Abstract
Aquaculture is responsible for more than 50% of global seafood consumption. Bacterial diseases are a major constraint to this sector and associated with misuse of antibiotics, pose serious threats to public health. Fish-symbionts, co-inhabitants of fish pathogens, might be a promising source of natural antimicrobial compounds (NACs) alternative to antibiotics, limiting bacterial diseases occurrence in aquafarms. In particular, sporeforming Bacillus spp. are known for their probiotic potential and production of NACs antagonistic of bacterial pathogens and are abundant in aquaculture fish guts. Harnessing the fish-gut microbial community potential, 172 sporeforming strains producing NACs were isolated from economically important aquaculture fish species, namely European seabass, gilthead seabream, and white seabream. We demonstrated that they possess anti-growth, anti-biofilm, or anti-quorum-sensing activities, to control bacterial infections and 52% of these isolates effectively antagonized important fish pathogens, including Aeromonas hydrophila, A. salmonicida, A. bivalvium, A. veronii, Vibrio anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, Photobacterium damselae, Tenacibaculum maritimum, Edwardsiela tarda, and Shigella sonnei. By in vitro quantification of sporeformers’ capacity to suppress growth and biofilm formation of fish pathogens, and by assessing their potential to interfere with pathogens communication, we identified three promising candidates to become probiotics or source of bioactive molecules to be used in aquaculture against bacterial aquaculture diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture industry is the world’s fastest growing animal protein producer. It supplies already 50% of the global seafood consumption, and it is indispensable to satisfy the world’s growing fish demand. However, frequent bacterial diseases occurring during fish farming can limit the worldwide growth of this sector (Plumb and Hanson 2011; Munn 2005; Lafferty et al. 2015). In fact, according to the United Nations, bacterial diseases are a major constraint to the economical profitability of the aquaculture industry (WHO 2014; Lafferty et al. 2015).
Vibriosis (Vibrio spp.), photobacteriosis (Photobacterium damselae sp.), tenacibaculosis (Tenacibaculum maritimum), edwardsiellosis (Edwardiella tarda), and furunculosis (Aeromonas salmonicida) are among the most common bacterial diseases known to affect important marine aquaculture fish species, such as turbot (Scophthalmus maximus), European seabass (Dicentrarchus labrax), Senegalese sole (Solea senegalensis), and gilthead seabream (Sparus aurata) (Menanteau-Ledouble et al. 2016; Janda and Abbott 2010; Frans et al. 2011; Avendaño-Herrera et al. 2006; Park et al. 2012; Lafferty et al. 2015; Plumb and Hanson 2011; Rivas et al. 2013; Andreoni and Magnani 2014). The symptomatology of these diseases include hemorrhagic septicemia, ulcers, gill inflammation, exophthalmia, multifocal necrosis, and white granulomas in the hematopoietic tissues (Menanteau-Ledouble et al. 2016; Janda and Abbott 2010; Frans et al. 2011; Avendaño-Herrera et al. 2006; Park et al. 2012; Andreoni and Magnani 2014). The exact pathogenetic mechanisms are not fully understood, but virulence factors of the reported Gram-negative bacteria, both extracellular (e.g., cytotoxins, proteases) and cell-wall associated (e.g., adhesins, invasins), are thought to play a prominent role in the development of diseases (Dallaire-Dufresne et al. 2014; Defoirdt 2013; Rasmussen-Ivey et al. 2016). Additionally, most of these pathogens form biofilms (mono- or multi- species communities attached to a surface and embedded in a polysaccharide matrix) that appear determinant for colonization and persistence inside the host (Avendaño-Herrera et al. 2006; Frans et al. 2011; Janda and Abbott 2010; Park et al. 2012; Defoirdt 2013; Wang et al. 2015; Jones and Oliver 2009; Andreoni and Magnani 2014). When associated in biofilms, bacteria are generally more resistant to antibiotics and innate immune defenses and also express more virulence factors as result of gene activation by quorum-sensing (Fey 2010; Defoirdt 2013; Moons et al. 2009). Quorum-sensing (QS) is the molecular strategy used by bacteria to monitor and regulate the size and density of their own population (Miller and Bassler 2001; Abisado et al. 2018). QS systems are mediated by small diffusible signalling molecules (e.g., acyl homoserine lactone, autoinducer-2) that regulate virulence, biofilm formation, inter- and intra-specific interactions, or antibiotic resistance (Miller and Bassler 2001; Chu and McLean 2016; Abisado et al. 2018).
Importantly, there is increasing evidence that Aeromonas spp., Vibrio spp., Photobacterium damselae sp., and Edwardsiella spp. might be responsible for emerging zoonoses which are dangerous for public health (Gauthier 2015; Khajanchi et al. 2010; Cabello et al. 2016; Igbinosa 2016). The routes of human infection are considered to be water (swimming or drinking) and water-associated food like fish, shellfish, and raw-edible products contaminated via irrigation. Aquaculture farmers are frequently exposed to fish; therefore, they are at higher risk of infection through open wounds, bites, or pincer injuries (Weir et al. 2012). Furthermore, the susceptibility of the abovementioned bacteria to traditional antibiotic treatments is not well documented and their resistance to different classes of antibiotics has been reported (Khajanchi et al. 2010; Gauthier 2015; Cabello et al. 2016; Igbinosa 2016). In this context, it is urgent to find alternative solutions to antibiotics that assure an advanced and integrated healthcare for humans, animals, and environment.
One of the most promising health-promoting strategies to improve fish resistance to diseases is the use of probiotics, defined as “live organisms which when administrated in adequate amounts confer a health benefit on the host” (Food and Agriculture Organization of the United Nations 2001). Probiotic bacteria may contribute to the gut microbial balance, enhance the host immune responses, compete with pathogens for adhesion sites and nutrients, or produce natural antimicrobial compounds (NACs). NAC can be metabolites, peptides, or proteins and might inhibit pathogen growth, biofilm formation, and QS (Hong et al. 2005; Hai 2015; Verschuere et al. 2000). Probiotic bacteria have also been implicated in bioremediation and water quality improvement and have been reported to reduce antibiotic residues in the environment, contributing to the sustainability of aquaculture (Verschuere et al. 2000).
Among bacteria currently used as probiotics, sporeforming species such as Bacillus spp. exhibit critical advantages for application in aquafeeds, as their spores are easily produced in large scale and can be dehydrated, thus facilitating feed incorporation and long-term storage without losing characteristics (Hong et al. 2005; Cutting 2011). Moreover, Bacillus spp. spores survive and transit through the gut since they are acid and bile tolerant (Tam et al. 2006). Further, Bacillus spp. are recognized to be NAC producers and are capable to antagonize important human and animal pathogens (Abriouel et al. 2011; Sahoo et al. 2016; Sumi et al. 2014). Indeed, we have recently isolated from the gut of European seabass sporeforming Bacillus spp. with promising probiotic characteristics, including a broad capacity to inhibit the growth of different fish pathogens (Serra et al. 2019). Thus, Bacillus spp. might be an ideal source of new NACs with anti-growth, anti-biofilm, or anti-QS activities, and thus a useful weapon against fish pathogens.
Here, we describe the isolation, identification, and characterization of sporeforming Bacillus spp. from the gut of fish with different feeding habits, and thus different gastrointestinal structures: the European seabass (Dicentrarchus labrax), white seabream (Diplodus sargus), and gilthead seabream (Sparus aurata). The bacterial isolates and their NACs were tested in vitro for their capacity to suppress growth and biofilm formation of fish pathogens, their potential to interfere with pathogens QS, and their suitability to become probiotics for aquaculture.
Material and Methods
Experimental Conditions
The trial was performed at the experimental facilities of the Marine Zoological Station, Faculty of Sciences, Porto University. White seabream (Diplodus sargus) and European sea bass (Dicentrarchus labrax) juveniles were obtained from IPMA, Olhão, Portugal, and gilthead seabream (Sparus aurata) juveniles were obtained from Atlantik Fish, Algarve, Portugal. After 15 days of quarantine, fish were transferred to the experimental tanks and acclimatized to the rearing conditions for 15 days. The experiment was performed in a recirculating water system equipped with 9 cylindrical fiberglass tanks of 100-L capacity and thermo-regulated to 22.0 ± 1.0 °C. Tanks were supplied with continuous flow of filtered seawater (2.5–3.5 l min−1), salinity average 34.0 ± 1.0 g l−1, and dissolved oxygen was kept near saturation (7 mg l−1). Seawater was filtered by passing through a biological filter followed by a sand filter and an UV light. Triplicate groups of 10 gilthead seabream (initial body weight (IBW) of 62 g), European seabass (IBW of 30 g), and white seabream (IBW of 40 g) were randomly distributed to each tank. The trial lasted 6 weeks, and during that period, fish were fed by hand twice daily, 6 days a week, until apparent visual satiation with a commercial diet (Skretting, Stavanger, Norway) containing 16% lipids and 47% protein. Fish handling and procedures were performed in compliance with the recommendations of the EEC Directive (2010/63/EU) for care and use of laboratory animals.
Sampling
At the end of the trial, nine fish of each species (3 fish per tank) were randomly captured 4 h after the morning meal (to guarantee that intestines were filled with digesta) and euthanized by a lethal dose (1 mL/L) of anesthetic (ethylene glycol monophenyl ether). Digesta contents were collected under aseptic conditions by squeezing the entire gut (without pyloric caeca), previously excised. To reduce inter-fish variation, each sample was composed of a pool of gut digesta contents from the 3 fish per tank.
Isolation, Selection, and Characterization of Sporeforming Bacteria
To select the aerobic bacterial sporeforming isolates, around 150 mg of each digesta were diluted in buffered peptone water (0.9%) and homogenized by vigorous vortexing. Then, serial dilutions (10 ̄ 0, 10 ̄ 1, 10 ̄ 2) of homogenate were prepared in Bott and Wilson (B&W) salts (1.24% K2HPO4, 0.76% H2PO4, 0.1% trisodium citrate, 0.6% [NH4]2SO4, pH6.7) and 100 µL aliquots were spread onto Luria Bertani (LB) agar plates (Becton, Dickinson and Company, USA) after 20-min heat treatment at 65 °C (Barbosa et al. 2005).
Following incubation at 37 °C for 48 h, colonies obtained from each digesta were counted and randomly selected by their different morphologies. All selected isolates were purified, numbered, and stored at −80 °C in 30% glycerol until used. Spore production was evaluated by phase-contrast microscopy observation of isolates grown for 48 h in solid Difco Sporulation Medium or DSM (Becton, Dickinson and Company, USA) (Barbosa et al. 2005), using a Nikon Eclipse Ci-L microscope, equipped with a CoolLED’s pE 300 lite illumination system, and images were obtained with a DS-Ri 2 camera. All isolates were further investigated for catalase activity by suspending a fresh colony in 3% hydrogen peroxide (H2O2) solution; the production of air bubbles was considered a positive response (Barbosa et al. 2005).
Molecular Identification and Phylogenetic Analysis
Genomic DNA of sporeformers was extracted from overnight LB liquid cultures, based on the method of Pitcher et al. (1989) with few modifications. Briefly, each bacterial pellet was homogenized with 50 mg/mL of lysozyme (Sigma-Aldrich, Germany), in TE buffer (10 mM Tris HCl, 1 mM EDTA) followed the addition of by 2 mg/mL of RNAse (Sigma-Aldrich, Germany). After incubation at 37 °C for 1 h, 10% SDS, and 0.2 mg/mL of proteinase K (Sigma-Aldrich, Germany) were added, and tubes incubated for 30 at 55 °C. Then, GES solution (5 M guanidine thiocyanate, 0.5 M EDTA, 10% N-lauroysarcosine) and 7.5 M ammonium acetate precipitated the remaining proteins prior to nucleic acids extraction with of phenol:chloroform:isoamyl-alcohol (25:24:1). After the aqueous phase collection, the addition of chloroform:isoamyl-alcohol (24:1) allowed a re-extraction of the nucleic acids. The DNA was precipitated with 0.6 volumes of isopropanol washed with ice-cold 70% ethanol and dissolved in ultrapure water.
PCR amplification of the 16S rRNA gene was accomplished using universal primers 16S-27F (Weisburg et al. 1991) and 16S-1492R (Weisburg et al. 1991), at an annealing temperature of 55 °C. Each 50 μL reaction contained 1 × DreamTaq Buffer (Thermo Scientific, Vilnius, Lithuania), 0.2 mM of each dNTP (Thermo Scientific, Vilnius, Lithuania), 0.2 μM of each primer (STAB Vida, Lisboa, Portugal), 1.25 U of DreamTaq DNA polymerase enzyme (Thermo Scientific, Vilnius, Lithuania), and 25 ng DNA template. The amplified products were sequenced with the primer 16S-27F (Weisburg et al. 1991) at STABVIDA (Caparica, Portugal).
Phylogenetic analysis was accomplished online, using the Sequence Match software package through the GenBank non-redundant nucleotide database with BLAST (http://www.ncbi.nlm.nih.gov) or by comparison with sequences in the Ribosomal Database Project 10 (http://rdp.cme.msu.edu/).
Bacterial Strains and Culture Conditions
The sporeforming bacterial strains isolated from fish gut were denominated “producer strains” and were routinely grown in LB medium at 37 °C, with constant agitation (120 rpm). The laboratory strain Bacillus subtilis 168 (Zeigler et al. 2008) (Table 1) was used as control for “producer strains”, and the Gram-positive Staphylococcus aureus LMG 2884, was used as control for “indicator strains” due to its inhibition by B. subtilis 168 which has been previously described (Serra et al. 2019). These controls were grown in the same conditions as the “producer strains” and “indicator strains,” respectively. Target fish pathogens presented in Table 1 were used as “indicator strains” and were grown aerobically in BHI medium (except for T. maritimum, which was grown in Marine medium) at 25 °C (A. hydrophila, A. salmonicida, A. veronii, A. bivalvium, V. anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, Photobacterium damselae subsp. damselae, Photobacterium damselae subsp. piscicida, and T. maritimum) or 37 °C (for E. tarda, S. sonnei, and S. aureus).
A strain of Chromobacterium violaceum (CECT 494) and the cvil::mini-Tn5 mutant of Chr. violaceum CV026 (CECT 5999) (Table 1) were used as biosensors for quorum-quenching (QQ) detection. Biosensors were routinely cultivated aerobically in LB media at 30 °C for 48 h. Pseudomonas aeruginosa PAO-1 was used as positive control for QQ activity and was grown aerobically at 37 °C for 24 h.
Anti-growth Activity Screening
The antimicrobial activity of all producer strains was first evaluated by colony overlay assay, as described by Barbosa et al. (2005). Briefly, producer strains were inoculated as 5 μL spots on LB plates, grown for 24 h and then killed with chloroform vapors. Plates were overlaid with soft BHI agar inoculated with the indicator strains (OD600 ~ 0.1) and incubated for 24 h at 25 °C or 37 °C (for S. sonnei and E. tarda). Growth inhibition zones around the colonies, after 24-h incubation at 25 °C or 37 °C as described above, were considered positive and the corresponding growth inhibition halos were measured (mm). All plates were photographed in Gel-Doc™ XR+ System, using Image Lab™ Software (Bio-Rad, EU).
The antimicrobial activity of cell-free supernatant containing NACs of the most promising producer strains (those exhibited a stronger antimicrobial activity against fish pathogens or “indicator strains”) was detected by two independent growth inhibition assays. First, a well-diffusion assay was performed as described by Serra et al. (2019). The indicator strains were inoculated (OD600 ~ 0.1) on BHI agar plates, assuring uniform coverage. Then, 9-mm-diameter wells were punched and filled with 100 µL cell-free supernatant of each producer strain, previously grown overnight at 37 °C and 150 rpm, centrifuged for 10 min at 11,800g, and sterilized by filtration with 0.22 µm cellulose acetate filter (VWR, Portugal). Growth inhibition zones around the wells after 24-h incubation at 25 °C or 37 °C were considered positive, and the corresponding growth inhibition halos were measured (mm). Plates were photographed as described above. For the second growth inhibition assay (microplate assay), 100 µL cell-free supernatant of each producer strain (obtained as described above) were added to 96-well flat-bottomed polystyrene plates and inoculated with 100 µL of indicator bacterial cultures (OD600 ~ 0.1). A positive control containing 100 µL of LB medium and 100 µL of the appropriate bacterial cultures (OD600 ~ 0.1) and a negative control containing only 200 µL of LB medium were also prepared. The 96-well microplate was aerobically incubated (120 rpm) at 25 ºC, and the optical density at 600 nm (OD600) was measured every hour for 24 h in a Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher Scientific Inc.). The data is composed by three independent experiments.
Anti-biofilm Formation Screening
The ability of sporeformers to inhibit biofilm formation of each pathogenic strain was tested by a modification of Papa et al. (2015) method. In brief, 100 µL of indicator cultures (OD600 ~ 0.1) were added to 96-well flat-bottomed polystyrene plates. Each well was filled with 100 µL of cell-free supernatant of each sporeforming strain. Moreover, 100 µL of LB medium and 100 µL of the appropriate bacterial cultures (OD600 ~ 0.1) was used as positive control, and 200 µL of LB medium was used as negative control. After 24 h of aerobic incubation at 25 °C, bacterial cells were removed and the wells were washed 3 times with 250 µL of phosphate-buffered saline (PBS) and allowed to dry in inverted position. The wells were stained with 250 µL of 0.1% crystal violet for 15 min at room temperature and rinsed twice with 250 µL of double-distilled water. After wells’ drying (in inverted position), the dye bound to adherent cells was solubilized in 250 µL of 20% glacial acetic acid and 80% ethanol for 30 min at room temperature. The final quantification of biomass was accessed by measuring the optical density at 590 nm in a Microplate Spectrophotometer (Multiskan™ GO, Thermo Fisher Scientific Inc.). The data is composed by three independent experiments.
Anti-quorum Sensing (Anti-QS) Screening (Quorum-Quenching-Potential)
Sporeformers QQ potential was evaluated as described by McLean et al. (2004) with few modifications. Briefly, producer strains were inoculated as 5 μL spots on LB plates and grown at 37 °C. After overnight incubation, bacterial spots were killed with chloroform vapors for 30 min, followed by plate covers replacement and plates aeration for 20 min. Then, producer strains were overlaid with LB soft agar (0.8% agar) previously inoculated with 100 μL of biosensor strains (OD600 ~ 0.1), followed by plates incubation for 48 h at 30 °C. In the case of Chr. violaceum CV026, the LB soft agar was supplemented with 25 μg/mL of kanamycin (Nzytech, Lisboa, Portugal) and N-(β-ketocaproyl)-L-homoserine lactone (3-Oxo-C6-HSL) (Sigma-Aldrich, Germany) to 5 µM of final concentration (McClean et al. 1997), prior to bacterial culture inoculation. A positive QQ result was represented by inhibition of purple pigmentation around the producer strains. The cell-free supernatant screening was performed by overlaying LB plates with LB soft agar (0.8% agar) previously inoculated with 100 µL of biosensor strains (OD600 ~ 0.1) in the same experimental conditions described above. Once the plates solidified, 9-mm-diameter wells were punched and filled with 100 µL cell-free supernatant of each producer strain (obtained as described above). Zones of purple pigmentation inhibition around the wells after incubation for 48 h at 30 °C were considered a positive result. P. aeruginosa PAO-1 and laboratory strain B. subtilis 168 were used as positive control for QQ activity and for bacterial growth, respectively. All digital photos were taken with a Sony IMX240 camera and zones (in mm) of pigment inhibition recorded.
Sporulation Capacity
Sporulation of each “producer strain” was induced by nutrient exhaustion in DSM (Difco Sporulation Medium) for 24 h at 37 °C in an orbital shaker at 150 rpm. Grown cultures were serial diluted in B&W isotonic buffer (Bott and Wilson salts: 1.24% K2HPO4, 0.76% H2PO4, 0.1% trisodium citrate, 0.6% [NH4]2SO4, pH6.7), and plated in LB agar before and after 20 min heat treatment at 80 °C, to determinate heat-resistant spores. After incubation at 37 °C for 24 h, visible colonies were counted and the sporulation efficiency determined by quantifying colony forming units (CFU/mL) titer before and after the heat treatment (Barbosa et al. 2005; Nicholson and Setlow 1990). The data is composed of three independent experiments.
Antibiotic Susceptibility Test
Antibiotic susceptibility of “producer strains” was determined by the disk diffusion method of Kirby-Bauer (Bauer et al. 1966), using antimicrobial susceptibility discs (Oxoid Limited, Thermo Fisher Scientific Inc.) containing teicoplanin 30 μg/disc (TEC30), vancomycin 30 μg/disc (VA30), chloramphenicol 30 μg/disc (C30), tetracycline 30 μg/disc (TE30), erythromycin 15 μg/disc (E15), gentamycin 10 μg/disc (CN10), kanamycin 30 μg/disc (K30), and streptomycin 10 μg/disc (S10). The antibiotics were tested followed the recommendations of the European Food Safety Authority Panel on Additives and Products or Substances used in Animal Feed (FEEDAP 2012). Bacterial inoculums were prepared in sterile saline solution, at an optical density adjusted to 0.5 McFarland standard units (OD600 ~ 0.1) and were spread with cotton swab onto 20 mL MH (Muller-Hinton, Oxoid) agar plates, assuring full coverage, and the antibiotics were distributed on the plates with a disk dispenser (Oxoid Limited, Thermo Fisher Scientific Inc.). After 24 h of incubation at 37 °C, bacteria were classified as sensitive (S), intermediate (I), and resistant (R), according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI 2013).
Statistical Analysis
The statistical analysis was done using the SPSS 23.0 software package for Windows. Before any test, data were checked for normal distribution and subject to Levene’s test to ensure homogeneity of variances that complies the requirements of ANOVA.
Repeated measures ANOVA and one-way ANOVA were performed to evaluate differences in the ability of sporeforming isolates to inhibit pathogens growth and biofilm formation, respectively. When p values were significant (p < 0.05), means were compared with Dunnet’s test.
Results
Culturable Aerobic Sporeformers Are Abundant in the Gut of Marine Fish
The heat-treated gut content of each fish species allowed selection, isolation, and purification of 176 bacterial isolates (61 from gilthead seabream, 51 from white seabream, and 64 from European seabass). Strain’s selection was based on macroscopic differences in colony morphologies and also on the collection of samples from different animal groups (tanks). Spore production analysis, induced by nutrient exhaustion in DSM, revealed that 172 strains (98%) produced endospores of different sizes and shapes (Table 2 and Fig. 1), and were catalase positive, indicating that these were probably Bacillus spp. and not aero-tolerant strains of Clostridium spp. (catalase negative) (Table 2).
a Morphological diversity of representative sporeforming isolates (FI numbers on top) obtained from fish intestinal contents. Photographs of colonies grown for 24 h at 37 °C in LB (Luria–Bertani) and DSM (Difco sporulation medium) agar medium were taken in a Gel-Doc™ XR + System, using the Image Lab™ Software (Bio-Rad, EU) and are at the same scale. b Sporulation capacity of the fish isolates, illustrating refractile sporulating cells and free spores. Spore production was evaluated by phase-contrast microscopy (PCM) using a Nikon Eclipse Ci-L microscope, equipped with a CoolLED’s pE 300 lite illumination system, of isolates cultured for 48 h in solid DSM. Images were obtained with a DS-Ri 2 camera, all in the same conditions and at the same scale. The laboratory strain B. subtilis 168 (Bsub, on top) was used as a control
Bacillus Sporeformers from Fish-Gut Are Promising Antagonists of Fish Pathogens
Among the 172 endospore-forming fish isolates (FIs) evaluated for their antimicrobial activity against a wide range of fish pathogens, 52% (90 isolates) were able to inhibit at least one of the tested pathogenic strains, and 23% (41 isolates) inhibited 2 or more pathogens. The later 41 isolates were identified by partial sequencing of the 16S rRNA gene (~ 700 kb), revealing a clear abundance of B. subtilis (54%) among the sporeformers isolated from the gut of gilthead seabream, white seabream, and European seabass (Table 2). B. licheniformis and B. methylotrophicus represented 9% and B. amyloliquefaciens represented 7% of the identified strains, whereas other bacterial species were present in smaller quantities. The identification of bacterial species was inconclusive for 10% of the strains (Table 2).
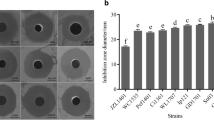
The colony-overlay assay allowed a progressive selection of the 8 most promising fish isolates according to the size of the inhibitory halos as well as the number of fish pathogens that they inhibited. The 8 FIs with the most promising antimicrobial activities, namely FI314, FI330, FI347, FI359, FI368, FI376, FI442, and FI480, were then tested simultaneously (via colony-overlay assay), in the exact same conditions (Fig. 2). Strains FI314, FI330, and FI442 showed antagonistic capacity against the growth of all pathogenic strains, except for S. sonnei. FI347 was active against A. hydrophila and A. veronii. FI359 inhibited the growth of A. hydrophila, A. veronii, V. harveyi, S. aureus and, in a smaller extent, of P. damselae subsp. piscicida. FI368, FI376, and FI480 inhibited the growth of S. aureus, V. harveyi, and A. veronii and, less markedly, that of P. damselae subsp. piscicida.
Growth inhibition zones for the indicator strains A. hydrophila, A. salmonicida, A. veronii, A. bivalvium, V. anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, P. damselae subsp. piscicida, P. damselae subsp. damselae, E. tarda, S. sonnei, and S. aureus around colonies of sporeforming fish isolates (FI numbers on top). The laboratory strain B. subtilis 168 was used as a control. All photos were taken in a Gel-Doc™ XR + System, using the Image Lab™ Software (Bio-Rad, EU) and are at the same scale
Antimicrobial Activity of Bacillus Sporeformers Is Mediated by Extracellular NACs
The antimicrobial activity of cell-free supernatant of the most promising Bacillus sporeformers (FI314, FI330, FI347, FI359, FI368, FI376, FI442, and FI480) is illustrated in Fig. 3. The extracellular compounds (hereinafter referred as extracellular NACs) of FI314 and FI330 produced clear inhibitory halos against A. salmonicida, V. anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, P. damselae subsp. piscicida, P. damselae subsp. damselae, T. maritimum, and S. aureus, and small inhibitory halos (2 mm, not clearly visible in Fig. 3) against A. bivalvium. FI442 extracellular NACs inhibited V. vulnificus and P. damselae subsp. damselae. The remaining strains (FI347, FI359, FI368, FI376, and FI480) did not inhibit the growth of the tested fish pathogens.
Growth inhibition zones for the indicator strains A. hydrophila, A. salmonicida, A. veronii, A. bivalvium, V. anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, P. damselae subsp. piscicida, P. damselae subsp. damselae, T. maritimum, E. tarda, S. sonnei, and S. aureus around the wells with cell-free supernatant of sporeforming fish isolates (FI numbers on top). All photos were taken in a Gel-Doc™ XR + System, using the Image Lab™ Software (Bio-Rad, EU) and are at the same scale. The laboratory strain B. subtilis 168 (Bsub on top) was used as a control
Based on the results of these experiments, six producer strains were selected having the best antimicrobial potential: FI314, FI330, FI359, FI376, FI442, FI480. The antimicrobial pattern of their extracellular NACs against the same fish pathogens was quantified via a growth-inhibition assay in liquid medium (microplate assay). FI314, FI330, and FI442 extracellular NACs were the most efficient in inhibiting bacterial growth (Fig. 4). In particular, the extracellular NACs of strains FI314 and FI330 reduced the growth of all the pathogens (p < 0.001). Furthermore, these two strains completely inhibited the growth of A. salmonicida, V. anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, P. damselae subsp. piscicida, P. damselae subsp. damselae, T. maritimum, and S. aureus for the entire duration of the assay (24 h). The extracellular NACs of FI442 extended the lag phase duration of V. anguillarum and P. damselae subsp. piscicida growth curves, and completely inhibited the growth of V. vulnificus and P. damselae subsp. damselae for 24 h. In general, the NACs of this isolate reduced the growth of A. salmonicida, V. anguillarum, V. parahaemolyticus, V. vulnificus, P. damselae subsp. piscicida, T. maritimum, S. aureus, and P. damselae subsp. damselae (p < 0.01). The extracellular NACs of FI376 reduced the growth of T. maritimum and S. aureus (p < 0.01), whereas those of FI480 reduced the growth of P. damselae subsp. damselae and T. maritimum (p < 0.01). Interestingly, the growth of A. salmonicida and V. harveyi was higher in the presence of FI359 and FI376 (p < 0.05) cell-free supernatants when compared to the control. FI480 cell-free supernatant also increased A. salmonicida growth (p < 0.05).
Growth inhibition assays of the indicator strains A. hydrophila, A. salmonicida, A. veronii, A. bivalvium, V. anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, P. damselae subsp. piscicida, P. damselae subsp. damselae, T. maritimum, E. tarda, S. sonnei, and S. aureus when cultured alone in brain heart infusion (BHI) or marine (MA) medium (in the case of T. maritimum), or supplemented with cell-free supernatant of the sporeforming isolates (FI numbers). Pathogenic strains growing without cell-free supernatant’s supplementation were used as positive control (control), and BHI or MA medium alone used as negative control (medium). The laboratory strain B. subtilis 168 was used as a control for bacterial growth. The data are obtained from three independent experiments. Significant differences (p < 0.05; p < 0.01; p < 0.001) in relation to control are represented by asterisks (*, **, ***, respectively)
Overall, only the fish isolates FI314, FI330, and FI442 were capable of suppressing fish pathogen’s growth for 24 h in microplate (Fig. 4) produce clear inhibitory halos against the bacteria in the well agar diffusion assay (Fig. 3).
Extracellular NACs of Bacillus Sporeformers Inhibit Fish-Pathogen Biofilms
Cell-free supernatants of the six isolates tested above (FI314, FI330, FI359, FI376, FI442, FI480) were also evaluated for their ability to interfere with biofilm formation of each fish pathogen (Fig. 5). The extracellular NACs of FI314 and FI330 decreased the biofilm formation of A. salmonicida, V. anguillarum, V. parahaemolyticus, P. damselae subsp. damselae, T. maritimum, and S. sonnei (p < 0.01). FI330 also reduced A. hydrophila biofilm (p < 0.05). The extracellular NACs of FI359 and FI376 were active on biofilms of A. hydrophila, A. salmonicida and S. sonnei, whereas those of FI442 and FI480 reduced the biofilm synthesis of A. salmonicida and S. sonnei (p < 0.05). The control B. subtilis 168 was also able to decrease the biofilm synthesis of T. maritimum (p < 0.05).
Biofilm formation of the fish pathogens A. hydrophila, A. salmonicida, A. veronii, A. bivalvium, V. anguillarum, V. harveyi, V. parahaemolyticus, V. vulnificus, P. damselae subsp. piscicida, P. damselae subsp. damselae, T. maritimum, E. tarda, S. sonnei, and S. aureus when cultured alone in brain heart infusion (BHI) or marine (MA) medium (in the case of T. maritimum), or supplemented with cell-free supernatant of the sporeforming isolates (FI numbers). The laboratory strain B. subtilis 168 was used as a control for bacterial growth. The data are obtained from three independent experiments. Significant differences (p < 0.05; p < 0.01; p < 0.001) in relation to control are represented by asterisks (*, **, ***, respectively)
Extracellular NACs of Bacillus Sporeformers Have QQ Activity
Chr. violaceum WT CECT 494 produces and senses several AHLs (e.g., N-(3-hydroxydecanoyl)-L-homoserine lactone and N-octanoyl-L-Homoserine lactone), whereas Chr. violaceum CV026 is unable to synthesize AHL, but responds to exogenous AHLs supplementation.
QQ was confirmed by the loss of purple violacein pigmentation around the sporeforming strains FI314, FI330, and FI442, suggesting the inactivation of AHLs produced by Chr. violaceum WT CECT 494 and of the 3-oxo-C6-HSL signal molecule externally added to Chr. violaceum CV026 biosensor (Fig. 6a). Additionally, the observed QQ activity of FI314 and FI330 was due to extracellular NACs, as their cell-free supernatants displayed activity against 3-oxo-C6-HSL signal molecule and interfered with the AHLs produced by Chr. violaceum WT CECT 494 to a lower extent (Fig. 6b). On the contrary, FI442 cell-free supernatant did not shown any QQ activity. The control P. aeruginosa PAO-1 effectively degraded the AHLs tested in all the experiments performed during the present study.
Degradation of the violacein pigment produced by Chr. violaceum biosensors around colonies of sporeforming fish isolates a or around the wells with cell-free supernatant of sporeforming fish isolates b. The laboratory strain B. subtilis 168 (Bsub) was used as a control for bacterial growth and P. aeruginosa PAO-1 as control for quorum-quenching (QQ) activity. All photos were taken with a Sony IMX240 camera and are at the same scale
Most Promising Bacillus Fish-Gut Sporeformers Have Probiotic Potential
Sporulation efficiency of the most promising strains (FI314, FI330, FI442) was determined due to their potential industrial application as probiotics. By comparison with the laboratory strain B. subtilis 168 (Zeigler et al. 2008), all the selected strains showed good spore titres (around 108 CFU/mL), and sporulating efficiency ≥ 79% (Table 3). All “producer trains” were also sensitive to all the antibiotics required by EFSA (FEEDAP 2012), with the exception of FI314 isolate that presented an intermediate susceptibility to streptomycin (S10) (Table 3).
Discussion
It is well established that gastrointestinal microbiota is a key player in human and animal health (Wang et al. 2018a, b), and the use of probiotics represents an excellent approach to improve gut microbial balance and host health (Dimitroglou et al. 2011). One important criterion for probiotic selection is the capacity to minimize pathogens’ growth by competitive exclusion or through the production of antimicrobial molecules (Verschuere et al. 2000; Dobson et al. 2012; Hai 2015; Banerjee and Ray 2017). The aquaculture sector growth is limited by frequent infection episodes (WHO 2014; Lafferty et al. 2015), but no specifically dedicated probiotics have so far been developed.
This study assumed that probiotics would be potentially more effective if originated from the gut of target animals and from the target pathogens ecological niche. Focus was on Bacillus species, which are natural habitants of the animal’s gut, including fish (Barbosa et al. 2005; Hong et al. 2005; Tam et al. 2006; Zhou et al. 2014; Midhun et al. 2017; Guo et al. 2016) and are known producers of NACs capable of inhibiting pathogen’s growth and proliferation (Abriouel et al. 2011; Caulier et al. 2019). In addition, different Bacillus spp. are commercially available probiotic products for human and animal use. Specifically, B. subtilis, B. vallismortis, B. mojavensis, B. atrophaeus, B. amyloliquefaciens, and B. licheniformis are included in the EFSA list of Qualified Presumption of Safety (QPS). However, no Bacillus probiotic is yet available for fish in the EU. To fulfil this market gap, we isolated several putative probiotic sporeforming Bacillus spp. from the gut of three marine aquaculture species, including gilthead seabream and European seabass (the most important fish species reared in the Mediterranean), and white seabream (a species with potential for aquaculture).
More than 50% of the Bacillus spp. isolates were active against at least one of the 14 tested bacterial fish pathogens, reinforcing their recognition, as producers of important bioactive metabolites (Caulier et al. 2019). Previous research reported that Bacillus spp. isolated from fish gut exhibited inhibitory activity against fish pathogens, including Aeromonas (Ramesh et al. 2015; Thankappan et al. 2015; Banerjee et al. 2016; Midhun et al. 2017; Ran et al. 2012; Guo et al. 2016; Meidong et al. 2018; Mukherjee et al. 2019; Serra et al. 2019; Askarian et al. 2012; Gao et al. 2017; Nandi et al. 2017; Kuebutornye et al. 2020) or Vibrio species (Chen et al. 2016; Serra et al. 2019; Askarian et al. 2012; Kuebutornye et al. 2020). The extent of the inhibitory actions observed in our study enlarges the potential role of fish-gut Bacillus community against furunculosis, vibriosis, photobacteriosis, tenacibaculosis and edwardsiellosis diseases, protecting the hosts against opportunistic bacteria.
Because Bacillus antimicrobial activity is usually a result of molecules produced and released to the surrounding environment (Abriouel et al. 2011; Caulier et al. 2019), the cell-free supernatants of each fish isolate were also tested for their antimicrobial activity. As expected, the anti-growth activity of Bacillus fish-gut isolates was mediated by extracellular NACs. In particular, the cell-free supernatants of strains FI314 and FI330 were highly effective in reducing the growth of all the tested fish pathogens. Unexpectedly, an increased growth of A. salmonicida and V. harveyi in the presence of FI359, FI376, and FI480 cell-free supernatants has been observed. A potential explanation of this evidence could be that cell-free supernatants might harbor substances that serve as nutrients for some bacteria (Moons et al. 2009; Hibbing et al. 2010). However, such hypothesis would need to be confirmed for our strains, under our experimental conditions. Another intriguing observation was the variation of results obtained when using two different antimicrobial assays for cell-free supernatant: only the extracellular NACs suppressing pathogen’s growth for 24 h in liquid medium produced a strong inhibitory halo against the same pathogen onto agar medium. For example, the FI314 and FI330 extracellular NACs inhibited the growth of S. sonnei, in liquid medium without a visible inhibitory halo onto agar medium. Such differences may reflect technical limitations, since agar-based tests can be influenced by compound’s diffusion into the agar, whereas the liquid-based assays allow full contact between the compounds and the target pathogenic strain. Thus, agar-based assays allow quick and less laborious screenings, but liquid-based assays are preferable when a quantitative analysis is to be done (Balouiri et al. 2016).
Bacterial biofilms are important key-parameters for bacterial proliferation and persistence, through the promotion of resistance and tolerance to external assaults, including antimicrobials (Flemming et al. 2016). The capacity of Bacillus spp. to regulate or inhibit pathogens biofilm formation has been already studied with the objective of controlling bacterial infections in medical and industrial fields (Nahar et al. 2018; Kalpana et al. 2012), including aquaculture (Hamza et al. 2016). Previous studies demonstrated Bacillus spp. potential in biofilm control of a broad range of aquatic pathogens, including Aeromonas, Pseudomonas, and Vibrio species (Vinoj et al. 2014; Chu et al. 2014; Hamza et al. 2018, 2016; Yatip et al. 2018; Kalpana et al. 2012). Accordingly, fish-gut isolates in the present study produced extracellular NACs capable of reducing biofilm formation of at least one of the tested pathogens, emphasizing the potential use of fish-gut Bacillus spp. in preventing the colonization and persistence of fish-pathogenic bacteria. Interestingly, and contrary to the anti-growth results, some fish-gut Bacillus extracellular NACs could reduce A. salmonicida biofilm formation. This was the case of isolates FI359, FI376, and FI480. Similarly, FI359, FI376, and FI442 extracellular NACs also inhibited the biofilm production by A. hydrophila, and S. sonnei without interfering with their growth. These observations open the possibility of developing new prophylactic or therapeutic approaches, to deal with problematic fish pathogens and zoonotic agents, such as A. salmonicida. Although not directly inhibiting the growth of fish bacterial pathogens, Bacillus spp. might control their proliferation by reducing their defense mechanisms, such as biofilm (Grassi et al. 2017; Pletzer et al. 2016).
Another promising pathogen-controlling strategy is interfering with their communication or quorum-sensing, in a process called quorum-quenching (QQ) (Grandclement et al. 2016). Indeed, members of the Bacillus genus are known as prominent producers of QQ molecules, such as AHL-lactonases (AiiA and homologues) and one AHL-oxireductase (CYP102A1, P450BM-3) (Chowdhary et al. 2007; Chu et al. 2014; Dong et al. 2000; Tinh et al. 2013; Torabi Delshad et al. 2018; Vinoj et al. 2014; Zhou et al. 2016). The present results showed that FI314, FI330, and FI442 produce compounds capable of interfering with externally added 3-oxo-C6-HSL (in the case of Chr. violaceum CV026 biosensor) and with AHLs produced by the wild type Chr. violaceum CECT 494, namely N-(3-hydroxydecanoyl)-L-homoserine lactone (3-hydroxy-C10-HSL), N-octanoyl-L-Homoserine lactone (C8-HSL) and other several minor AHLs (Morohoshi et al. 2008). The cell-free supernatant QQ-activity of these 3 promising Bacillus strains suggests an extracellular nature of the QQ molecules from FI314 and FI330. On the contrary, FI442 cell-free supernatant did not show any QQ activity, which indicates that its corresponding QQ molecules might possess an intracellular or cell-wall location, as reported in literature (Torabi Delshad et al. 2018; Cao et al. 2012; Chu et al. 2014; Pande et al. 2015; Romero et al. 2011; Zhou et al. 2018) or were degraded/inactivated under our experimental conditions.
Based on the results of the in vitro tests described in this study, the fish-gut isolates FI314, FI330, and FI442, identified as B. subtilis, were considered the most promising to be used as probiotics to incorporate in aquafeeds, or to become source of bioactive molecules able to antagonize important bacterial fish pathogens. Despite belonging to the same species (B. subtilis), the three selected isolates correspond to different strains. FI314 and FI330 were isolated from gilthead seabream (Sparus aurata), but from different digesta samples (different fish from different tanks) and FI442 was isolated from European seabass (Dicentrarchus labrax). Adding to the different origin and different colony morphologies, all three strains possess different phenotypes (e.g., results on antimicrobial activities or sporulation efficiency), an indication of own genotypes.
Fish pathogens’ infection routes are skin, gills, and gastrointestinal tract. In fact, some authors report the gastrointestinal tract as the main route of colonization in Aeromonas sp. (Lødemel et al. 2001; Ringø et al. 2004), Vibrio sp. (Oisson et al. 1996; Caruffo et al. 2015), and Edwardsiella sp. (Baldwin and Newton 1993), and imaging tools are being developed to visualize the exact dissemination of several pathogens inside the fish host (Bartkova et al. 2017; O'Toole et al. 2004). Thus, the selected Bacillus isolates and their NACs are expected to constitute a barrier against pathogens proliferation inside the animal’s gut. This may be achieved indirectly through the regulation of inflammatory pathways by enhancing the host immune system (Canny and McCormick 2008) or directly either by killing the pathogens, by preventing pathogens’ biofilm formation and subsequent gut colonizing, or by acting as quenching molecules shutting down pathogens communication or QS (Dobson et al. 2012; Wang et al. 2008; Abriouel et al. 2011; Sahoo et al. 2016). Importantly, NACs are directed to non-essential functions like biofilms or QS, and thus are less likely to induce resistance than common antibiotics (Sumi et al. 2014).
To confirm their potential probiotic application, the Bacillus fish-gut isolates were also evaluated for their capacity to produce spores. This feature is not only important from the production point of view, as the best sporeformers are the most attractive for high-yield fermentations, but also because spores allow bacteria to survive passage through the acidic stomach conditions, acting as a form of propagation inside the animal (Tam et al. 2006). All the isolated Bacillus strains tested showed a good sporulation efficiency, equivalent to the control laboratory strain B. subtilis 168. More importantly, all Bacillus isolates were susceptible to a series of antibiotic classes, including those demanded by EFSA as mandatory to comply with minimal safety requirements (Cabello et al. 2016; FEEDAP 2012) to be considered a probiotic.
The NACs produced by these B. subtilis strains, which are responsible for the observed anti-growth, anti-biofilm, and anti-QS activities, are currently being further studied for their isolation, identification, and full characterization. This is a challenging task, as Bacillus spp. produce a wide variety of antimicrobial molecules, from ribosomal peptides (RPs), volatile compounds, polyketides (PKs), and non-ribosomal peptides (NRPs) to hybrids between PKs and NRPs (Caulier et al. 2019). Interestingly, studies reporting the antimicrobial activity of B. subtilis against Gram-negative bacteria (the ones we target in this work) are scarce, when compared to the investigations on Gram-positive targets (Caulier et al. 2019; Olishevska et al. 2019). For example, seven compounds produced by B. subtilis sensu lato exhibited activity against gram-negative bacteria [Subtilosin A (Shelburne et al. 2007), Sonorensin (Chopra et al. 2014), CAMT2 (An et al. 2015), Subtulene A (Thasana et al. 2010), Lichenysin (Jenny et al. 1991), Lichenysin A (Yakimov et al. 1995), and Pumilacidin (Xiu et al. 2017)]. Among these compounds, only Subtilosin A and Subtulene A were isolated from B. subtilis sensu strictu, and their antimicrobial activity against fish pathogens is underexplored. Taking into account that 4–5% of the B. subtilis genome is dedicated to the production of antimicrobial compounds (Caulier et al. 2019) and the lack of studies on fish-gut associated Bacillus as producers of antimicrobial compounds (Soltani et al. 2019), the present investigation emphasizes the importance of described screening and bacterial collection, for the identification of new NACs with disease-preventive potential in aquaculture.
Data Availability
16S rRNA gene sequences of fish isolates described in this manuscript have been deposited in GenBank with the accession numbers provided in Table 2. Authors confirm that all relevant data are included in the article.
References
Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR (2018) Bacterial quorum sensing and microbial community interactions. mBio 9:e02331-17.
Abriouel H, Franz CM, Ben Omar N, Galvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35:201–232
An J, Zhu W, Liu Y, Zhang X, Sun L, Hong P, Wang Y, Xu C, Xu D, Liu H (2015) Purification and characterization of a novel bacteriocin CAMT2 produced by Bacillus amyloliquefaciens isolated from marine fish Epinephelus areolatus. Food Control 51:278–282
Andreoni F, Magnani M (2014) Photobacteriosis: prevention and diagnosis. J Immunol Res 2014:793817
Askarian F, Zhou Z, Olsen RE, Sperstad S, Ringø E (2012) Culturable autochthonous gut bacteria in Atlantic salmon (Salmo salar L.) fed diets with or without chitin. Characterization by 16S rRNA gene sequencing, ability to produce enzymes and in vitro growth inhibition of four fish pathogens. Aquac 326–329:1–8
Avendaño-Herrera R, Toranzo AE, Magariños B (2006) Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: a review. Dis Aquat Organ
Baldwin TJ, Newton JC (1993) Pathogenesis of enteric septicemia of channel catfish, caused by Edwardsiella ictaluri: bacteriologic and light and electron microscopic findings. J Aquat Anim Health 5:189–198
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79
Banerjee G, Nandi A, Ray AK (2016) Assessment of hemolytic activity, enzyme production and bacteriocin characterization of Bacillus subtilis LR1 isolated from the gastrointestinal tract of fish. Arch Microbiol: 30 71:255–266
Banerjee G, Ray AK (2017) The advancement of probiotics research and its application in fish farming industries. Res Vet Sci 115:66–77
Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO (2005) Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol 71:968–978
Bartkova S, Kokotovic B, Dalsgaard I (2017) Infection routes of Aeromonas salmonicida in rainbow trout monitored in vivo by real-time bioluminescence imaging. J Fish Dis 40:73–82
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Cabello FC, Godfrey HP, Buschmann AH, Dölz HJ (2016) Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect Dis 16:127–133
Canny GO, Mccormick BA (2008) Bacteria in the intestine, helpful residents or enemies from within? Infect Immun 76:3360–3373
Cao Y, He S, Zhou Z, Zhang M, Mao W, Zhang H, Yao B (2012) Orally administered thermostable N-acyl homoserine lactonase from Bacillus sp. strain AI96 attenuates Aeromonas hydrophila infection in zebrafish. Appl Environ Microbiol 78:1899–1908
Caruffo M, Navarrete N, Salgado O, Díaz A, López P, García K, Feijóo CG, Navarrete P (2015) Potential probiotic yeasts isolated from the fish gut protect zebrafish (Danio rerio) from a Vibrio anguillarum challenge. Front Microbiol 6.
Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J (2019) Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front Microbiol 10.https://doi.org/10.3389/fmicb.2019.00302
Chen Y, Li J, Xiao P, Li GY, Yue S, Huang J, Zhu WY, Mo ZL (2016) Isolation and characterization of Bacillus spp. M001 for potential application in turbot (Scophthalmus maximus L.) against Vibrio anguillarum. Aquacult Nutr 22:374–381
Chopra L, Singh G, Choudhary V, Sahoo DK (2014) Sonorensin: an antimicrobial peptide, belonging to the heterocycloanthracin subfamily of bacteriocins, from a new marine isolate, Bacillus sonorensis MT93. Appl Environ Microbiol 80:2981–2990
Chowdhary PK, Keshavan N, Nguyen HQ, Peterson JA, Gonzalez JE, Haines DC (2007) Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochem 46:14429–14437
Chu W, Mclean RJ (2016) Quorum signal inhibitors and their potential use against fish diseases. J Aquat Anim Health 28:91–96
Chu W, Zhou S, Zhu W, Zhuang X (2014) Quorum quenching bacteria Bacillus sp QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Sci Rep 4:5446
Clsi (2013) Clinical and Laboratory Standards Institute
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28:214–220
Dallaire-Dufresne S, Tanaka KH, Trudel MV, Lafaille A, Charette SJ (2014) Virulence, genomic features, and plasticity of Aeromonas salmonicida subsp. salmonicida, the causative agent of fish furunculosis. Vet Microbiol 169:1–7
Defoirdt T (2013) Virulence mechanisms of bacterial aquaculture pathogens and antivirulence therapy for aquaculture. Rev Aquacult 6:100–114
Diaz-Rosales P, Chabrillon M, Morinigo MA, Balebona MC (2003) Survival against exogenous hydrogen peroxide of Photobacterium damselae subsp. piscicida under different culture conditions. J Fish Dis 26:305–308
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels C, Guroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production–a Mediterranean perspective. Fish Shellfish Immunol 30:1–16
Dobson A, Cotter PD, Ross RP, Hill C (2012) Bacteriocin production: a probiotic trait? Appl Environ Microbiol 78:1–6
Dong YH, Xu JL, Li XZ, Zhang H (2000) AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci 97:3526
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance, European Food Safety Authority Panel on Additives and Products or Substances used in Animal Feed (EFSA-FEEDAP). EFSA J 10:2740
Fey PD (2010) Modality of bacterial growth presents unique targets: how do we treat biofilm-mediated infections? Curr Opin Microbiol 13:610–615
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Micro 14:563–575
Food and Agriculture Organization of the United Nations, W.H.O (2001) Report of the joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba, Argentina
Frans I, Michiels CW, Bossier P, Willems KA, Lievens B, Rediers H (2011) Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J Fish Dis 34:643–661
Gao XY, Liu Y, Miao LL, Li EW, Sun GX, Liu Y, Liu P (2017) Characterization and mechanism of anti-Aeromonas salmonicida activity of a marine probiotic strain, Bacillus velezensis V4. Appl Microbiol Biotechnol 101:3759–3768
Gauthier DT (2015) Bacterial zoonoses of fishes: a review and appraisal of evidence for linkages between fish and human infections. Vet J 203:27–35
Grandclement C, Tannieres M, Morera S, Dessaux Y, Faure D (2016) Quorum quenching: role in nature and applied developments. FEMS Microbiol Rev 40:86–116
Grassi L, Maisetta G, Esin S, Batoni G (2017) Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front Microbiol 8:2409–2409
Guo X, Chen DD, Peng KS, Cui ZW, Zhang XJ, Li S, Zhang YA (2016) Identification and characterization of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish Immunol 52:74–84
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119:917–935
Hamza F, Kumar AR, Zinjarde S (2016) Antibiofilm potential of a tropical marine Bacillus licheniformis isolate: role in disruption of aquaculture associated biofilms. Aquac Res 47:2661–2669
Hamza F, Kumar AR, Zinjarde S (2018) Efficacy of cell free supernatant from Bacillus licheniformis in protecting Artemia salina against Vibrio alginolyticus and Pseudomonas gessardii. Microb Pathog 116:335–344
Hibbing ME, Fuqua C, Parsek MR, Peterson SB (2010) Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 8:15–25
Hong HA, Duc LH, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835
Igbinosa EO (2016) Detection and antimicrobial resistance of Vibrio isolates in aquaculture environments: implications for public health. Microb Drug Resist 22:238–245
Janda JM, Abbott SL (2010) The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23:35–73
Jenny K, Käppeli O, Fiechter A (1991) Biosurfactants from Bacillus licheniformis: structural analysis and characterization. Appl Microbiol Biotechnol 36:5–13
Jones MK, Oliver JD (2009) Vibrio vulnificus: disease and pathogenesis. Infect Immun 77:1723
Kalpana BJ, Aarthy S, Pandian SK (2012) Antibiofilm activity of α-amylase from Bacillus subtilis S8–18 against biofilm forming human bacterial pathogens. Appl Biochem Biotechnol 167:1778–1794
Khajanchi BK, Fadl AA, Borchardt MA, Berg RL, Horneman AJ, Stemper ME, Joseph SW, Moyer NP, Sha J, Chopra AK (2010) Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: suggestive evidence of water-to-human transmission. Appl Environ Microbiol 76:2313–2325
Kuebutornye FKA, Lu Y, Abarike ED, Wang Z, Li Y, Sakyi ME (2020) In vitro assessment of the probiotic characteristics of three bacillus species from the gut of nile tilapia, Oreochromis niloticus. Probiotics Antimicrob Proteins 12:412–424
Lafferty KD, Harvell CD, Conrad JM, Friedman CS, Kent ML, Kuris AM, Powell EN, Rondeau D, Saksida SM et al (2015) Infectious diseases affect marine fisheries and aquaculture economics. Ann Rev Mar Sci 7:471–496
Lødemel JB, Mayhew TM, Myklebust R, Olsen RE, Espelid S, Ringø E (2001) Effect of three dietary oils on disease susceptibility in Arctic charr (Salvelinus alpinus L.) during cohabitant challenge with Aeromonas salmonicida ssp. salmonicida. Aquac Res 32:935–945
Mcclean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P et al (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiol 143(Pt 12):3703–3711
Mclean RJ, Pierson LS 3rd, Fuqua C (2004) A simple screening protocol for the identification of quorum signal antagonists. J Microbiol Methods 58:351–360
Meidong R, Khotchanalekha K, Doolgindachbaporn S, Nagasawa T, Nakao M, Sakai K, Tongpim S (2018) Evaluation of probiotic Bacillus aerius B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla-mong Pangasius bocourti. Fish Shellfish Immunol 73:1–10
Menanteau-Ledouble S, Kumar G, Saleh M, El-Matbouli M (2016) Aeromonas salmonicida: updates on an old acquaintance. Dis Aquat Organ 120:49–68
Midhun SJ, Neethu S, Vysakh A, Sunil MA, Radhakrishnan EK, Jyothis M (2017) Antibacterial activity of autochthonous bacteria isolated from Anabas testudineus (Bloch, 1792) and it’s in vitro probiotic characterization. Microb Pathog 113:312–320
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199
Moons P, Michiels CW, Aertsen A (2009) Bacterial interactions in biofilms. Crit Rev Microbiol 35:157–168
Morohoshi T, Kato M, Fukamachi K, Kato N, Ikeda T (2008) N-acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol Lett 279:124–130
Mukherjee A, Banerjee G, Mukherjee P, Ray AK, Chandra G, Ghosh K (2019) Antibacterial substances produced by pathogen inhibitory gut bacteria in Labeo rohita: Physico-chemical characterization, purification and identification through MALDI-TOF mass spectrometry. Microb Pathog. https://doi.org/10.1016/j.micpath.2019.02.028
Munn C (2005) Pathogens in the Sea: An Overview. In: Belkin S, Colwell R (eds) Oceans and Health: Pathogens in the Marine Environment. Springer US
Nahar S, Mizan MFR, Ha AJW, Ha SD (2018) Advances and future prospects of enzyme-based biofilm prevention approaches in the food industry. Compr Rev Food Sci Food Saf 17:1484–1502
Nandi A, Dan SK, Banerjee G, Ghosh P, Ghosh K, Ringø E, Ray AK (2017) Probiotic potential of autochthonous bacteria isolated from the gastrointestinal tract of four freshwater teleosts. Probiotics Antimicrob Proteins 9:12–21
Nicholson W, Setlow P (1990) Sporulation, germination and outgrowth. Harwood CR, Cutting SM (Eds), Molecular biological methods for Bacillus. Chichester, UK: John Wiley & Sons Ltd
O’toole R, Von Hofsten J, Rosqvist R, Olsson PE, Wolf-Watz H (2004) Visualisation of zebrafish infection by GFP-labelled Vibrio anguillarum. Microb Pathog 37:41–46
Oisson JC, Jöborn A, Westerdahl A, Blomberg L, Kjelleberg S, Conway PL et al (1996) Is the turbot, Scophthalmus maximus (L.), intestine a portal of entry for the fish pathogen Vibrio anguillarum? J Fish Dis 19:225–234
Olishevska S, Nickzad A, Déziel E (2019) Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl Microbiol Biotechnol 103:1189–1215
Pande GS, Natrah FM, Flandez AV, Kumar U, Niu Y, Bossier P, Defoirdt T (2015) Isolation of AHL-degrading bacteria from micro-algal cultures and their impact on algal growth and on virulence of Vibrio campbellii to prawn larvae. Appl Microbiol Biotechnol 99:10805–10813
Papa R, Selan L, Parrilli E, Tilotta M, Sannino F, Feller G, Tutino ML, Artini M (2015) Anti-biofilm activities from marine cold adapted bacteria against staphylococci and Pseudomonas aeruginosa. Front Microbiol 6:1333
Park SB, Aoki T, Jung TS (2012) Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res 43:67–67
Pitcher DG, Saunders NA, Owen RJ (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156
Pletzer D, Coleman SR, Hancock RE (2016) Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr Opin Microbiol 33:35–40
Plumb JA, Hanson LA (2011) Health maintenance and principal microbial diseases of cultured fishes, 3rd edn. Blackwell Publishing Ltd., Ames, Iowa, USA
Ramesh D, Vinothkanna A, Rai AK, Vignesh VS (2015) Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 45:268–276
Ran C, Carrias A, Williams MA, Capps N, Dan BCT, Newton JC, Kloepper JW, Ooi EL, Browdy CL, Terhune JS, Liles MR (2012) Identification of Bacillus strains for biological control of catfish pathogens. PLoS One 7:e45793
Rasmussen-Ivey CR, Figueras MJ, Mcgarey D, Liles MR (2016) Virulence factors of Aeromonas hydrophila: in the wake of reclassification. Front Microbiol 7:1337
Ringø E, Jutfelt F, Kanapathippillai P, Bakken Y, Sundell K, Glette J, Mayhew TM, Myklebust R, Olsen RE (2004) Damaging effect of the fish pathogen Aeromonas salmonicida ssp. salmonicida on intestinal enterocytes of Atlantic salmon (Salmo salar L.). Cell Tissue Res 318:305–311
Rivas AJ, Lemos ML, Osorio CR (2013) Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front Microbiol 4:283
Romero M, Martin-Cuadrado AB, Roca-Rivada A, Cabello AM, Otero A (2011) Quorum quenching in cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiol Ecol 75:205–217
Sahoo TK, Jena PK, Patel AK, Seshadri S (2016) Bacteriocins and their applications for the treatment of bacterial diseases in aquaculture: a review. Aquac Res 47:1013–1027
Serra CR, Almeida EM, Guerreiro I, Santos R, Merrifield DL, Tavares F, Oliva-Teles A, Enes P (2019) Selection of carbohydrate-active probiotics from the gut of carnivorous fish fed plant-based diets. Sci Rep 9:6384
Shelburne CE, An FY, Dholpe V, Ramamoorthy A, Lopatin DE, Lantz MS (2007) The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J Antimicrob Chemother 59:297–300
Soltani M, Ghosh K, Hoseinifar SH, Kumar V, Lymbery AJ, Roy S, Ringø E (2019) Genus bacillus, promising probiotics in aquaculture: aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev Fish Sci Aquac 27:331–379
Sumi CD, Yang BW, Yeo IC, Hahm YT (2014) Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can J Microbiol 61:93–103
Tam NK, Uyen NQ, Hong HA, Le Duc H, Hoa TT, Serra CR, Henriques AO, Cutting SM (2006) The intestinal life cycle of Bacillus subtilis and close relatives. J Bacteriol 188:2692–2700
Thankappan B, Ramesh D, Ramkumar S, Natarajaseenivasan K, Anbarasu K (2015) Characterization of Bacillus spp. from the gastrointestinal tract of Labeo rohita: towards to identify novel probiotics against fish pathogens. Appl Biochem Biotechnol 175:340–353
Thasana N, Prapagdee B, Rangkadilok N, Sallabhan R, Aye SL, Ruchirawat S, Loprasert S (2010) Bacillus subtilis SSE4 produces subtulene A, a new lipopeptide antibiotic possessing an unusual C15 unsaturated beta-amino acid. FEBS Lett 584:3209–3214
Tinh NTN, Dung NV, Trung CT, Thuy VT (2013) In vitro characterization of a recombinant AHL-lactonase from Bacillus cereus isolated from a striped catfish (Pangasianodon hypophthalmus) pond. Indian J Microbiol 53:485–487
Torabi Delshad S, Soltanian S, Sharifiyazdi H, Haghkhah M, Bossier P (2018) Identification of N-acyl homoserine lactone-degrading bacteria isolated from rainbow trout (Oncorhynchus mykiss). J Appl Microbiol 125:356–369
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol R 64:655–671
Vinoj G, Vaseeharan B, Thomas S, Spiers AJ, Shanthi S (2014) Quorum-quenching activity of the AHL-lactonase from Bacillus licheniformis DAHB1 inhibits Vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Mar Biotechnol (NY) 16:707–715
Wang AR, Ran C, Ringø E, Zhou ZG (2018a) Progress in fish gastrointestinal microbiota research. Rev Aquacult 10:626–640
Wang H, Wei CX, Min L, Zhu LY (2018b) Good or bad: gut bacteria in human health and diseases. Biotechnol Biotechnol Equip 32:1075–1080
Wang R, Zhong Y, Gu X, Yuan J, Saeed AF, Wang S (2015) The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front Microbiol 6:144
Wang YB, Li JR, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquac 281:1–4
Weir M, Rajić A, Dutil L, Uhland C, Bruneau N (2012) Zoonotic bacteria and antimicrobial resistance in aquaculture: opportunities for surveillance in Canada. Can Vet J 53:619–622
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
WHO (2014) Antimicrobial Resistance: Global Report on Surveillance
Xiu P, Liu R, Zhang D, Sun C (2017) Pumilacidin-like lipopeptides derived from marine bacterium Bacillus sp. strain 176 suppress the motility of Vibrio alginolyticus. Appl Environ Microbiol 83:e00450-e517
Yakimov MM, Timmis KN, Wray V, Fredrickson HL (1995) Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl Environ Microbiol 61:1706–1713
Yatip P, Teja DNC, Flegel TW, Soowannayan C (2018) Extract from the fermented soybean product Natto inhibits Vibrio biofilm formation and reduces shrimp mortality from Vibrio harveyi infection. Fish Shellfish Immunol 72:348–355
Zeigler DR, Prágai Z, Rodriguez S, Chevreux B, Muffler A, Albert T, Bai R, Wyss M, Perkins JB (2008) The origins of 168, W23, and other Bacillus subtilis legacy strains. J Bacteriol 190:6983–6995
Zhou S, Xia Y, Zhu C, Chu W (2018) Isolation of marine Bacillus sp. with antagonistic and organic-substances-degrading activities and its potential application as a fish probiotic. Mar Drugs 16
Zhou S, Zhang A, Yin H, Chu W (2016) Bacillus sp. QSI-1 modulate quorum sensing signals reduce Aeromonas hydrophila level and alter gut microbial community structure in fish. Front Cell Infect Microbiol 6:184
Zhou Z, Zhou X, Zhong Z, Wang C, Zhang H, Li D, He T, Li C, Liu X, Yuan H, Ji H, Luo Y, Gu W, Fu H, Peng G (2014) Investigation of antibacterial activity of Bacillus spp. isolated from the feces of Giant Panda and characterization of their antimicrobial gene distributions. World J Microbiol Biotechnol 30:3129–3136
Acknowledgments
The authors thank F. Tavares (CIBIO-Centro de Investigação em Biodiversidade e Recursos Genéticos, InBIO Laboratório Associado, Portugal), M. A. Morinigo (Universidad de Málaga, Spain) for the gift of pathogenic bacterial strains, and A. O. Henriques (Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, ITQB-NOVA, Portugal) for the gift of B. subtilis 168.
Funding
RAS is the recipient of a PhD grant (SFRH/BD/131069/2017) from FCT (Foundation for Science and Technology), under the POCH program; PE and CRS have a scientific employment contract supported by national funds through FCT–Portuguese Foundation for Science and Technology. This research was partially supported by the Strategic Funding to UIDB/04423/2020 and UIDP/04423/2020, UIDB/04033/2020 and UIDB/00772/2020 through national funds provided by FCT and European Regional Development Fund (ERDF), in the framework of the program PT2020.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: RAS, AO-T, MJS, PE, CRS. Performed the fish trial: RAS, PP-F, PE. Performed the experiments and analyzed the data: RAS, CRS. Critically evaluated all the data and edited manuscript: RAS, AO-T, RJ, MJS, PE, CRS. Wrote the paper: RAS, CRS. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Animal experiments were approved by the Animal Welfare Committee of the Interdisciplinary Centre of Marine and Environmental Research (CIIMAR) and carried out in a registered installation (N16091.UDER) and were performed by trained scientists (following FELASA category C recommendations) in full compliance with national rules and following the European Directive 2010/63/EU of the European Parliament and the European Union Council on the protection of animals used for scientific purposes.
Conflict of Interest
RJ is an employee of Epicore Inc., a subsidiary of Archer Daniels Midland, that supported this research by completing a commercial licensing agreement for the potential utilization of the probiotic bacteria FI314, FI330, and FI442 identified in this research, in commercial products for aquaculture. The opinions expressed in the manuscript are those of the authors and do not necessarily reflect company policies. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santos, R.A., Oliva-Teles, A., Pousão-Ferreira, P. et al. Isolation and Characterization of Fish-Gut Bacillus spp. as Source of Natural Antimicrobial Compounds to Fight Aquaculture Bacterial Diseases. Mar Biotechnol 23, 276–293 (2021). https://doi.org/10.1007/s10126-021-10022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-021-10022-x