Abstract
Inactivation of quorum sensing (QS) signal molecules, such as acylhomoserine lactones (AHLs) of pathogenic bacteria, has been proposed as a novel method to combat bacterial diseases in aquaculture. Despite the importance of micro-algae for aquaculture, AHL degradation by bacteria associated with micro-algal cultures has thus far not been investigated. In this study, we isolated Pseudomonas sp. NFMI-T and Bacillus sp. NFMI-C from open cultures of the micro-algae Tetraselmis suecica and Chaetoceros muelleri, respectively. An AHL degradation assay showed that either monocultures or co-cultures of the isolates were able to degrade the AHL N-hexanoyl-l-homoserine lactone. In contrast, only Bacillus sp. NFMI-C was able to inactivate N-hydroxybutanoyl-l-homoserine lactone, the AHL produced by Vibrio campbellii. The isolated bacteria were able to persist for up to 3 weeks in conventionalized micro-algal cultures, indicating that they were able to establish and maintain themselves within open algal cultures. Using gnotobiotic algal cultures, we found that the isolates did not affect growth of the micro-algae from which they were isolated, whereas a mixture of both isolates increased the growth of Tetraselmis and decreased the growth of Chaetoceros. Finally, addition of Bacillus sp. NFMI-C to the rearing water of giant river prawn (Macrobrachium rosenbergii) larvae significantly improved survival of the larvae when challenged with pathogenic V. campbellii, whereas it had no effect on larval growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture, the farming of aquatic animals and plants (algae) in marine, brackish and freshwater environments, is the fastest-growing food-producing industry worldwide (Bostock et al. 2010). The giant river prawn, Macrobrachium rosenbergii, is an important crustacean species from an economic perspective, with an annual global production of more than 200 000 t (New et al. 2010; FAO 2013). Disease outbreaks are considered to be amongst the major obstacles to produce healthy and high quality seed for the further expansion of giant river prawn culture (Nhan et al. 2010). Previous studies have shown that Vibrio spp., including Vibrio campbellii, are a major cause of disease in the early life stages (larvae and postlarvae) of M. rosenbergii (Tonguthai 1997; Kennedy et al. 2006; New et al. 2010; FAO 2013).
Bacterial diseases in aquaculture have thus far mainly been tackled by using antibiotics, but unfortunately, the use of these compounds has not been very successful and has led to the development and spread of resistant pathogens (Defoirdt et al. 2011a). Therefore, there is a need for novel methods to control bacterial disease. As virulence gene expression in many bacterial pathogens is controlled by quorum sensing, interference with this cell-to-cell communication mechanism has been proposed as a novel biocontrol strategy (Defoirdt et al. 2004). V. campbellii contains a three-channel quorum sensing system, with three different types of signal molecules, namely Harveyi autoinducer-1 (HAI-1), autoinducer-2 (AI-2) and Cholerae autoinducer-1 (CAI-1), which feed a shared signal transduction cascade (Ruwandeepika et al. 2012). Quorum sensing has been reported to control the expression of different virulence genes in V. campbellii, and we recently reported that the HAI-1 and the AI-2-mediated channels of the V. campbellii quorum sensing system are essential for full virulence to giant river prawn larvae (Pande et al. 2013).
The use of signal molecule-degrading bacteria is one of the most intensively studied strategies to interfere with quorum sensing (LaSarre and Federle 2013; Tang and Zhang 2014). The ability to inactivate acylhomoserine lactones (AHLs), one of the types of quorum sensing molecules, is widely distributed in the bacterial kingdom (Dong et al. 2007). Two major classes of AHL-inactivating enzymes have been described: lactonases, which are, e.g. produced by Bacillus spp., cleave the lactone ring of the signal molecules into acylated homoserine, whereas acylases, e.g. produced by Pseudomonas spp., cleave the AHL molecule into homoserine lactone and a fatty acid (Fast and Tipton 2012). As V. campbellii HAI-1 is an AHL and as this signal is essential for full virulence towards giant river prawn larvae (Pande et al., 2013), the use of AHL-degrading bacteria might be an effective strategy to protect the larvae from the pathogen. This kind of bacteria has been isolated from various environments, including the digestive tract of healthy shrimp (Tinh et al. 2007) and fish (Cam et al. 2009a).
Micro-algae are an important constituent of many aquaculture systems, especially the so-called greenwater systems, in which the animals are cultured in water containing 105 to 107 cells of micro-algae per ml (Coutteau and Sorgeloos 1992; Hargreaves 2006). Greenwater systems are used to culture various animals, including giant river prawn larvae (FAO 2013). Micro-algal cultures and greenwater used in aquaculture are not axenic and contain bacteria. However, the potential beneficial effects of bacteria associated with micro-algae remain largely unexplored (Natrah et al. 2014). In this study, we aimed at investigating (1) whether AHL-degrading bacteria can be isolated from micro-algal cultures, (2) whether they have any impact on algal growth, and (3) whether they can protect giant river prawn larvae against V. campbellii.
Materials and methods
Micro-algal strains and culture conditions
Axenic Tetraselmis suecica CCAP66/4 and Chaetoceros muelleri CCMP1316 were obtained from the Culture Collection of Algae and Protozoa (CCAP, Dunstaffnage Marine Laboratory, Scotland) and the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (CCMP, USA), respectively. The algae were grown in Guillard’s F/2 medium (Sigma) (with silicate addition for C. muelleri) in sterile 250-ml Schott bottles provided with 0.22-μm filtered aeration. All parameters for algal culture were kept constant (pH 7, continuous light of 100 μmol photons m−2s−1, temperature of 24 °C and 30 g l−1 salinity). The density of axenic cultures was measured using a Bürker haemocytometer and a spectrophotometer (Thermo Spectronic) at OD550 nm. Axenity tests were done by plating the supernatant on marine agar and Luria-Bertani (LB) agar 30 g l−1 salinity (bacterial contamination test) and potato dextrose agar (fungal contamination test). Samples were also routinely checked microscopically at ×1000 magnification with oil immersion, immediately before harvesting.
Isolation of AHL-degrading bacteria from micro-algal cultures
Open cultures of T. suecica and C. muelleri were grown under similar conditions as described above for strains CCAP66/4 and CCMP1316. Fifty millilitres of the cultures was transferred to sterile Erlenmeyer flasks containing 5 ml of sterile NaCl solution (30 g l−1) containing 50 mg l−1 N-hexanoyl-l-homoserine lactone (HHL). The Erlenmeyer flasks were covered with aluminium foil to prevent the growth of the micro-algae and were incubated on a shaker (120 rpm) at 24 °C. The isolation was performed in four consecutive cycles (72 h for the first cycle and 48 h for the second to fourth cycle). At the end of each cycle, 50 μl of the suspension was transferred to a new flask. At the end of the fourth cycle, the suspensions were spread-plated on Luria-Bertani agar containing 30 g l−1 NaCl (LB30), and after 48 h incubation at 24 °C, colonies were picked, suspended in a 30 g l−1 NaCl solution and plated again. After three rounds of purification, isolates were grown in LB30 broth for 24 h at 24 °C and grown cultures were stored at −80 °C in 40 % glycerol. Two isolates, NFMI-T and NFMI-C isolated from T. suecica and C. muelleri, respectively, were used in further experiments. The isolates were submitted to the BCCM-LMG culture collection (http://bccm.belspo.be) under the numbers LMG 28858 (Bacillus sp. NFMI-C) and LMG 28859 (Pseudomonas sp. NFMI-T).
Bacterial strains and culture conditions
V. campbellii BB120 (ATCC BAA-1116) (Bassler et al. 1997), its mutant JAF548 (Freeman and Bassler 1999) and the AHL-degrading isolates were stored at −80 °C in 40 % glycerol. The stocks were streaked onto LB agar, and after 24 h of incubation at 28 °C, a single colony was picked and inoculated into 5 ml of fresh LB broth and incubated overnight at 28 °C under constant agitation (100 min−1). For the preparation of inocula for giant river prawn challenge tests, strains were grown in LB broth containing 12 g l−1 NaCl (LB12).
Selection of natural rifampicin-resistant mutants of the isolates
Rifampicin-resistant mutants of the isolates were selected as described by Pande et al. (2013). Briefly, 100 μl of densely grown cultures (OD600 of 1) was inoculated into 5 ml of fresh LB12 broth containing 50 mg l−1 rifampicin (Sigma) and incubated for 5 days at 24 °C under constant agitation (100 min−1). The grown cultures were inoculated into fresh LB broth with 50 mg l−1 rifampicin. The grown cultures were stored at −80 °C in 40 % glycerol until use.
Quantification of N-hexanoyl-l-homoserine lactone
A stock solution of HHL was prepared by dissolving HHL (Fluka) in 200 μl of ethanol (95 %) and then further diluted to a final concentration of 2500 mg l−1 by adding sterile distilled water. A plate diffusion method was used for quantitative detection of HHL using Chromobacterium violaceum CV026 as a reporter (Defoirdt et al. 2011b). Briefly, CV026 was grown to an optical density of around 2 at 550 nm in buffered (2 g/l MOPS) LB medium (pH 6.5) containing 20 mg l−1 kanamycin and spread over buffered (pH 6.5) LB plates. Subsequently, 10 μl of sample solution was applied to the centre of the plates and the plates were incubated at 28 °C for 48 h. After the incubation, the zone of purple-pigmented CV026 was measured and the concentration of HHL in the sample was calculated based on a standard curve.
AHL degradation assay
AHL degradation by the isolates was studied as reported previously (Defoirdt et al. 2011b). Briefly, the isolates (either single isolates or a 1:1 mixture) were inoculated at 108 CFU ml−1 in buffered LB30 medium (pH 6.5) supplemented with 10 mg l−1 HHL. At regular time intervals, 1-ml samples from each culture was taken and filtered over a 0.2-μm filter. The HHL concentration in the cell-free supernatants was determined as described above using C. violaceum CV026. Pseudomonas sp. P3/pME6000 and Pseudomonas sp. P3/pME6863 (= pME6000 + the Bacillus AHL lactonase gene aiiA; Molina et al. 2003), grown under the same conditions, were used as negative and positive control, respectively.
Identification of the isolates by 16S rRNA gene sequencing
PCR targeting a 1500-bp fragment of the 16S rRNA gene of the isolates was performed according to Boon et al. (2002) using the primer pair GM3f and GM4r (Biolegio, Nijmegen, The Netherlands). PCR was performed with a GeneAmp PCR system 2700 thermal cycler (PE Applied Biosystems, Nieuwerkerken a/d Ijssel, The Netherlands) using the program: 95 °C for 5 min, 32 cycles of 94 °C for 1 min, 42 °C for 1 min, 72 °C for 3 min and finally an extension period of 72 °C for 10 min. DNA sequencing of the obtained PCR products (467 and 462 bp for NFMI-C and NFMI-T, respectively) was carried out at IIT Biotech (Bielefeld, Germany). The nucleotide sequences of the isolates were deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/Genbank) under the accession numbers KM525666 and KM525667 for NFMI-C and NFMI-T, respectively. Homology searches were completed with the BLAST server of the National Centre for Biotechnology Information for the comparison of the nucleotide query sequence against a nucleotide sequence database (blastn).
Impact of the isolates on the growth of gnotobiotic micro-algae
Axenic T. suecica CCAP66/4 and C. muelleri CCMP1316 were inoculated in 250-ml Erlenmeyer flasks containing 50 ml F/2 medium (with silica for C. muelleri) at 104 cells ml−1, with and without the isolates (either single isolates or a 1:1 mixture) at 102 CFU ml−1. As controls, the isolates were inoculated in flasks without algae. The flasks were incubated on a shaker (120 rpm) with constant illumination of 100 μmol photons m−2s−1 at 24 °C for 15 days. All treatments were performed in triplicate. Growth of the micro-algae was monitored by measuring in vivo chlorophyll a fluorescence (exCitation 430 nm, emission 670 nm) using a Tecan Infinite 200 microplate reader (Tecan, Mechelen, Belgium).
Persistence of the isolates in conventionalized micro-algal cultures
Conventionalized cultures of T. suecica CCAP66/4 and C. muelleri CCMP1316 were obtained by inoculating axenic cultures with 105 CFU ml−1 of microbiota taken from the open cultures of the respective micro-algae (obtained by taking the supernatant of cultures centrifuged at 300×g for 5 min). The micro-algae were inoculated in 250-ml Erlenmeyer flasks containing 50 ml F/2 medium (with silica for Chaetocerosmuelleri) at 104 cells ml−1. A 1:1 mixture of the rifampicin-resistant mutants of NFMI-C and NFMI-T was inoculated at the start of the experiment at a total density of 105 CFU ml−1. The flasks were incubated on a shaker (120 rpm) with constant illumination (4000 lx) at 24 °C. All treatments were performed in triplicates. The density of the AHL degraders was determined after 2 and 3 weeks of incubation by plating on LB30 agar containing 100 mg l−1 rifampicin. Conventionalized algal cultures without the addition of the isolates were used as controls.
Giant river prawn challenge test
Giant river prawn challenge tests were performed as described by Pande et al. (2013). Briefly, larvae were obtained from a single oviparous female breeder. A matured female which had just completed its pre-mating molt was mated with a hard-shelled male. The female with fertilized eggs was then maintained for 20 to 25 days to undergo embryonic development. When fully ripe (indicated by dark grey colour of the eggs), the female was transferred to a hatching tank (30 l) containing slightly brackish water (containing 6 g l−1 Instant Ocean synthetic sea salt, Aquarium System Inc., Sarrebourg, France). The water temperature was maintained at 28 °C by a thermostat heater. After hatching, the newly hatched larvae with yolk were left for 24 h in the hatching tank. The next day, prawn larvae with absorbed yolk were distributed in groups of 25 larvae in 200-ml glass cones containing 100 ml fresh autoclaved brackish water (12 g l−1 synthetic sea salts). The glass cones were placed in a rectangular tank containing water maintained at 28 °C and was provided with aeration. The larvae were fed daily with 5 Artemia nauplii/larva and acclimatized to the experimental conditions for 24 h.
During the experiments, water quality parameters were kept at minimum 5 mg l−1 dissolved oxygen, maximum 0.5 mg l−1 ammonium-N and maximum 0.05 mg l−1 nitrite-N. Larvae were challenged by adding 106 CFU ml−1 of V. campbellii BB120 to the rearing wateron the day after first feeding, and Bacillus sp. NFMI-C was added at 105 CFU ml−1. Survival was counted daily in the treatment receiving V. campbellii BB120 only, and the challenge test was stopped when more than 50 % mortality was achieved. At this time point, larval survival was determined in all treatments by considering that only those larvae presenting movement of appendages were alive.
The larval stage index (LSI) was determined according to Maddox and Manzi (1976) by randomly sampling five larvae from each treatment and calculated as:
LSI = Σ Si/N
Si : stage of the larva (i = 1 to 12)
N : the number of larvae examined
Statistical data analysis
Statistical analyses were performed using the SPSS software, version 20. Giant river prawn survival data were arcsin transformed in order to satisfy normal distribution and homoscedasticity requirements. Data were analysed by one-way ANOVA, followed by Tukey multiple range tests with a significance level set at 0.05.
Results
Isolation of AHL-degrading bacteria from micro-algal cultures
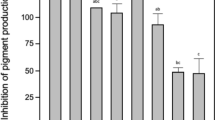
AHL-degrading strains were isolated from open cultures of T. suecica and C. muelleri by sequentially culturing the mixed microbial community in a medium containing N-hexanoyl-l-homoserine lactone (HHL) as the sole carbon and nitrogen source, followed by selecting individual colonies on LB agar plates. Two isolates, NFMI-T and NFMI-C (isolated from T. suecica and C. muelleri, respectively), were used in further experiments. The isolates were inoculated at 108 CFU ml−1 in buffered LB medium supplemented with 10 mg l−1 HHL in order to determine whether they were able to degrade AHLs in a nutrient-rich background, simulating the presence of high levels of other nutrients as is the case in a gastrointestinal environment. Both isolates were able to degrade HHL to below detection limit within 12 h when grown in monoculture (Fig. 1). However, degradation proceeded faster when inoculated in a 1:1 mixture (total density 108 CFU ml−1), with the HHL level being decreased to below detection limit after 6 h (Fig. 1). The HHL degradation rates of NFMI-C monoculture, NFMI-T monoculture and an NFMI-C NFMI-T co-culture were 0.75, 0.77 and 1.67 mg l−1 h−1, respectively.
N-hexanoyl-l-homoserine lactone (HHL) degradation by the isolates NFMI-C and NFMI-T, either alone or as a 1:1 mixture, in Luria-Bertani broth containing 10 mg l−1HHL. Pseudomonas sp. P3/pME6000 was used as a negative control; Pseudomonas sp. P3/pME6863 (containing the Bacillus sp. AHL lactonase gene aiiA) was used as a positive control. All strains were inoculated at a total density of 108 CFU ml−1
Identification of the isolates
The isolates were identified by sequencing of the 16S rDNA. An NCBI BLAST search revealed that isolate NFMI-C was most closely related to Bacillus spp. and that isolate NFMI-T was most closely related to Pseudomonas spp. (Table 1).
Impact of the isolates on growth of axenic micro-algae
Axenic T. suecica CCAP66/4 and C. muelleri CCMP1316 were inoculated in fresh algal growth medium, with and without the isolates (either single isolates or a 1:1 mixture) and growth of the micro-algae was monitored by measuring in vivo chlorophyll a fluorescence. Pseudomonas sp. NFMI-T alone had no effect on the growth of T. suecica CCAP66/4, whereas the addition of a mixture of both isolates resulted in higher chlorophyll fluorescence after 12 and 15 days of culture (Fig. 2a). In case of C. muelleri CCMP1316, growth was not affected by Bacillus sp. NFMI-C alone, whereas the mixture of both isolates resulted in a decreased chlorophyll fluorescence throughout the experiment (Fig. 2b). Because differences could only be observed when using both isolates and because the mixture showed the best HHL degrading capacity, a 1:1 mixture of the isolates was used in further co-culture experiments with micro-algae.
Impact of the isolates on growth of micro-algae in Guillard’s F/2 medium. a Chlorophyll fluorescence of Tetraselmis suecica CCAP66/4 (Tetra), with or without Pseudomonas sp. NFMI-T or a 1:1 mixture of both isolates. b Chlorophyll fluorescence of Chaetoceros muelleri CCMP1316 (Chaeto), with or without Bacillus sp. NFMI-C or a 1:1 mixture of both isolates. Cultures of the isolates without micro-algae were used as controls
Persistence of the isolate mixture in conventionalized micro-algal cultures
Conventionalized cultures of T. suecica CCAP66/4 and C. muelleri CCMP1316 were obtained by inoculating axenic cultures with 105 CFU ml−1 of microbiota taken from the open cultures of the respective micro-algae. A 1:1 mixture of natural rifampicin-resistant mutants of Bacillus sp. NFMI-C and Pseudomonas sp. NFMI-T was inoculated at the start of the experiment at a total density of 105 CFU ml−1, and the density of the AHL degraders was determined after 2 and 3 weeks of incubation by plating on LB30 agar containing 100 mg l−1 rifampicin. The isolates were detected after 2 and 3 weeks of culture in both conventionalized T. suecica CCAP66/4 and C. muelleri CCMP1316 cultures (Table 2), where they had increased to approximately 106 CFU ml−1.
Impact of the isolates on AHL quorum sensing in V. campbellii
Because bioluminescence is one of the phenotypes that are regulated by quorum sensing in V. campbellii, we used bioluminescence as a read-out of quorum sensing activity and determined the impact of the isolates on bioluminescence of wild-type V. campbellii BB120. In order to compensate for the competition for nutrients, we mixed BB120 with its mutant JAF548 as a control. JAF548 has a completely inactive quorum sensing system (and therefore is not luminescent; Freeman and Bassler 1999). The results revealed that Bacillus sp. NFMI-C, but not Pseudomonas sp. NFMI-T, decreased quorum sensing-regulated luminescence of V. campbellii in co-culture (Fig. 3). However, there were no such effects when V. campbellii was grown in the presence of cell-free supernatants of the isolate (data not shown). Importantly, neither of the isolates affected the growth of V. campbellii in co-culture (Table 3) and the cell-free supernatants of the isolates also did not affect the growth of the pathogen (data not shown).
Impact of Bacillus sp. NFMI-C on the survival and growth of giant river prawn larvae challenged with V. campbellii
Our previous research showed that AHL quorum sensing is essential for full virulence of V. campbellii towards giant river prawn larvae (Pande et al. 2013). Hence, since Bacillus sp. NFMI-C was able to interfere with AHL quorum sensing in V. campbellii, we went further to investigate whether this isolate was able to protect giant river prawn larvae from the pathogen. Addition of Bacillus sp. NFMI-C to the rearing water indeed resulted in a significantly improved survival of challenged prawn larvae when compared to untreated challenged larvae (Table 4). Consistent with our previous work, we found no differences in growth (as assessed by determining the larval stage index) between the different treatments.
Discussion
The ability to interfere with bacterial quorum sensing by degrading AHL molecules is widely distributed in the bacterial kingdom (LaSarre and Federle 2013), and because of their potential as novel disease control agents, AHL-degrading bacteria have been isolated from various aquatic environments, including the water column, sediment, seaweed and the intestinal tract of healthy aquatic organisms (Tang and Zhang 2014). However, despite the importance of micro-algae for, e.g. aquaculture (Natrah et al. 2014), AHL degradation by bacteria associated with micro-algal cultures has thus far not been investigated. In this study, we report the isolation of two AHL-degrading strains, Bacillus sp. NFMI-C and Pseudomonas sp. NFMI-T, from open cultures of C. muelleri and T. suecica, respectively. Both strains showed HHL degradation rates that were similar to those of Bacillus sp. strains isolated from the intestinal tract of shrimp and fish (0.7–0.9 mg l−1 h−1) (Defoirdt et al. 2011b). However, a co-culture of both strains degraded HHL approximately twice as fast. HHL was used as test compound because it is relevant to aquaculture. Indeed, it is produced by pathogenic bacteria such as Aeromonas hydrophila, Aeromonas salmonicida, Edwardsiella tarda and Vibrio salmonicida (Swift et al. 1997; Morohoshi et al. 2004; Bruhn et al. 2005). Furthermore, Bacillus sp. NFMI-C was also found to interfere with quorum sensing in V. campbellii (most probably by degradation of the AHL N-3-hydroxybutanoyl-l-homoserine lactone) in co-culture.
Several Bacillus species have been reported to produce AHL lactonases, which inactivate AHLs by hydrolysing the lactone ring. Lactonases are intracellular enzymes capable of inactivating a wide range of AHLs, varying in acyl chain length and substitution (Dong et al. 2007). Pseudomonas species, in contrast, have been reported to produce AHL acylases, which cleave AHLs by amino hydrolysis. Unlike lactonases, acylases exhibit substrate specificity depending on the acyl side chain length and the substitution at the β-position of the acyl chain (Tang and Zhang 2014). This might explain why Pseudomonas sp. NFMI-T was not able to interfere with quorum sensing in V. campbellii although it was able to degrade HHL.
In addition to the AHL degradation capacity of the isolates, we also investigated their impact on micro-algal growth. Bacillus sp. NFMI-C and Pseudomonas sp. NFMI-T had no effect on growth of C. muelleri and T. suecica, respectively, when added alone. However, a mixture of both strains increased the growth of T. suecica, whereas it decreased the growth of C. muelleri. Interactions between bacteria and micro-algae are fairly complex and not yet completely understood. The exact mechanisms by which bacteria stimulate micro-algae are largely unknown, although a few compounds responsible for such effects (including vitamins and hormones) have been identified (Natrah et al. 2014). The negative impact of the NFMI-C NFMI-T mixture on the growth of C. muelleri might be due to competition for nutrients. Indeed, bacteria have been reported to decrease the growth of another diatom, Cylindrotheca fusiformis, at low phosphate concentrations (suggesting that the bacteria scavenge phosphate better than the micro-algae) (Amin et al. 2012). Another possibility is that Pseudomonas sp. NFMI-T produces a compound that inhibits the growth of Chaetoceros, without affecting the growth of Tetraselmis (from which it was isolated). Indeed, some bacteria (including Pseudomonas aeruginosa) are able to produce algicidal compounds (Natrah et al. 2014). This algicidal activity can be caused either by the release of algicidal compounds in the environment or by lysis of the micro-algal cells following attachment (Mayali and Azam 2004).
Our in vivo challenge test revealed that Bacillus sp. NFMI-C significantly increased the survival of giant river prawn larvae challenged with V. campbellii, whereas the isolate had no effect on larval growth. This is consistent with our previous work showing that AHL quorum sensing is essential for full virulence of V. campbellii towards giant river prawn larvae (Pande et al. 2013) and previous reports documenting that AHL degraders are able to improve the survival of prawn larvae (Cam et al. 2009b) and turbot larvae (Scophthalmus maximus L.) (Tinh et al. 2008) in the presence of exogenous AHL (which caused mortality in both species; probably by triggering pathogenicity mechanisms in pathogenic bacteria that were naturally present in the cultures). We found that isolate NFMI-C was most closely related to Bacillus spp. Strains belonging to Bacillus species such as B. subtilis, B. cereus, B. coagulans, B. clausii, B. megaterium and B. licheniformis are the most frequently used probiotics in aquaculture (Wang et al. 2008). Hence, the use of Bacillus sp. strains able to degrade AHL molecules might be an interesting new type of probiotics for aquaculture with a defined mode of action (which does not necessarily mean that AHL degradation is the sole beneficial effect). Along this line, AHL-degrading Bacillus sp. have been shown to inhibit the protease production, haemolytic activity and biofilm formation of A. hydrophila strain YJ-1 and to significantly improve the survival of zebrafish (Danio rerio) challenged with this pathogen (Chu et al. 2014). AHL-degrading enzymes could be administered to the aquaculture system in several ways, and this will depend on the nature of the specific system. Indeed, AHL-degrading bacteria capable of establishing themselves in algal cultures (as described in this study) might be the agents of choice in greenwater systems, whereas in other systems, it might be more efficient to use purified (preferentially heat stable) AHL-degrading enzymes that are administered via the feed (as e.g. described in Chen et al., 2010; Cao et al., 2012).
In conclusion, in this study, we isolated two AHL-degrading strains, Bacillus sp. NFMI-C and Pseudomonas sp. NFMI-T, from cultures of the micro-algae C. muelleri and T. suecica, respectively. Both strains were able to quickly degrade AHLs in a nutrient-rich background. The isolates were able to maintain themselves in conventionalized algal cultures, and their impact on algal growth was dependent on the tested micro-algal species. Bacillus sp. NFMI-C, but not Pseudomonas sp. NFMI-T, was able to decrease quorum sensing-regulated luminescence of V. campbellii and improved the survival of giant river prawn larvae when challenged with V. campbellii. These AHL-degrading isolates might have potential as novel biocontrol strains for use in aquaculture. Further research will reveal the efficacy of the isolates in different aquaculture host-pathogen systems. Indeed, although Pseudomonas sp. NFMI-T was not able to interfere with quorum sensing in V. campbellii, it might be well capable of protecting aquatic hosts from bacteria that use another type of AHL molecule such as Aeromonas spp. and Edwardsiella spp., which use N-hexanoyl-l-homoserine lactone (HHL). Furthermore, a combination of both isolates might even be more effective since a co-culture showed a higher HHL degradation rate than the single cultures of the isolates.
References
Amin SA, Parker MS, Armbrust EV (2012) Interactions between diatoms and bacteria. Microbiol Mol Biol Rev 76:667–680
Bassler BL, Greenberg EP, Stevens AM (1997) Cross-species induction of luminescence in the quorum sensing bacterium Vibrio harveyi. J Bacteriol 179:4043–4045
Boon N, De Windt W, Verstraete W, Top EM (2002) Evaluation of nested PCR-DGGE (denaturing gradient gel electrophoresis) with group-specific 16 S rRNA primers for the analysis of bacterial communities from different wastewater treatment plants. FEMS Microbiol Ecol 39:101–112
Bostock J, McAndrew B, Richards R, Jauncey K, Telfer T, Lorenzen K, Little D, Ross L, Handisyde N, Gatward I, Corner R (2010) Aquaculture: global status and trends. Phil Trans R Soc B365:2897–2912
Bruhn JB, Dalsgaard I, Nielsen KF, Buccholtz C, Larsen JL, Gram L (2005) Quorum sensing signal molecules (acylated homoserine lactones) in Gram-negative fish pathogenic bacteria. Dis Aquat Org 65:43–52
Cam DTV, Hao NV, Dierckens K, Defoirdt T, Boon N, Sorgeloos P, Bossier P (2009a) Novel approach of using homoserine lactone-degrading and poly-beta-hydroxybutyrate-accumulating bacteria to protect Artemia from the pathogenic effects of Vibrio harveyi. Aquaculture 291:23–30
Cam DTV, Nhan DT, Ceuppens S, Hao NV, Dierckens K, Wille M, Sorgeloos P, Bossier P (2009b) Effect of N-acyl homoserine lactone-degrading enrichment cultures on Macrobrachium rosenbergii larviculture. Aquaculture 294:5–13
Cao YA, He SX, Zhou ZG, Zhang MC, Mao W, Zhang HT, Yao B (2012) Orally administered thermostable N-acyl homoserine lactonase from Bacillus sp strain AI96 attenuates Aeromonas hydrophila infection in zebrafish. Appl Environ Microbiol 78:1899–1908
Chen RD, Zhou ZG, Cao BYG, Yao B (2010) High yield expression of an AHL-lactonase from Bacillus sp B546 in Pichia pastoris and its application to reduce Aeromonas hydrophila mortality in aquaculture. Microb Cell Factories 9:39
Chu W, Zhou S, Zhu W, Zhuang X (2014) Quorum quenching bacteria Bacillus sp. QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Sci rep 4:5446.DOI: 10.1038/srep05446
Defoirdt T, Boon N, Bossier P, Verstraete W (2004) Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture 240:69–88
Defoirdt T, Sorgeloos P, Bossier P (2011a) Alternatives to antibiotics for the control of bacterial diseases in aquaculture. Curr Opin Microbiol 14:251–258
Defoirdt T, Thanh LD, Van Delsen B, De Schryver P, Sorgeloos P, Boon N, Bossier P (2011b) N-acylhomoserine lactone degrading Bacillus strains isolated from aquaculture animals. Aquaculture 311:258–260
Dong YH, Wang LH, Zhang LH (2007) Quorum-quenching microbial infections: mechanisms and implications. Philos Trans R Soc B362:1201–1211
FAO, 2013. Cultured aquatic species information programme. Macrobrachium rosenbergii.
Fast W, Tipton PA (2012) The enzymes of bacterial census and censorship. Trends Biochem Sci 37:7–14
Freeman JA, Bassler BL (1999) Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J Bacteriol 181:899–906
Hargreaves JA (2006) Photosynthetic suspended-growth systems in aquaculture. Aquac Eng 34:344–363
http://www.fao.org/fishery/culturedspecies/Macrobrachium_rosenbergii/en. Accessed 27 March 2013
Kennedy B, Venugopal MN, Karunasagar I, Karunasagar I (2006) Bacterial flora associated with the giant freshwater prawn Macrobrachium rosenbergii, in the hatchery system. Aquaculture 261:1156–1167
LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77(1):73–111
Maddox MB, Manzi JJ (1976) The effects of algal supplements on static system culture on Macrobrachium rosenbergii (de Man) larvae. Proc World Maricult Soc 7:677–698
Mayali X, Azam F (2004) Algicidal bacteria in the sea and their impact on algal blooms. J Eukary Microbiol 51:139–144
Molina L, Constantinescu F, Michel L, Reimmann C, Duffy B, Defago G (2003) Degradation of pathogen quorum-sensing molecules by soil bacteria: a preventive and curative biological control mechanism. FEMS Microbiol Ecol 45:71–81
Morohoshi T, Inaba T, Kato N, Kanai K, Ikeda T (2004) Identification of quorum-sensing signal molecules and the LuxRI homologs in fish pathogen Edwardsiella tarda. J Biosci Bioeng 98:274–281
Natrah FMI, Bossier P, Sorgeloos P, Yusoff FM, Defoirdt T (2014) Significance of microalgal-bacterial interactions for aquaculture. Rev Aquacult 5:1–14
New MB, Valenti WC, Tidwell JH, D’Abramo LR, Kutty MN (2010) Freshwater prawn: biology and farming. Blackwell Publishing Ltd
Nhan DT, Cam DTV, Wille M, Defoirdt T, Bossier P, Sorgeloos P (2010) Quorum quenching bacteria protect Macrobrachium rosenbergii larvae from Vibrio harveyi infection. J Appl Microbiol 109:1007–1016
Pande GSJ, Natrah FMI, Sorgeloos P, Bossier P, Defoirdt T (2013) The Vibrio harveyi quorum sensing signals have a different impact on virulence of the bacterium towards different hosts. Vet Microbiol 167:540–545
Ruwandeepika HAD, Jayaweera TSP, Bhowmick PP, Karunasagar I, Bossier P, Defoirdt T (2012) Pathogenesis, virulence factors and virulence regulation of vibrios belonging to the Harveyi clade. Rev Aquacult 4:59–74
Swift S, Karlyshev AV, Fish L, Durant EL, Winson MK., Chhabra SR, Williams P, Macintyre S, Stewart GSAB (1997) Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J Bacteriol 179:5271–5281
Tang K, Zhang XH (2014) Quorum quenching agents: resources for antivirulence therapy. Mar Drugs 12:3245–3282. doi:10.3390/md12063245
Tinh NTN, Gunasekara RAYSA, Boon N, Dierckens K, Sorgeloos P, Bossier P (2007) N-acyl homoserine lactone-degrading microbial enrichment cultures isolated from Penaeus vannamei shrimp gut and their probiotic properties in Brachionus plicatilis cultures. FEMS Microbiol Ecol 62:45–53
Tinh NTN, Yen VHN, Dieckens K, Sorgeloos P, Bossier P (2008) An acyl homoserine lactone-degrading microbial community improves the survival of first-feeding turbot larvae (Scophthalmus maximus L.). Aquaculture 285:56–62
Tonguthai K (1997) Diseases of the freshwater prawn, Macrobrachium rosenbergii. Aquat Anim Health Res Inst Newslett 4:1–9
Wang YB, Li JR, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281:1–4
Acknowledgments
This work was funded by the Research Foundation of Flanders (FWO-Vlaanderen; project no. 1.5.013.12N), by the Bijzonder Onderzoeksfonds (BOF)—special research fund for a PhD finalization grant from Ghent University (Code: 01DI0114) and by the Directorate General of Higher Education of Indonesia through a doctoral scholarship to G.S.J.P. by the University Putra Malaysia and Malaysian Ministry of Higher Education (MOHE) through doctoral grant to N.F.M.I.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gde Sasmita Julyantoro Pande and Fatin Mohd Ikhsan Natrah contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pande, G.S.J., Natrah, F.M.I., Flandez, A.V.B. et al. Isolation of AHL-degrading bacteria from micro-algal cultures and their impact on algal growth and on virulence of Vibrio campbellii to prawn larvae. Appl Microbiol Biotechnol 99, 10805–10813 (2015). https://doi.org/10.1007/s00253-015-6918-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6918-1