Abstract
Probiotics used in aquaculture are mostly from non-fish sources, as a result ineffective in eliciting the desired effects in aquatic animals. In this study, three Bacillus species were isolated from the digestive tract of freshwater fish Oreochromis niloticus and characterised based on their morphological, biochemical and evolutionary relationships. Their probiotic potentials were evaluated based on their ability to tolerate low pH, bile salt concentration, high temperatures, adhesion ability (auto-aggregation and hydrophobicity), haemolytic activity and antimicrobial activity including biosafety assay. Three Bacillus strains identified as Bacillus velezensis TPS3N (MK130897), Bacillus subtilis TPS4 (MK130899) and Bacillus amyloliquefaciens TPS17 (MK130898) were designated as TPS3N, TPS4 and TPS17, respectively. TPS3N and TPS17 were α-haemolytic, while TPS4 was γ-haemolytic. The three isolates had higher viability ability after exposure to higher temperatures (80 °C, 90 °C and 100 °C) and were resistant to low pH (1) and bile salt concentration (0.5%) as well as high cell surface hydrophobicity and auto-aggregation. The three isolates were compatible with one another and thus can be used in consortia. These strains were susceptible to gentamicin, cephalexin, ampicillin, ceftriaxone, kanamycin, amikacin, penicillin, cefoperazone, chloramphenicol, erythromycin, tetracycline, doxycycline, ciprofloxacin, clindamycin (except TPS4) and furazolidone (except TPS17). The antimicrobial assessment showed that among the three isolates, TPS3N and TPS17 exhibited good antimicrobial activity against the three fish pathogens (Streptococcus agalactiae, Aeromonas hydrophila, Vibrio harveyi), while TPS4 was effective against Streptococcus agalactiae only. The results of this work suggest that Bacillus strains TPS3N, TPS4 and TPS17 could be considered as potential probiotics in tilapia aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia is one of the most cultured freshwater aquaculture species second to carps with regard to total global aquaculture production [1, 2]. It is an important source of protein for people in most developed and developing countries with China having the highest production percentage [3, 4]. Tilapia aquaculture has increased dramatically over the years due to increasing demand and the wide adaptation of intensive culture practices. However, increased production of tilapia has led to the proliferation of diseases [5]. The use of probiotics to combat diseases is well elucidated in aquaculture [6]. Research into the use of probiotics came to the fore due to the adverse effects of antibiotics such as a change in the microbiota of aquaculture systems and resistance of the microorganism to the antibiotics in fighting diseases in aquaculture [7, 8]. Probiotics are considered effective and environmentally friendly substitutes to antibiotics [9] as have been shown over the years not only to boost fish immunity and response to stress but also to improve growth and enhance the rearing water quality [10]. Probiotics when ingested in the right dose can stimulate the growth of other useful microorganisms and a reduction in pathogens thus improving the intestinal microbial balance of the host and lowering the risk of gastrointestinal diseases [11].
The digestive tract of fish provides a conducive environment for bacteria growth and survival, aiding the bacteria community to exhibit a variety of enzymatic potentials which in turn aids the fish’s digestion [12, 13]. The enzymes synthesised by this microbial community include amylolytic, proteolytic, lipolytic and cellulolytic enzymes which are involved in the digestion of carbohydrates, proteins, lipids and cellulose, respectively [14, 15]. To be able to produce the enzymes mentioned above, the bacteria must be able to survive the gastrointestinal conditions and thus should be resistant to low pH and tolerant to gastric juice in order to transit through the stomach and the intestines [16, 17]. To be used as feed additives, probiotic bacteria are required to withstand high temperature since the feed production process requires high temperatures [18]. Haemolytic bacteria are considered unsafe for use as probiotics due to the virulent factor of haemolysin which causes anaemia and oedema in the host. Above all, probiotic bacteria should be able to synthesise proteins or bacteriocins that inhibit the growth of pathogenic bacteria [19]. Therefore, to promote production and to reduce disease symptoms in aquaculture for sustainable development, screening and selection of probiotics are crucial [19].
Among the numerous probiotics, Bacillus species have proven to be very useful due to their special qualities such as the production of non-pathogenic and non-toxic compounds, improvement of water quality and a sporulation capacity which gives them an advantage in terms of survival (heat-tolerance and longer shelf-life) in diverse environments compared to other probiotics [7, 11, 20, 21]. These qualities have translated into their ability to improve growth, and enhance immunity and response to stress, improving the rearing water quality and above all enhancing the resistance of fish to diseases [5, 10, 22].
Bacillus species as probiotics used in aquaculture have many sources including soil, water, decaying matter, commercial sources, and the gastrointestinal tract of fish and other vertebrates [23,24,25]. However, as stated by Kavitha et al. [7] and Ghosh et al. [26], most of the probiotics used in aquaculture are commercial probiotics which are often relatively ineffective because they are isolated from non-fish sources hence ineffective in the colonisation process in the fish gut [19]. It was also established that those isolated from the intestinal tract of fish are more effective on their host in comparison to others [22, 27, 28]; thus, it is advantageous to isolate probiotics from fish to be used in aquaculture. Taking all of the above into consideration, in the present study, we have isolated, identified and characterised three probiotic Bacillus species based on their probiotic traits including antagonism to selected fish pathogens, resistance to low pH and high temperature, non-haemolytic nature and bile tolerance from the intestines of Nile tilapia Oreochromis niloticus. We hope that the information provided in this study could be further used to test their efficacy in tilapia culture.
Materials and Methods
Sample Collection
Healthy (i.e. without any symptoms of infection (i.e., haemorrhage, ascites, lethargic, and detachment of scales) [5] samples of Nile tilapia, O. niloticus, of average weight 150 ± 5 g were obtained from a local fish farm (Zhanjiang, Guangdong Province, China) and transported alive to the laboratory in polythene bags containing oxygenated water for immediate use.
Isolation of Gut Bacteria
Fish were anesthetised by exposure to an overdose of ethyl 3-aminobenzoate methanesulfonate, tricaine methanesulfonate (Sigma-Aldrich, 150 mg L−1MS-222) and then killed by a blow to the head. Fish were cleaned externally with cotton dipped into 75% ethanol to remove or kill any external bacteria on their bodies. The fish guts were then dissected using sterile scissors, and the intestines removed and stripped carefully to remove all digesta content and washed three times using a physiological saline solution (PBS). The weight of the intestines was then taken, and equal proportions of PBS by volume added. The content was then homogenised using 15 ml Borosilicate glass tissue homogeniser (Shanghai Lenggu Instrument Company, Shanghai, China) under sterile conditions in ice to keep it cold. Afterwards, 0.5 ml of the gut homogenate was diluted with 4.5 ml PBS. This mixture was serially diluted using PBS, and 0.1 ml of the aliquot was spread on Luria-Bertani (LB) agar plates. The plates were incubated for 24 h at 30 °C. Single colonies were randomly selected and inoculated into LB media for mass culture under the same culture conditions. Repeated streaking of the isolates was done to obtain very pure colonies. Potential probiotic strains were characterised based on their basic morphology and identified by 16S rDNA gene sequence analysis using universal bacterial primers 27F (AGAGTTTGATCCTGGCTCAG) and 1492R (GGTTACCTTGTTACGACTT) through polymerase chain reaction (PCR) [29]. The PCR reaction system contained 1 μL of each primer, 1 μL template of each isolate, 12.5 μL of 10 × Extaq buffer and 9.5 μL of double distilled water. For negative control, double distilled water was used as template, and for positive control, Vibrio harveyi (previously isolated in our laboratory) [30] was used as template. The PCR amplification was initiated with denaturation at 96 °C for 5 min followed by 33 cycles of denaturation at 96 °C for 30 s, annealing at 55 °C for 45 s, and extension at 72 °C for 1 min 30 s; the amplification was completed by holding the reaction mixture at 72 °C for 10 min. The PCR products were analysed by agarose gel (1% w/v) electrophoresis and later sequenced by Sangon Biotech Co., Ltd. (Guangzhou, China). The sequence homology was compared with 16S rDNA gene sequences available in the National Center for Biotechnology Information (NCBI) using Basic Local Alignment Search Tool (BLAST) program from the National Center for Biotechnology Information (NCBI). Similarity analysis was carried out to help identify the types of probiotic strains. Also, a phylogenetic tree was constructed using Mega 7 software to establish evolutionary relationships. Identified probiotic strain sequences were then submitted to NCBI and accession numbers obtained.
Biochemical Characterisation
Biochemical characterisation (Table 2) of the selected probiotic strains was assayed using commercial kits (Huankai Microbial, Guangzhou, China) following the manufacturer’s protocol and confirmed using Bacillus cereus (HBIG07-1) identification bar (Qingdao Hope Bio-Technology co., Ltd., Qingdao, China).
Growth of Bacteria in Luria-Bertani Broth
A single colony of probiotic bacteria from an LB agar plate was selected and inoculated into 5.0 ml of LB broth and incubated at 37 °C overnight. The next morning, 1 ml of the culture was inoculated into 100 ml sterile LB broth in 500-ml Erlenmeyer flasks and incubated at 37 °C with shaking (150 rpm) while monitoring their growth by measuring the absorbance at 600 nm at 2 h interval for 24 h [31].
Biosafety Assay
In order to assess the possible harmful effects of the probiotic bacteria isolates in Nile tilapia, 0.1 ml (108 CFU/ ml), each of the bacteria, was intraperitoneally injected into groups of three (each consisting of 10 Nile tilapia fish with an average body weight of 100 g) fish. A control group (10 fish) was injected with the same volume of sterile PBS (pH 7.2). The culture condition of fish was similar as previously described [32]. Fish were monitored daily to detect any clinical signs, and mortality rate was recorded for 10 days.
Antibiotic Susceptibility
Antibiotic susceptibility of the three selected isolates was evaluated against some antibiotics (listed in Table 3) using commercial antibiotics discs purchased from Hangzhou Microbial Reagent Co., Ltd., Hangzhou, China. Antibiotic discs were carefully placed on to Mueller-Hinton agar plates previously spread with the probiotic bacteria and incubated for 24 h at 37 °C. Susceptibility was observed by measuring (mm) the zone of inhibition as previously described by Patel et al. [33].
Resistance to Bile Salts
Resistance to bile was determined according to modified methods described by Argyri et al. [34]. Briefly, bacterial cells from an overnight culture were harvested (9000 g, 5 min, 4 °C), washed twice with PBS buffer (pH 7.2) and re-suspended in PBS solution (pH 7.4), containing 0.5% (w/v) bile salts (BBI Life Sciences, Shanghai, China). Resistance was evaluated in triplicates by counting viable colonies after incubation at 37 °C for 1 h, 2 h, 3 h and 4 h.
Resistance to High Temperature
The ability of the bacteria isolates was assessed according to Guo et al. [18] with slight modification to determine their resistivity to different temperatures since the processing of fish feed at times requires high temperatures. Overnight culture of isolates was washed twice with PBS (pH 7.4) and afterwards exposed to 80 °C, 90 °C and 100 °C temperature for 2 min, 5 min and 10 min, respectively, after which equal volume of sterile LB broth was added to the heat-treated isolates to determine their ability to grow after heat treatment. Growth was monitored by measuring absorbance at 600 nm after 12 h of incubation at 37 °C with continuous shaking (150 rpm).
Compatibility of the Three Isolates
Compatibility study was done according to methods described by Rajyalakshmi et al. [35]. In brief, the three probiotic isolates were vertically streaked on LB agar plate 5 mm apart followed by perpendicular streak 10 mm apart from each other. The plates were incubated for 24 h at 37 °C, and compatibility was determined by observing the zone of inhibition among the isolates.
Antimicrobial Activity
Three pathogenic bacterial strains were previously isolated from diseased fish from Langye fish farms in the Gaozhu City of the Guangdong Province and identified as Streptococcus agalactiae, Aeromonas hydrophila and Vibrio harveyi for use in our laboratory. These pathogens were tested against the Bacillus strains isolated from the fish used in the present study using the cross streak method and agar well diffusion method [36].
Auto-aggregation
The auto-aggregation of the selected Bacillus strains was analysed in accordance with the modified method by Lee et al. [37]. Bacterial cells were harvested by centrifugation at 9400g for 3 min, washed with PBS twice, re-suspended in the supernatant and then vortexed for 30 s. The absorbance was measured at 600 nm using a spectrophotometer (Shanghai Inesa Analytical Instrument Company, shanghai, China) at 0 h, 1 h, 2 h, 3 h and 24 h.
Auto-aggregation (%) = (1 − At/A0) × 100
A0 = Absorbance at 0 h at 600 nm.
At = Absorbance at 1, 2, 3, 24 h at 600 nm.
Cell Hydrophobicity
The cell hydrophobicity of the selected Bacillus strains was analysed according to Lee et al. [37]. Briefly, a 24 h culture was centrifuged at 9400g for 3 min, and the cells were washed and re-suspended with 2 ml of phosphate buffer saline (PBS, pH 7.4) twice. To determine the percentage hydrophobicity, its absorbance was measured at 600 nm and recorded as A0. An equal volume of the cell suspension was mixed with each solvent, namely chloroform (an acidic solvent), xylene (a nonpolar solvent) or ethyl acetate (a basic solvent), and vortexed for 5 min. The mixture was allowed to separate into two phases for 30 min. The absorbance was measured at 600 nm and recorded as A1 and hydrophobicity (%) calculated as:
A0 = Absorbance before mixing with solvent at 600 nm.
A1 = Absorbance after mixing with solvent at 600 nm.
Haemolytic Activity
The selected probiotic Bacillus species were subjected to a haemolytic assay by streaking them onto agar plates supplemented with 7% sheep blood. The plates were incubated at 37 °C for 48 h, and the haemolytic zones were observed. The isolates were subsequently classified as α, β or γ-haemolysis. Isolates with a green zone around the colony were recorded as α-haemolysis, while those with a clear zone were denoted as β-haemolysis and those that did not produce any zone around the colony was referred to as no haemolysis [37, 38].
Determination of Optimal Growth and pH
Optimal growth and pH was assessed according to Kavitha et al. [7]. In brief, fresh overnight cultures of bacteria isolates were inoculated into LB broth with variable pH (1–10). The pH was adjusted with acetic acid (99%) and 5 N NaOH. The inoculated broths were incubated at 37 °C for 24 h and growth monitored using a spectrophotometer (Shanghai Inesa Analytical Instrument Company, shanghai, China) at 600 nm (OD) against the uninoculated broth.
Detection of Biofilm Formation (Congo Red Agar Method)
Biofilm production was detected according to the methods described by Kavitha et al. [7]. Briefly, the isolates were streaked on Mueller Hinton agar supplemented with 0.8 g/l of Congo red dye and incubated at 37 °C for 48 h. The presence of black colonies with a dry crystalline consistency indicated biofilm production, and red colonies indicated non-biofilm-producing strains.
Statistical Analysis
All the experiments were performed in triplicates, and the results were subjected to one-way analysis of variance (ANOVA). The differences in mean values were identified by Tukey’s HSD tests (P < 0.05). Data were expressed as a mean ± standard error (SE). Data were analysed by SPSS (IBM SPSS STATISTICS, 16.0 package, IBM Corporation, New York, USA) for Windows version 7.0 (SPSS, Chicago, USA).
Results
Identification of Gut Bacteria
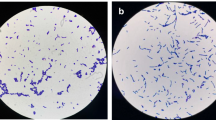
Three potential probiotic bacteria, TPS3N, TPS4 and TPS17, were selected following morphological and biochemical characterisation (Tables 1 and 2). 16S rDNA PCR (Fig. 1) gene sequence analysis revealed that the three isolates were Bacillus species (refer to supplemental data for sequences). The three isolates, TPS3N, TPS4 and TPS17, showed close sequence homology (99%) with Bacillus velezensis, Bacillus subtilis and Bacillus amyloliquefaciens, respectively. Phylogenetic analysis (Fig. 2) confirmed that the isolates TPS3N, TPS4 and TPS17 were closest to Bacillus velezensis, Bacillus subtilis and Bacillus amyloliquefaciens, respectively. The 16S rDNA gene sequences were submitted to GenBank (NCBI) and accession numbers obtained; thus, the isolates TPS3N, TPS4 and TPS 17 were designated as Bacillus velezensis TPS3N (MK130897), Bacillus subtilis TPS4 (MK130899) and Bacillus amyloliquefaciens TPS17 (MK130898), respectively.
Biochemical and Morphological Characterisation
Tables 1 and 2 summarise the biochemical and morphological characteristics of the three isolates. The three isolates had similar biochemical characters and thus can use almost all the carbon sources examined except TPS3N which did not demonstrate an ability to use citrate as a carbon source. All isolates were negative for lactose fermentation, hippuric acid, gelatin liquefaction, methyl red and VP test. Only TPS4 was able to grow in lysozyme broth and was mannitol positive; thus, it is halophilic. However, they have different morphological characteristics (Fig. 3).
Pictorial overview of the morphological, biochemical characteristics and antimicrobial activities of the three isolates. Lane 1: morphology of TPS3N, TPS4, TPS17. Lane 2: antimicrobial activities of TPS3N, TPS4, TPS17 (cross streak method). Lane 3: evidence of biochemical test of the isolates (3i: citrate reduction test, 3ii: urease test, 3iii: arginine dihydrolase test, 3iv: confirmatory test using Bacillus cereus identification bar). Lane 4: antimicrobial activities of TPS3N, TPS4, TPS17 (agar well diffusion method). SA: Streptococcus agalactiae, AH: Aeromonas hydrophila, VH: Vibrio harveyi
Growth Curve (in LB) Using OD
All the three isolates had their log phase beginning at approximately 2 h after incubation at 37 °C with continuous shaking (150 rpm). TPS3N attained its stationary phase earlier than TPS4 and TPS17 (Fig. 4).
Biosafety Assay
There were no pathological symptoms (i.e. oedema, haemorrhage, lesions, loss of scale and mucus) observed in both experimental and control fish after in vivo biosafety assay. Also, no mortalities were recorded. This confirmed that the isolates were non-pathogenic.
Antibiotic Susceptibility
The results of antibiotic susceptibility of the selected isolates are shown in Table 3. All the isolates were highly susceptible to most of the tested antibiotics except TPS3N and TPS17 which were resistant to ceftazidime. The isolate TPS4 and TPS17 were found to be moderately susceptible to ceftazidime and clindamycin and furazolidone, respectively. With regard to polymyxin, all the isolates were moderately susceptible.
Resistance to Bile Salt
The isolates were subjected to 0.5% bile salt resistance assay and survivability monitored by counting the number of colony forming units after 4 h of exposure and expressed as a percentage. The result revealed that more than 50% of the isolates survived after 3 h of exposure. However, after 4 h of bile salt exposure, the percentage survivability of TPS3N dropped to 46.2%, while TPS4 and TPS17 remained above 50% (Fig. 5). Regarding TPS3N, significant (P < 0.05) reduction in percentage survival was observed at each hour after 2 h of exposure to bile salt. Significant reduction in percentage survival was observed in TPS4 after 1 h and 2 h (83.8% and 62.2%, respectively) after which the reduction remained insignificant to the fourth hour of exposure. In TPS17, a significant reduction in percentage survival was observed after 2 h and remained unchanged afterwards.
Resistance to High Temperature
After exposure to different temperatures (80 °C, 90 °C, 100 °C for 2 min, 5 min, 10 min, respectively), the three isolates gave promising results. High growth (OD) was observed in all the isolates exposed to the various temperatures in comparison to the control (isolates without exposure to higher temperatures). However, no significant (P < 0.05) differences were observed at the different times of exposure among the different temperatures (Fig. 6).
Compatibility of the Three Isolates
In the present study, when the isolates were characterised for their compatibility, no definite sign of suppression of the three bacteria isolates was observed on each other suggesting that they were compatible.
Antimicrobial Activity
In the present study, the three isolates were evaluated for their antimicrobial traits against three fish pathogens (viz. Streptococcus agalactiae, Aeromonas hydrophila, Vibrio harveyi). It was noted that all the three selected isolates were found to inhibit at least one of the three tested pathogens (Table 4). TPS4 could not inhibit Vibrio harveyi and Aeromonas hydrophila both in the cross streak method and in the agar well diffusion method (Fig. 3).
Auto-aggregation
Auto-aggregation assay which is strongly correlated with cell adhesion to the digestive tract revealed that all the three isolates TPS3N, TPS4 and TPS17 had low cell adhesion ability (less than 30%) at the first 3 h. However, after 24 h, cell adhesion increased to 92.97%, 84.83% and 89.13%, respectively (Fig. 7).
Cell Hydrophobicity
Adhesion of the selected isolates (TPS3N, TPS4, TPS17) to ethyl acetate, chloroform and xylene was tested to determine the adhesion capability of the bacterial to cell surfaces, and the results are shown in Fig. 8. Cell surface hydrophobicity of the isolates TPS3N and TPS17 to ethyl acetate was significantly lower (P < 0.05) than that of chloroform and xylene.
Haemolytic Activity
Regarding haemolytic activities, TPS3N and TPS17exhibited α-haemolysis, while TPS4 exhibited γ-haemolysis (Table 2).
Determination of Optimal Growth and pH
After exposure to different pH, TPS3N, TPS4 and TPS17 gave promising tolerance results. Though irregular, gradual increase in the growth of the isolates was observed within the pH range of 1.0–7.0 (TPS3N and TPS17) and 1.0–8.0 (TPS4). Decreased growth was observed as pH increased from 7.0 to 10.0 suggesting that the isolates could survive in extreme acidic as well as alkaline conditions. All the Bacillus strains displayed a significant difference (P < 0.05) in different pH at some points (Fig. 9).
Detection of Biofilm Formation (Congo Red Agar Method)
Congo red agar method was used to screen and ascertain the ability of the isolates to produce biofilm. None of the isolates formed black colonies indicating negative biofilm production.
Discussion
Attempts to curb the situation of fish diseases in aquaculture have led to the discovery and use of probiotics as a safer alternative to the widely used antibiotics due to their adverse effects [8, 39]. Bacillus species as probiotics have characteristics that make them outstanding among all other probiotics [11, 21]. Also, the positive effects of Bacillus species in tilapia aquaculture have been confirmed by many researchers [5, 10, 22], and those isolated from the gastrointestinal tract of a healthy fish are considered the best source for controlling many infectious diseases in fish [27].
In this current investigation, we isolated and assessed the probiotic potentials of three Bacillus species, viz. Bacillus velezensis TPS3N (MK130897), Bacillus subtilis TPS4 (MK130899) and Bacillus amyloliquefaciens TPS17 (MK130898) from the gut of Nile tilapia using in vitro methods. The isolated strain TPS3N, TPS4 and TPS17 were identified using morphological characteristics and biochemical tests and further confirmed by 16S rDNA gene sequence. The three isolates utilised a wide range of carbon sources namely rhamnose, sorbitol, inositol, adonitol, citrate, glucose, starch, and mannitol as well as the amino acid arginine which suggests that they could be helpful in the digestion of carbohydrates, and the hydrolysis of amino acids thus can be used as probiotics and for the production of value-added products in food industries as have been reported in other studies [7, 27, 37].
Unlike other probiotics, Bacillus species produce spores that are more heat tolerant [18, 40], resistant to low pH and a high percentage of bile concentration [41, 42], and have the abilities to germinate and survive in the gut of fish [41, 43, 44]. Gastric (low pH) and intestinal (high bile concentration) tolerance are the prerequisites for probiotics to survive and colonise the gut to produce beneficial traits [45, 46]. Also, heat treatment is an essential process during feed production to increase the palatability and kill pathogenic vegetative cells [18]. In this study, isolates TPS3N, TPS4 and TPS17 exhibited sporulation ability which translated into their ability to withstand pH as low as 1 (Fig. 9), 0.5% bile concentration (Fig. 5) and higher viability after heat treatment compared to the control (Fig. 6). It could, therefore, be deduced that higher temperatures (80 °C, 90 °C, 100 °C) activated the bacteria strains, hence the higher growth rates. Similar studies support the sporulation capacity of Bacillus velezensis [47, 48], Bacillus subtilis [18] and Bacillus amyloliquefaciens [28]. The higher viability of the strains after heat treatment strongly suggests that they could be used as feed additives and hence are good potential probiotics.
Colonisation and adhesion to epithelial cells and mucosal surfaces are important characteristics of good probiotics since it guarantees the ability to resist the fluctuation of the intestinal content and it also inhibits the pathogenic bacteria adhesion by occupying all the space of the intestine as well as inhibits inflammatory reactions [49,50,51]. An indirect method of determining the adhesion ability of probiotic bacteria is the determination of the auto-aggregation and the hydrophobicity of the bacteria [52, 53]. In this study, TPS17 showed much higher hydrophobicity (97.5% and 97.1%) with chloroform and xylene respectively indicating bacterial adhesion to hydrocarbons compared to TPS3N (92.8% and 95.8%) and TPS4 (85.1% and 96.6%). With regard to ethyl acetate, however, the highest hydrophobicity was observed in TPS4 (90.6%) as against TPS3N (78.1%) and TPS17 (74.9%). The hydrophobicity results in this study are relatively higher than that of Bacillus species in a study conducted by Lee et al. [37] and Manhar et al. [54] suggesting higher electron donation (chloroform) and acceptance (ethyl acetate) [55] of our strains hence higher adhesion to epithelial cells. Also, there is a strong correlation between auto-aggregation and cell adhesion to the digestive tract, which is one of the prerequisites for a good probiotic bacteria. TPS3N, TPS4 and TPS17 showed high auto-aggregation (93.0%, 84.8% and 89.1%, respectively) after 24 h which agreed with a similar study conducted by Liu et al. [56].
The safety prerequisites for the selection of a probiotic strain are the absence of haemolytic activity, and antibiotic resistance [34] as haemolysin is considered a virulent factor due to its ability to initiate infection by entering small lesions in the mucous membranes and skin of any host [19, 27]. No or γ-haemolysis and α-haemolysis are considered to be safe, and β-haemolysis is considered harmful [57]. In this study, isolates TPS3N and TPS17 exhibited α-haemolysis, while TPS4 showed γ-haemolysis. A similar observation was made by Lee et al. [37] and Kavitha et al. [7]. Out of the 17 antibiotics tested, all the three isolates were highly susceptible to 15. Intermediate susceptibility was observed in polymyxin for all the isolates, also in clindamycin and ceftazidime for TPS4 and furazolidone for TPS17.
In a previous study by Saarela et al. [58], it was revealed that food produced using mono species probiotics had a sour and acidic taste. This has inspired us to investigate the compatibility of our strains in order to be used as multispecies probiotics, and the results showed that all the three isolates are compatible with one another which is in agreement with Rajyalakshmi et al. [35] who also observed compatibility among some Bacillus species.
Biofilms have great significance for public health, as biofilm-forming microorganisms exhibit dramatically reduced susceptibility to antimicrobial agents [59] despite the advantages associated with biofilm formation [60, 61]. Meanwhile, antibiotics-resistant strains are considered unsafe for use as probiotics. In this study, isolates TPS3N, TPS4 and TPS17 tested negative for biofilm formation test. Similarly, in an experiment conducted by Kavitha et al. [7], only one isolate out of three isolates formed biofilm.
Fish diseases caused by Streptococcus [62], Aeromonas [63] and Vibrio [64] species have been reported in aquaculture. Earlier reports indicated that several species of Bacillus have antimicrobial properties against several Gram-positive and Gram-negative pathogenic bacteria. In this study, aside from TPS4 which was effective against Streptococcus agalactiae only, TPS3N and TPS17 showed great antimicrobial effects against the three pathogenic bacteria, viz. Streptococcus agalactiae, Aeromonas hydrophila and Vibrio harveyi suggesting that the three Bacillus strains TPS3N, TPS4 and TPS17 could be used to fight fish disease in aquaculture.
Conclusion
The study showed that all three strains, TPS3N, TPS4 and TPS17 isolated from the gut of Nile tilapia, O. niloticus, possess characteristics such as high survivability after heat treatment, non-haemolytic nature, wide antimicrobial activities and safety confidence including antibiotic susceptibility. Overall, the features identified in these bacteria strains show that they might have great potential as probiotics for aquaculture use. However, in vivo assessment is required to ascertain their applications in aquaculture environment especially in tilapia culture.
References
Narimbi J, Mazumder D, Sammut J (2018) Stable isotope analysis to quantify contributions of supplementary feed in Nile Tilapia Oreochromis niloticus (GIFT strain) aquaculture. Aquac Res 49:1866–1874. https://doi.org/10.1111/are.13642
Trosvik KA, Webster CD, Thompson KR, Metts LA, Gannam A, Twibell R (2013) Effects on growth performance and body composition in Nile tilapia, Oreochromis niloticus, fry fed organic diets containing yeast extract and soyabean meal as a total replacement of fish meal without amino acid supplementation. Biol Agric Hortic 29:173–185. https://doi.org/10.1080/01448765.2013.810123
Nguyen NH, Ponzoni RW, Yee HY, Abu-Bakar KR, Hamzah A, Khaw HL (2010) Quantitative genetic basis of fatty acid composition in the GIFT strain of Nile tilapia (Oreochromis niloticus) selected for high growth. Aquaculture 309:66–74. https://doi.org/10.1016/j.aquaculture.2010.08.034
Mo WY, Man YB, Wong MH (2018) Use of food waste, fish waste and food processing waste for China’s aquaculture industry: needs and challenge. Sci Total Environ 613–614:635–643. https://doi.org/10.1016/j.scitotenv.2017.08.321
Abarike ED, Cai J, Lu Y, Yu H, Chen L, Jian J, Tang J, Jun L, Kuebutornye FKA (2018) Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 82:229–238. https://doi.org/10.1016/j.fsi.2018.08.037
Van Hai N (2015) Research findings from the use of probiotics in tilapia aquaculture: a review. Fish Shellfish Immunol 45:592–597. https://doi.org/10.1016/j.fsi.2015.05.026
Kavitha M, Raja M, Perumal P (2018) Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquac Reports 11:59–69. https://doi.org/10.1016/j.aqrep.2018.07.001
Resende JA, Silva VL, Fontes CO et al (2012) Multidrug-resistance and toxic metal tolerance of medically important bacteria isolated from an aquaculture system. Microbes Environ 27:449–455. https://doi.org/10.1264/jsme2.ME12049
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Elsabagh M, Mohamed R, Moustafa EM, Hamza A, Farrag F, Decamp O, Dawood MAO, Eltholth M (2018) Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac Nutr 24:1–10. https://doi.org/10.1111/anu.12797
Buruiană CT, Profir AG, Vizireanu C (2014) Effects of probiotic Bacillus species in aquaculture – an overview. Ann Univ Dunarea Jos Galati, Fascicle VI Food Technol 38:9–17
Kar N, Ghosh K (2008) Enzyme producing bacteria in the gastrointestinal tracts of Labeo rohita (Hamilton) and Channa punctatus (Bloch). Turkish J Fish Aquat Sci 1:115–120
Ray AK, Roy T, Mondal S, Ringø E (2010) Identification of gut-associated amylase, cellulase and protease-producing bacteria in three species of Indian major carps. Aquac Res 41:1462–1469. https://doi.org/10.1111/j.1365-2109.2009.02437.x
Ray AK, Ghosh K, Ringø E (2012) Enzyme-producing bacteria isolated from fish gut: a review. Aquac Nutr 18:465–492. https://doi.org/10.1111/j.1365-2095.2012.00943.x
Falcón-Hidalgo B, Forrellat-Barrios A, Farnés OC, Hernández KU (2011) Digestive enzymes of two freshwater fishes (Limia vittata and Gambusia punctata) with different dietary preferences at three developmental stages. Comp Biochem Physiol - B Biochem Mol Biol 158:136–141. https://doi.org/10.1016/j.cbpb.2010.10.009
Balcázar JL, Vendrell D, de Blas I, Ruiz-Zarzuela I, Muzquiz JL, Girones O (2008) Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 278:188–191. https://doi.org/10.1016/j.aquaculture.2008.03.014
Kim HJ, Shin HS, Ha WK, Yang HJ, Lee SW (2006) Characterization of lactic bacterial strains isolated from raw milk. Asian-Australasian J Anim Sci 19:131–136. https://doi.org/10.5713/ajas.2006.131
Guo X, Chen DD, Peng KS, Cui ZW, Zhang XJ, Li S, Zhang YA (2016) Identification and characterization of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish Immunol 52:74–84. https://doi.org/10.1016/j.fsi.2016.03.017
Nandi A, Dan SK, Banerjee G, Ghosh P, Ghosh K, Ringø E, Ray AK (2017) Probiotic potential of autochthonous bacteria isolated from the gastrointestinal tract of four freshwater teleosts. Probiotics Antimicrob Proteins 9:12–21. https://doi.org/10.1007/s12602-016-9228-8
Kuebutornye FKA, Abarike ED, Lu Y (2019) A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol 87:820–828. https://doi.org/10.1016/j.fsi.2019.02.010
Geng X, Dong XH, Tan BP et al (2012) Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquac Nutr 18:46–55. https://doi.org/10.1111/j.1365-2095.2011.00875.x
Van Doan H, Hoseinifar SH, Khanongnuch C et al (2018) Host-associated probiotics boosted mucosal and serum immunity, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture 491:94–100. https://doi.org/10.1016/j.aquaculture.2018.03.019
Sumon MS, Ahmmed F, Khushi SS, Ahmmed MK, Rouf MA, Chisty MAH, Sarower MG (2018) Growth performance, digestive enzyme activity and immune response of Macrobrachium rosenbergii fed with probiotic Clostridium butyricum incorporated diets. J King Saud Univ - Sci 30:21–28. https://doi.org/10.1016/j.jksus.2016.11.003
Adorian TJ, Jamali H, Farsani HG, Darvishi P, Hasanpour S, Bagheri T, Roozbehfar R (2018) Effects of probiotic bacteria Bacillus on growth performance, digestive enzyme activity, and hematological parameters of Asian Sea bass, Lates calcarifer (Bloch). Probiotics Antimicrob Proteins 11:1–8. https://doi.org/10.1007/s12602-018-9393-z
Sankar H, Philip B, Philip R, Singh ISB (2017) Effect of probiotics on digestive enzyme activities and growth of cichlids, Etroplus suratensis (Pearl spot) and Oreochromis mossambicus (Tilapia). Aquac Nutr 23:852–864. https://doi.org/10.1111/anu.12452
Ghosh S, Sinha A, Sahu C (2007) Isolation of putative probionts from the intestines of Indian major carps. Isr J Aquac - Bamidgeh 59:127–132
Ramesh D, Vinothkanna A, Rai AK, Vignesh VS (2015) Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 45:268–276. https://doi.org/10.1016/j.fsi.2015.04.018
Reda RM, El-Hady MA, Selim KM, El-Sayed HM (2018) Comparative study of three predominant gut Bacillus strains and a commercial B. amyloliquefaciens as probiotics on the performance of Clarias gariepinus. Fish Shellfish Immunol 80:416–425. https://doi.org/10.1016/j.fsi.2018.06.031
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
Kuebutornye FKA, Liao J, Pang H, Lu Y, Ayiku S, Sakyi ME (2018) Molecular cloning and bioinformatics analysis of T3SS inner membrane ring HrpQ from Vibrio harveyi. Genomics Appl Biol 9:40–47. https://doi.org/10.5376/gab.2018.09.0007
Xie F, Quan S, Liu D, Ma H, Li F, Zhou F, Chen G (2014) Purification and characterization of a novel α-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochem 49:47–53. https://doi.org/10.1016/j.procbio.2013.09.025
Abarike ED, Jian J, Tang J, Cai J, Yu H, Lihua C, Jun L (2018) Influence of traditional Chinese medicine and Bacillus species (TCMBS) on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac Res 49:2366–2375. https://doi.org/10.1111/are.13691
Patel AK, Ahire JJ, Pawar SP, Chaudhari BL, Chincholkar SB (2009) Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res Int 42:505–510. https://doi.org/10.1016/j.foodres.2009.01.013
Argyri AA, Zoumpopoulou G, Karatzas KAG, Tsakalidou E, Nychas GJE, Panagou EZ, Tassou CC (2013) Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol 33:282–291. https://doi.org/10.1016/j.fm.2012.10.005
Rajyalakshmi K, Roopa B, Saikat DM, Priyanka D, Vadlamudi S, Subramaniam G (2016) Characterization of potential probiotic bacteria isolated from sorghum and pearl millet of the semi-arid tropics. African J Biotechnol 15:613–621. https://doi.org/10.5897/AJB2016.15212
Lertcanawanichakul M, Sawangnop S (2008) A comparison of two methods used for measuring the antagonistic activity of Bacillus species. Walailak J Sci Technol 5:161–171. https://doi.org/10.2004/wjst.v5i2.86
Lee S, Lee J, Jin YI, Jeong JC, Chang YH, Lee Y, Jeong Y, Kim M (2017) Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT - Food Sci Technol 79:518–524. https://doi.org/10.1016/j.lwt.2016.08.040
Engel RR, Matsen JM, Chapman SS, Schwartz S (1972) Carbon monoxide production from heme compounds by bacteria. J Bacteriol 112:1310–1315
Magnadottir B (2010) Immunological control of fish diseases. Mar Biotechnol 12:361–379. https://doi.org/10.1007/s10126-010-9279-x
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64:548–572. https://doi.org/10.1128/MMBR.64.3.548-572.2000
Barbosa TM, Serra CR, La Ragione RM et al (2005) Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol 71:968–978. https://doi.org/10.1128/AEM.71.2.968-978.2005
Spinosa MR, Braccini T, Ricca E, de Felice M, Morelli L, Pozzi G, Oggioni MR (2000) On the fate of ingested Bacillus spores. Res Microbiol 151:361–368. https://doi.org/10.1016/S0923-2508(00)00159-5
Hoa NT, Baccigalupi L, Huxham A, Smertenko A, van PH, Ammendola S, Ricca E, Cutting SM (2000) Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl Environ Microbiol 66:5241–5247. https://doi.org/10.1128/AEM.66.12.5241-5247.2000
Hong HA, To E, Fakhry S et al (2009) Defining the natural habitat of Bacillus spore-formers. Res Microbiol 160:375–379. https://doi.org/10.1016/j.resmic.2009.06.006
Guglielmotti DM, Marcó MB, Golowczyc M, Reinheimer JA, Quiberoni AL (2007) Probiotic potential of Lactobacillus delbrueckii strains and their phage resistant mutants. Int Dairy J 17:916–925. https://doi.org/10.1016/j.idairyj.2006.11.004
Cartman ST, La Ragione RM, Woodward MJ (2008) Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl Environ Microbiol 74:5254–5258. https://doi.org/10.1128/AEM.00580-08
Gao XY, Liu Y, Miao LL, Li EW, Sun GX, Liu Y, Liu ZP (2017) Characterization and mechanism of anti-Aeromonas salmonicida activity of a marine probiotic strain, Bacillus velezensis V4. Appl Microbiol Biotechnol 101:3759–3768. https://doi.org/10.1007/s00253-017-8095-x
Ye M, Tang X, Yang R, Zhang H, Li F, Tao F, Li F, Wang Z (2018) Characteristics and application of a novel species of Bacillus: Bacillus velezensis. ACS Chem Biol 13:500–505. https://doi.org/10.1021/acschembio.7b00874
Guo XH, Kim JM, Nam HM, Park SY, Kim JM (2010) Screening lactic acid bacteria from swine origins for multistrain probiotics based on in vitro functional properties. Anaerobe 16:321–326. https://doi.org/10.1016/j.anaerobe.2010.03.006
Kos B, Šušković J, Vuković S, Šimpraga M, Frece J, Matošić S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987. https://doi.org/10.1046/j.1365-2672.2003.01915.x
Sim I, Koh JH, Kim DJ, Gu SH, Park A, Lim YH (2015) In vitro assessment of the gastrointestinal tolerance and immunomodulatory function of Bacillus methylotrophicus isolated from a traditional Korean fermented soybean food. J Appl Microbiol 118:718–726. https://doi.org/10.1111/jam.12719
Meidong R, Doolgindachbaporn S, Jamjan W, Sakai K, Tashiro Y, Okugawa Y, Tongpim S (2017) A novel probiotic Bacillus siamensis B44v isolated from Thai pickled vegetables (Phak-dong) for potential use as a feed supplement in aquaculture. J Gen Appl Microbiol 63:246–253. https://doi.org/10.2323/jgam.2016.12.002
Collado MC, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226:1065–1073. https://doi.org/10.1007/s00217-007-0632-x
Manhar AK, Saikia D, Bashir Y, Mech RK, Nath D, Konwar BK, Mandal M (2015) In vitro evaluation of celluloytic Bacillus amyloliquefaciens AMS1 isolated from traditional fermented soybean (Churpi) as an animal probiotic. Res Vet Sci 99:149–156. https://doi.org/10.1016/j.rvsc.2015.01.008
Bellon-Fontaine MN, Rault J, Van Oss CJ (1996) Microbial adhesion to solvents: a novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surfaces B Biointerfaces 7:47–53. https://doi.org/10.1016/0927-7765(96)01272-6
Liu H, Wang S, Cai Y, Guo X, Cao Z, Zhang Y, Liu S, Yuan W, Zhu W, Zheng Y, Xie Z, Guo W, Zhou Y (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 60:326–333. https://doi.org/10.1016/j.fsi.2016.12.003
Shin HJ, Choi H, Kim DW et al (2012) Probiotic potential of Pediococcus pentosaceus BCNU 9070. J Life Sci 22:1194–1200. https://doi.org/10.5352/JLS.2012.22.9.1194
Saarela M, Mogensen G, Fondén R, Mättö J, Mattila-Sandholm T (2000) Probiotic bacteria: safety, functional and technological properties. J Biotechnol 84:197–205. https://doi.org/10.1016/S0168-1656(00)00375-8
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392. https://doi.org/10.1086/322972
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79. https://doi.org/10.1146/annurev.micro.54.1.49
Morikawa M (2006) Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J Biosci Bioeng 101:1–8. https://doi.org/10.1263/jbb.101.1
Shoemaker CA, Klesius PH, Evans JJ (2001) Prevalence of Streptococcus iniae in tilapia, hybrid striped bass, and channel catfish on commercial fish farms in the United States. Am J Vet Res 62:174–177. https://doi.org/10.2460/ajvr.2001.62.174
Amal MNA, Koh CB, Nurliyana M, Suhaiba M, Nor-Amalina Z, Santha S, Diyana-Nadhirah KP, Yusof MT, Ina-Salwany MY, Zamri-Saad M (2018) A case of natural co-infection of Tilapia Lake virus and Aeromonas veronii in a Malaysian red hybrid tilapia (Oreochromis niloticus×O. mossambicus) farm experiencing high mortality. Aquaculture 485:12–16. https://doi.org/10.1016/j.aquaculture.2017.11.019
Bluford J, Gauthier D, Colasanto M, Rhodes M, Vogelbein W, Haines A (2017) Identification of virulence genes in Vibrio spp. isolates from the 2009 Bermuda reef fish mortality event. J Fish Dis 40:597–600. https://doi.org/10.1111/jfd.12532
Funding
Shenzhen strategic emerging and future industrial development funds (20170426231005389) supported this work.
Author information
Authors and Affiliations
Contributions
Yishan Lu and Felix K.A. Kuebutornye conceived and designed the experiment. Felix K.A. Kuebutornye, Zhiwen Wang and Yuan Li carried out field experiments and laboratory analysis of data. Emmanuel D. Abarike drafted and proofread the manuscript and Michael Essien Sakyi analysed some data, edited and proofread the revised manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Animal Rights
All fish were handled following the U.K animal act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article to be considered for publication has not been published previously and is not under consideration for publication elsewhere.
Rights and permissions
About this article
Cite this article
Kuebutornye, F.K., Lu, Y., Abarike, E.D. et al. In vitro Assessment of the Probiotic Characteristics of Three Bacillus Species from the Gut of Nile Tilapia, Oreochromis niloticus. Probiotics & Antimicro. Prot. 12, 412–424 (2020). https://doi.org/10.1007/s12602-019-09562-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09562-5