that activate the coagulation cascade and make patients especially prone to thromboembolism [7]. Indeed, migratory superficial thrombophlebitis (Trousseau syndrome) has anecdotally been a watch word for occult pancreatic malignancy [8]. Another potential reason may be the nature of our patient population: our institution is a major referral center for pancreatic disorders (including pancreatic cancer). This may have enriched our population with a higher rate of splenic infarctions related to pancreatic cancer compared with a community hospital.
The high association of acute splenic infarction with active malignancy differs from what has been reported in the literature in (previously) the two largest case series of acute splenic infarction. In a study of 59 patients with acute splenic infarction by Nores et al. in 1998, 35 cases were due to hematologic causes (60 %), most commonly myelofibrosis (present in 15 cases or 25 %) [9]. A second study of 66 patients with acute splenic infarction in 2004 by Gorg et al. demonstrated only 10 patients with underlying malignancy (15 %) [10]. One potential reason for the differences observed may be the increased utilization of cross-sectional imaging (mainly CT) in the emergency room in the years following publication of the study by Nores et al. [11]. This finding may also account for the larger number of patients in our study (currently the largest series on acute splenic infarction reported in the literature): more imaging means more acute splenic infarctions are being detected. The increasing number of cancer survivors may have also played a smaller role in our findings [12]. The rate of cancer deaths in the USA was 215 per 100,000 in 1990, and dropped to 166 per 100,000 in 2012 [12]. An increase in the number of patients living with cancer means more patients living long enough to undergo imaging of the secondary complications of malignancy such as splenic infarction.
Eleven percent of the cases of acute splenic infarction occurred within 2 weeks of a major hospital procedure. The most common procedure was a mesenteric angiogram (eight cases). While the temporal proximity of the two events does not imply causation, it is conceivable that interrogation of mesenteric vessels may have dislodged a small cholesterol plaque or embolus in a tortuous atherosclerotic vessel. Analysis of this association is complicated by the fact that patients undergoing mesenteric angiograms (and solid organ transplantation) had multiple other co-morbidities.
Our study also demonstrated a high positivity rate for a central cause of arterial embolization when additional infarcts were present. Overall, a central cause of arterial embolism was identified on echocardiography in 12 out of 18 patients (67 %) with acute splenic infarction and additional infarcts in other vascular beds. The yield for a potential cause of acute splenic infarction is increased in the presence of additional visceral infarcts: such patients should therefore be screened more thoroughly for a central cause of arterial embolization. A recent study had demonstrated that infarcts in multiple visceral vascular beds could be caused by left-sided cardiac thrombus [13], so a close inspection of the heart on CT in patients with nontraumatic acute splenic infarcts could prove to be a fruitful endeavor.
Interestingly, a filling defect in the main splenic artery was only visualized on CT in 4 out of 123 patients with acute splenic infarction (3 %), which is unsurprising given that portal venous phase scanning is less sensitive for arterial thrombi. Additionally, nonvisualization of a splenic artery thrombus may be due to interval recanalization and distal embolization of the clot into more distal branches. Alternatively, arterial occlusion may have occurred at more distal splenic artery branches. Interestingly, splenic vein thrombosis may cause variceal hemorrhage and splenomegaly, but it is not classically associated with splenic infarction [14].
One important limitation of our study was that it was retrospective in nature. There could be an inherent bias in the decision-making process regarding which patients get cross-sectional imaging in the emergency room (ER): cancer patients may be more likely to receive a CT of the abdomen when presenting to the ER with abdominal complaints. Additionally, all our cases of acute splenic infarct were diagnosed on contrast-enhanced CT. However, many patients with left flank pain are scanned in the emergency room with noncontrast CTs looking for nephrolithiasis. At least one study has shown unsuspected splenic infarcts in patients suspected of having a renal stone, who underwent repeat CT scanning with intravenous contrast administration [15]. We may have also missed some cases of acute splenic infarcts in patients who only received a noncontrast CT of the abdomen and pelvis in our emergency room. Another potential issue was that all the cases of acute splenic infarction were diagnosed on CT and no histopathologic correlation was obtained. However, contrast-enhanced CT is considered the gold-standard for diagnosis of acute splenic infarction, and histopathologic confirmation would be unreasonable in most cases (Table 3).
Conclusion
Patients with nontraumatic splenic infarctions appear to have a relatively high association with active malignancy. Pancreatic disorders, malignant and inflammatory, also appear to be an important cause of splenic infarction, presumably due to the close proximity of the pancreas to the splenic vessels. The presence of multiple infarcts in addition to an acute splenic infarct should trigger the search for a central cause of arterial embolism.
References
Lawrence Y, Pokroy R, Berlowitz D et al (2010) Splenic Infarction: an update on William Osler’s observations. Israeli Med Assoc J 12:362
Catalano O, Cusati B, Nunziata A et al (2004) Real-time, contrast-specific, sonography of acute splenic disorders: pictorial review. Emerg Radiol 11:15–21
Tonolini M, Bianco R (2013) Nontraumatic splenic emergencies: cross-sectional imaging findings and triage. Emerg Radiol 20:323–332
Gayer G, Galperin-Eizenberg M (2008) Iatrogenic splenic injuries in postoperative patients: a series of case reports. Emerg Radiol 15:109–113
Sanon S, Lenihan D, Mouhayar E (2010) Peripheral arterial ischemic events in cancer patients. Vasc Med 16:119–130
Thomas R (2001) Hypercoagulability syndromes. Arch Intern Med 161:2433–2439
Kakkar A, DeRuvo N, Chinswangwatanakul V et al (1995) Extrinsic-pathway activation in cancer with high factor VIIs and tissue factor. Lancet 346:1004–1005
Pinzon R, Drewinko B, Trujillo J et al (1986) Pancreatic cancer and Trousseau’s Syndrome: experience at a large cancer center. J Clin Oncol 4:509–514
Nores M, Phillips E, Morgenstern L et al (1998) The clinical spectrum of splenic infarction. Am Surg 64:182–188
Gorg C, Gorg K (2004) Acute, complete splenic infarction in cancer patients is associated with a fatal outcome. Abdom Imaging 29:224–227
Rao VM, Levin DC, Parker L et al (2011) Trends in utilization of various imaging modalities in emergency departments: nationwide Medicare data from 2000–2008. J Am Coll Radiol 8:706–709
Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/ Accessed 11/28/2015.
Cox M, Balasubramanya R, Hou A et al (2015) Incidental left atrial and ventricular thrombi on routine CT: outcome and influence on subsequent management at an urban tertiary care referral center. Emerg Radiol 22:657–660
Schwartz S, Stubbs A, Taljanovic M et al (2008) Pancreatitis-associated splenic vein thrombosis with intrasplenic venous thrombosis: a case report. Emerg Radiol 15:433–436
Agarwal M, Levenson R, Siewert B et al (2015) Limited utility of performing follow-up contrast-enhanced CT in patients undergoing initial non-enhanced CT for evaluation of flank pain in the emergency department. Emerg Radiol 22:109–115
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
IRB statement
This retrospective review study was approved by the Thomas Jefferson University Hospitals Institutional Review Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cox, M., Li, Z., Desai, V. et al. Acute nontraumatic splenic infarctions at a tertiary-care center: causes and predisposing factors in 123 patients. Emerg Radiol 23, 155–160 (2016). https://doi.org/10.1007/s10140-016-1376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-016-1376-3