Abstract
Mastitis is one of the most important causes of loss of cattle production, burdening producers due to the increased cost of milk production and decreased herd productivity. The development of alternative methods for the treatment and prevention of mastitis other than traditional chemical antibiotic therapy needs to be implemented to meet international pressures to reduce the use of these drugs and promote the elimination of multiresistant microbial strains from the environment. Treatment with probiotic bacteria or yeast strains offers a possible strategy for the control of mastitis. The objective of this work was to isolate, identify, and characterize lactic bacteria from milk and the intramammary duct of Gyr, Guzerat, Girolando 1/2, and Holstein cattle breeds from Brazil. Samples of 115 cows were taken, a total of 192 bacteria isolates belonging to 30 species were obtained, and 81 were selected to evaluate their probiotic potential in in vitro characterization tests. In general, bacteria isolated from the mammary gland have low autoaggregation, cell surface hydrophobicity, and co-aggregation with mastitis etiological bacteria Staphylococcus aureus and Escherichia coli. Also, they have biofilm assembly capacity, inability to produce exopolysaccharides, high production of H2O2, and strong antagonism against mastitis pathogens. Ten lactic bacteria isolates were used in co-culture with human MDA-MB-231 breast epithelial cells to assess their adhesion capacity and impairment of the S. aureus invasion. Our results, therefore, contribute to the future production of new prevention and treatment tools for bovine mastitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Livestock occupies 30% of the ice-free land surface, respecting the environment and animal welfare, aiming to deliver safe food to humans (Gaggìa et al. 2010). The dairy industry contributes to the economies of several populations in many countries. An increasing demand worldwide has been noticeable, leading to an increase in global milk production (IDF, 2013). The milk production has the interference of several geographic, management, and biological factors, especially those related to animal genetic and health (Begum et al. 2014).
Mastitis is an inflammation of the mammary gland caused by the invasion by infectious agents, such as Staphylococcus, Streptococcus, and Gram-negative bacteria (Watts 1988). Subclinical mastitis shows no clinical signs but results in increased somatic cell count (SCC) in milk above 200,000 cells/mL (NMC, 2001). In dairy farming, this is the most common disease and has the most significant economic impact (Contreras and Rodríguez 2011). Costs related to mastitis treatment vary from € 112 to € 1006/case (Heikkilä et al. 2012). However, the principal loss caused by this illness is the reduction of milk production in animals with subclinical infection since a sick quarter presents a decrease of 10 to 12% in their production capacity (Akers and Nickerson 2011).
Standard mastitis treatment uses penicillin, cephalosporin, lincosamides, and macrolides. However, pathogens have developed resistance to these antimicrobials (Thaker et al. 2013). Besides, these medications imply the presence of antibiotic residues in milk (Dalton 2006), and there is a growing concern about the residual effect of antimicrobials on food in the human health (Mentem 2001). Therefore, safeguards were imposed by some governments (34th Codex Alimentarius Commission 2009 and European Union Council Regulation 37/2010/EC) proposing to limit the residual concentration of antibiotics that will be tolerated in the milk (Klostermann et al. 2008). In this scenario, the research on alternatives to increase the natural defense mechanisms of farm animals is emerging, aiming to reduce antibiotic usage (Verstegen and Williams 2002).
Several pathologies in the gastrointestinal and urogenital tract derive from the perturbation of their healthy microbiota (Walker and Iyengar 2015). Such dysbiosis can be prevented or treated by the administration of commensal members of the respective microbiota (Van den Elsen et al. 2017). Hence, bacteriotherapy is the use of beneficial bacteria to prevent or treat colonization of the host by pathogens (Huovinen 2001; Strauss 2000). Beneficial bacteria are known as probiotics that are living microorganisms that, when administered in adequate amounts, confer benefits to the health of the host (FAO/WHO 2002). Mastitis is associated with disturbances in the mammary quarter microbiota (Falentin et al. 2016; Oikonomou et al. 2012). So, it seems possible that the prevention or treatment of mastitis can be made using probiotics, like in different pathologies in humans and animals (Hunt et al. 2011).
In cattle, lactic acid bacteria (LAB) such as Lactococcus, Lactobacillus, Leuconostoc, Enterococcus, Streptococcus, and Weissella are among the most abundant in fresh milk (Martín et al. 2010). Also, they are safety employed in food technology (Abdullah and Osman 2010). Therefore, some LAB has the status of Generally Recognized as Safe, according to the Food and Drug Administration. Moreover, LAB strains have several probiotic effects already described (Schmitz and Suchodolski 2016).
LAB appears to prevent intramammary infections caused by Staphylococcus aureus, one of the major mastitis pathogens (Heikkilä and Saris 2003). Lactobacillus fermentum CECT5716 and L. salivarius CECT5713 show anti-inflammatory and anti-infective roles on the S. aureus infection (Arroyo et al. 2010; Jiménez et al. 2008). Lactococcus lactis DPC3147 produces a bacteriocin that inhibits the growth of mastitis pathogens (Ryan et al. 1998) and increases the expression of IL-8 and IL-1β (Beecher et al. 2009), leading to recruitment of neutrophils to the mammary gland (Crispie et al. 2008). Lactobacillus casei strains, isolated from the udder canal, reduced invasion of mammary cells by S. aureus (Bouchard et al. 2013).
More than a hundred microorganisms are listed as microbiota stabilizers in the Register of Feed Additives of the European Union, which illustrates the importance of prospecting new probiotics to be used in animal production (Ducatelle et al. 2015). However, few bacteria have been characterized so far as possible probiotics for bovine mastitis bacteriotherapy. LAB, resident members of the bovine mammary gland microbiota, should be associated with a healthy udder, as they produce a healthy state in other ecosystems. Therefore, due to the few studies conducted so far, there is a niche to be explored in the prospection of new potential bacteria to be used for the prevention and/or treatment of mastitis. Thereby, the objective of this work was to isolate, identify, and evaluate the probiotic potential of LAB strains isolated from bovine mammary gland samples.
Materials and methods
Animal sampling
Milk and intramammary swab samples were collected from 115 dairy cows that were within the peak of 1st to 5th lactation (with at least 60 days postpartum) belonging to Holstein, Guzerat, Gyr, and Girolando 1/2 breeds. Average 30% of the lactating cows in each herd were randomly sampled on four farms, located in different regions of the Minas Gerais state, Brazil. These animals were free of any antibiotic therapy at least 30 days before sampling, which was taken in November/2013 and January/2014 (rainy season) only in the morning milking (4 to 6 AM). During collection, animals with features suggestive of clinical mastitis were discarded. One quarter per cow was sampled, corresponding to the right rear quarter. Teats were thoroughly washed with a potassium permanganate solution (1:1000 w/v) and subjected to cleaning with iodized alcohol solution and dried using paper towels. By manual milking, first milk jets were discarded, and 100 mL of milk was collected in 50-mL sterile tubes. Milk aliquots of 50 mL took in bronopol tubes were used for SCC. COPAN Venturi Transystems (Copan Diagnostics IN, Murrieta, CA, USA) was used for an intramammary swab sampling. The swab was inserted 2 cm inside the teat apex and turned three times before removal (Bouchard et al. 2015). The swabs were immediately introduced in tubes containing a sterile transport medium. All samples were stored on ice until processing in the laboratory.
SCC analysis of milk samples was performed in the Bentley Combi System 2300® (Bentley Instruments Incorporated, Chaska, USA), and used as a classification criterion to healthy and subclinical mastitis animals. The cut-off point for the determination of subclinical mastitis was SCC > 200,000 cells/mL (Harmon 1994).
Collection techniques performed in this study are part of a standard routine veterinary practice in farms. According to the European Directive 2010/63/EU, this type of experiment does not require authorization application, since all the procedures performed here are part of routine farm care and were accompanied by a veterinarian. Permission for sample collection was received from animal owners who also consented to the publication of this research. Furthermore, no animals were sacrificed or suffered any aggression for this study.
Isolation of LAB strains
At the laboratory, swab and milk samples were homogenized, diluted serially in phosphate-buffered saline (PBS), plated onto de Man, Rogosa, and Sharpe (MRS; Acumedia, Baltimore, MD, USA) agar (Difco, BD Biosciences, Franklin Lakes, NJ, USA), added 100 mg/L of cycloheximide (Sigma-Aldrich, St. Louis, MO, USA), and incubated for 48 h at 37 °C in an anaerobic chamber (Forma Scientific Inc., Marietta, OH, USA) containing an atmosphere of 85% N2, 10% H2, and 5% CO2. Randomly, 10% of the colonies from each plate (Chen et al. 2008) were transferred into MRS broth and incubated at 37 °C for 24 h in anaerobiosis. Gram-positive isolates with a rod, coccoid, and coccobacilli shape, and catalase-negative were selected as presumed LAB and purified by two subcultures in MRS agar and preserved in MRS added with 20% glycerol and stored at − 80 °C. The LAB was regularly cultured by inoculation in MRS broth with 1% v/v of a fresh stationary culture and incubation in anaerobic conditions at 37 °C for 18 h.
Genetic identification of LAB isolates
LAB isolates were identified at the species level by rRNA ARDRA (amplified ribosomal DNA restriction analysis) or, when necessary, by 16S rRNA gene sequencing as described by Sandes et al. (2014). Species-level identification was made using the Seqmatch algorithm (RDP—Ribosomal Database Project), considering the similarity of at least 98% identity in the multiple alignments (Maidak et al. 2000).
Bacterial lineages were clustered by rep-PCR (repetitive extragenic polymorphic-based polymerase chain reaction) fingerprinting, using the (GTG)5 primer (5′-GTG GTG GTG GTG GTG-3′) following the procedures described by Gevers et al. (2001).
In vitro criteria for selection of LAB probiotic candidates
Potentially probiotic microorganisms must meet selective criteria evaluated in in vitro testing to access antimicrobial activity, cell surface characteristics, and ability to adhere to epithelial host cells (Gaggìa et al. 2010). Therefore, a new probiotic bacterium for mastitis bacteriotherapy is desirable to have a hydrophobic cell surface and autoaggregation, produce hydrogen peroxide, and display antagonism against mastitis pathogens, only have intrinsically resistance to antibiotics, produce exopolysaccharides (EPS) and to form a biofilm, as well as to be non-pathogenic, non-toxigenic, and non-invasive (Silva et al. 2013; 2017).
LAB surface properties
Autoaggregation assays were adapted from Del Re et al. (2000). The LAB cultures in stationary phase were centrifuged, washed twice in PBS, and adjusted to an OD600 nm of 0.6 in PBS (A0). Then, LAB suspensions could stand for 4 h at room temperature. After that, an aliquot of the suspension was gently collected, and the OD600 nm was measured (At). Autoaggregation percentage is expressed as: [1 − (At/A0)] × 100, where At represents the absorbance at time t = 4 h and A0 represents the absorbance at t = 0. The level of autoaggregation was quantified, and the strains were classified as high (H: 67–100%), mid (M: 34–66%), or low (L: 0–33%) autoaggregative (Nader-Macías et al. 2008).
The ability of LAB to co-aggregate with mastitis pathogens, S. aureus ATCC 29,213 and Escherichia coli ATCC 25,922, was evaluated. The cell suspensions for co-aggregation assay were done in the same way as for autoaggregation assay. Equal volumes (0.5 mL) of each cell suspension were mixed in pairs by vortexing for 10 s. Control tubes were set up at the same time, containing 1 mL of each bacterial suspension on its own. OD600 nm (A) of the suspension was measured after 4-h incubation at room temperature. Co-aggregation percentage, for each pathogen, is expressed as follows: {[(Ax + Ay)/2 − A(x + y)] / (Ax + Ay/2)} × 100, where x and y represent each analyzed strains (x = LAB and y = pathogen), and (x + y) represents the absorbance data of the mixture of both. The degree of co-aggregation was quantified, and the strains classified according to the criteria established by Nader-Macías et al. (2008).
The hydrophobicity of the cellular surface was assessed by the microbial adhesion to the solvents (MATS) approach, described by Kos et al. (2003). The LAB cultures in stationary phase were centrifuged, washed twice in PBS, and adjusted to an OD600 nm of 0.6 with 0.1 M KNO3 pH 6.2 (A0). Next, xylene was added to the bacterial suspensions, forming a two-phase system. The aqueous phase was removed, and the OD600 nm was measured (A1). The MATS was calculated as the percentage of LAB associated with xylene according to the formula: [(A0 − A1/A0)] × 100. The hydrophobicity was quantified, and the strains were classified as high (H), middle (M), or low (L) hydrophobics (Nader-Macías et al. 2008). The adhesiveness capability of LAB strains, indirectly assessed by autoaggregation, co-aggregation, and cell surface hydrophobicity, was chosen because they are less time-consuming and less expensive.
LAB exopolysaccharide and biofilm production
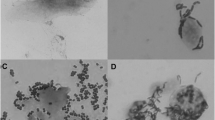
EPS production is described as an indication of biofilm formation on surfaces under natural conditions (Van der Meulen et al. 2007). The LAB cultures in stationary phase were seeded in Petri dishes containing modified MRS medium by replacing 20 g/L glucose with 80 g/L sucrose and Tween-20 free (Waldherr et al. 2010). EPS producers were identified by their slimy colony appearance.
LAB biofilm formation was verified according to Pérez-Ibarreche et al. (2014) with modifications. Basically, 200 μL of MRS medium was added to each well of 96-well polystyrene microplates (TPP, Trasadingen, Suisse). LAB cultures in stationary phase were used as inoculum (5% v/v) and incubated without shaking at 37 °C for 72 h in anaerobic conditions. The biofilm production was quantified after washing the wells with PBS and staining the attached bacteria for 30 min with 200 μL 0.1% (w/v) crystal violet in isopropanol-methanol-PBS solution (1:1:18, v/v/v). Excess stain was rinsed, the wells dried, and the dye bound to the adherent cells solubilized with 200 μL 30% (v/v) acetic acid. The OD570 nm of 135 μL of each well was measured. The sterile medium was included as a negative control to ensure that the influence on biofilm formation was not due to a non-specific binding of crystal violet. As a selection criterion for biofilm producer LAB, a cut-off OD of three standard deviations above the mean OD of the negative control was defined, and the strains were classified as high (H), mid (M), or low (L) biofilm producers according to the criteria established by Milanov et al. (2010).
LAB antimicrobial activity
Hydrogen peroxide (H2O2) is known to have a bactericidal effect against several pathogenic bacteria. H2O2 production was assessed following the assay described by Juárez Tomás et al. (2004). LAB strains in stationary phase were spotted onto TMB-plus MRS agar (Acumedia Neogen Corp., Lansing, USA) supplemented with 1 mM 3,3′,5,5′-tetramethylbenzidine (TMB, Sigma-Aldrich) and 2 U/mL horseradish peroxidase (Sigma-Aldrich), and incubated for 48 h at 37 °C in anaerobic conditions. After that period, the plates were exposed to atmospheric air for 10 min. Strains were qualitatively classified as high (H), mid (M), or low (L) H2O2 producers according to the blue color intensity of the colonies.
Detection of LAB antagonist effect over mastitis pathogens was performed by an agar double diffusion assay. Five microliters of LAB cultures in the stationary phase was spotted onto MRS agar and incubated in anaerobic conditions for 24 h at 37 °C. After, the cells were killed by exposure to chloroform vapor for 30 min. Twelve pathogenic bacteria causing mastitis (S. aureus and Streptococcus uberis clinical bovine mastitis isolates; S. agalactiae 8710, Listeria innocua 5830, S. capitis 8246, S. xylosus 8671, S. sciuri 8583, Listeria monocytogenes ATCC 15,313, S. aureus ATCC 29,213, S. agalactiae ATCC 1381, and E. coli ATCC 25,922) were cultured to the stationary phase in brain heart infusion (BHI; Acumedia Neogen Corp., Lansing, MI) medium. Except for ATCC strains, all pathogens used were isolated from bovine mammary gland samples. These microorganisms were inoculated in BHI soft agar, which was overlaid on the surface of MRS plates containing chloroform-killed LAB. After incubation at 37 °C for 24 h, the LAB antagonistic activity was determined by measuring the growth inhibition zone using a digital pachymeter (Mitutoyo Sul Americana Ltd, São Paulo, Brazil). Pathogens considered inhibited in this assay were those that had any visible inhibition zone in their growth around the LAB spot. LAB antagonism was expressed as percent antagonism, defined as the number of pathogens that were inhibited divided by the total number of pathogens tested, multiplied by 100, using the following formula: % antagonism = (X/Y) × 100, where X is the pathogen number inhibited by the LAB isolate and Y is the total number of pathogens tested in the antagonism assay.
Susceptibility to antimicrobials
The disc diffusion method was used to verify the sensitivity to antimicrobials of each LAB. Bacterial suspensions were adjusted to 108 viable cells according to the McFarland scale and spread onto MRS agar, and antibiotic discs were dispensed onto the agar surface using an antibiotic disc dispenser (amikacin 30 µg, ampicillin 45 µg, ceftriaxone 30 µg, chloramphenicol 30 µg, erythromycin 15 µg, oxacillin 1 µg, penicillin G 10 U, and vancomycin 30 µg; Oxoid Ltd, Basingstoke, UK). After incubation at 37 °C for 24 h, inhibition zones around the discs were measured using a digital pachymeter (Mitutoyo Sul Americana Ltd). The LAB strains were qualitatively classified as resistant, susceptible, or moderately susceptible strains according to the cut-off levels proposed by Charteris et al. (1998).
Presence of virulence factor genes for mastitis
The existence of agg, gelE, efa, cylA, and cad genes encoding virulence factors in the mastitis pathogens was evaluated in LAB by PCR. For this, 100 ng of total DNA from the strains was amplified using Master Mix PCR (Promega) and 1-μM primer pairs for each gene and cycling conditions described by Espeche et al. (2012). The amplicons were resolved in 1.4% agarose gel electrophoresis and visualized in UV transilluminator after staining with ethidium bromide. Samples that showed amplified products for any of the genes tested were sequenced to confirm the identity of the amplicons using the same primers used in the PCR, followed by the similarity search performed through of the BLASTn tool 2.125 from the National Centre of Biotechnology Information GenBank database (Altschul et al. 1997).
LAB adhesion and invasion to epithelial human MDA-MB-231 cell line
The established human MDA-MB-231 ATCC® HTB-26™ (MDA-MB-231) cell line was cultured in T25 cell culture flasks in RPMI 1640 (Sigma-Aldrich) with 10% heat-inactivated fetal calf serum (FCS). Cells were incubated at 37 °C in a humidified incubator with 5% CO2. They were cultured until reaching a confluence of 80%, detached treating with 0.05% trypsin (Sigma-Aldrich), and suspended in fresh RPMI 1640 medium at a concentration of 1.5 × 105 cells/mL.
Confluent monolayers of MDA-MB-231 (1.5 × 105 cells/well) were grown in 24-well cell culture plates (TPP) and inoculated with LAB at a ratio of interaction (ROI) of 100 LAB:1 cell. After 1 h of co-cultivation at 37 °C, in a CO2 incubator with 5% CO2, cells were washed three times with pre-warmed PBS. After that, the monolayers were treated with 0.25% trypsin (Sigma-Aldrich) and lysed with 1 mL of 0.1% Triton X-100 in PBS for 10 min. Bacteria were enumerated by the standard plating technique after lysates have been serially diluted in 0.9% saline and plated on MRS agar, and colonies were counted after incubation for 48 h at 37 °C. The adhered LAB was expressed as CFU/mL recovered from each well. This assay was done in quadruplicate for each LAB.
For quantification of internalized LABs in MDA-MB-231 cells, confluent monolayers of this cell (~ 1.5 × 105 cells/well) were grown in 24-well cell culture plates (TPP) and were infected with LAB at a ROI of 100 LAB:1 cell. After 1 h of co-culture, the cells were washed 3 times with PBS preheated to 37 °C and then incubated for an additional 1 h with the complete RPMI 1640 medium supplemented with 100 µg/mL of streptomycin and 100 U/mL of penicillin (Sigma-Aldrich). Then, washes and cell lysis were performed, followed by plating of the lysate, as previously described. The internalized LAB was expressed as CFU/mL recovered from each well. This assay was done in quadruplicate for each LAB.
LAB interference on the S. aureus adhesion and invasion of human MDA-MB-231 cell line
Confluent monolayers of MDA-MB-231 (1.5 × 105 cells/well) were grown in 24-well cell culture plates (TPP) and were infected with LAB at a ROI of 100 LAB:1 cell and simultaneously with pathogenic bacteria at an infection rate (MOI, multiplicity of infection) of 10 pathogens:1 cell. The control of the experiment was carried out in wells infected with only one of the pathogens, without LAB. It was used as a pathogen S. aureus ATCC 2921. After 1 h of co-cultivation at 37 °C, in a CO2 incubator with 5% CO2, cells were washed three times with pre-warmed PBS. Then, the monolayers were washed and lysed by the procedures described for the assays using only LAB. The lysates were serially diluted in 0.9% saline and plated on mannitol agar (Acumedia) and the colonies were counted after incubating the plates for 48 h in a microbiological incubator at 37 °C. For the assay of LAB interference in S. aureus invasion in MDA-MB-231 cells, an additional step incubation was performed, after simultaneous co-infection of pathogen and LAB for 1 h, with complete medium RPMI 1640 supplemented with 100 µ/mL of streptomycin and 100 U/mL of penicillin (Sigma-Aldrich) for 1 h at 37 °C, in a CO2 incubator with 5% CO2, prior to cell lysis. Previously, it was found that LABs are unable to grow on mannitol agar, which is selective for the pathogenic bacteria (data not shown). The adhesion or invasion of S. aureus was expressed as CFU/mL of pathogen recovered from each well (controls and experimental, the latter also containing LAB). This assay was done in quadruplicate for each LAB.
Statistical analyses
GraphPad Prism® 6 software (GraphPad Software Inc.) was used for the statistical analyses of LAB isolation data, evaluation of the probiotic potential of LAB isolates by the in vitro tests, and the co-culture assays. Data obtained were tested by the Kolmogorov–Smirnov test to verify the normality of the distribution. The means, standard deviations, and coefficients of variation were obtained for each dataset. The data that presented a normal distribution were analyzed by the one-way ANOVA test for each dataset in each type of test, to verify if there were statistically significant differences between the variances, and the Tukey post-test of multiple comparisons, to check which means were significantly different (P < 0.05). For the data that did not present normal distribution, the Kruskal–Wallis test was used with the Dunn post-test, of multiple comparisons, with the same objectives. Also, correlation analyses between some of the potential probiotic characteristics were done using the Pearson correlation test, with variables considered to be significantly correlated with P < 0.05. Directly correlated variables showed a positive Pearson correlation coefficient signal, and those inversely correlated showed a negative Pearson correlation coefficient signal.
Results
Cows and milk quality
Forty cows had a milk SCC of more than 200,000 cells/mL (3 Guzerat, 13 Holstein, 11 Gyr, and 13 Girolando 1/2), corresponding to 35% of the total (115 animals). Overall, SCC and TBC mean counts were 456,790 ± 567,672 cells/mL and of 29,437 ± 39,186 CFU/mL, respectively. Among the 75 healthy animals, the mean SCC was 70,489 ± 78,510 and the mean TBC was 7572 ± 5387 CFU/mL, while cows with subclinical mastitis had mean SCC of 1,158,662 ± 959,983 and mean TBC of 69,364 ± 96,031 CFU/mL.
LAB counts in milk and intramammary swab samples
Lactic acid bacteria count in MRS agar ranged from 0 to 4.75 log10 CFU/mL and from 0 to 4.68 log10 CFU/swab, with average counts of 2.09 ± 1.3 log10 CFU/mL and 3.22 ± 1.04 log10 CFU/swab, in raw milk and intramammary swab samples, respectively. No statistically significant differences were found between the counts of animals from different farms, breeds, and udder health states.
LAB isolation and species identification
Three hundred and fourteen bacterial isolates with LAB typical colonies in MRS agar (211 swab; 103 milk) from 81 cows (9 Guzerat, 14 Holstein, 25 Gyr, and 33 Girolando 1/2) were obtained. Among these LABs, 45 (14.3%) have morphology of coccobacillus, 47 (15.4%) rod, and 222 (70.3%) coccus. To cluster LAB isolates of the same sampled animal, we done a rep-PCR fingerprinting analysis (GTG)5 and discarded 122 isolates (39%) representing replicated bacterial strain. Therefore, after this filtering, 192 isolates were submitted to the species-level identification based on the 16S rRNA gene analysis.
The 192 isolates were identified as belonging to 30 different LAB species with the following relative abundance: Pediococcus pentosaceus (36%), P. stilesii (0.5%), Enterococcus hirae (12%), E. camelliae (1.6%), E. casseliflavus/gallinarum (1.6%), E. faecalis (1.1%), E. italicus (1.1%), E. saccharolyticus (0.5%), E. faecium (0.5%), E. pseudoavium (0.5%), E. durans (0.5%), Lactococcus lactis (8.2%), L. garvieae (6.8%), Weissella paramesenteroides (7.8%), W. confusa (1.5%), W. cibaria (1.1%), Streptococcus lutetiensis (7.8%), S. bovis (1.1%), S. salivarius (1.1%), S. infantarius (1.1%), S. henryi (0.5%), S. gallolyticus (0.5%), S. equinus (0.5%), S. pseudoporcinus (0.5%), S. parasanguinis (0.5%), Lactobacillus plantarum (3.1%), L. pentosus (0.5%), L. mucosae (0.5%), L. brevis (0.5%), L. paracasei (0.5%).
A higher number of LAB species were recovered from raw milk (22 species) than to that got in swab (16 species). Also, the number of species exclusively isolated from raw milk was higher than those isolated only with swab, 14 and 8 species, respectively. Lactobacillus and Weissella strains were mainly recovered from raw milk, whereas Streptococcus and Pediococcus from a swab. Interestingly, 99% of the Pediococcus isolates were obtained in a single farm and from intramammary swab samples. On average, we got 7 to 10 different species in each farm or breed.
After molecular identification of the 192 isolates, their rep-PCR fingerprinting (GTG)5 patterns were again evaluated for the identification of isolates of the same species belonging to the same strain. Ninety-three different strains were identified. Eighty-one strains were obtained from the biological material of cows with SCC < 200,000 cells/mL and chosen for the characterization of their probiotic potential on in vitro tests.
LAB cell surface properties
The mean percentage of autoaggregation of the 81 LAB isolates was 42.45 ± 18.30%, ranging from 0 to 89.5%. Half of the strains showed low autoaggregation (53.66%), and only 18.29% of them were considered as displaying high autoaggregation. The mean percentage of co-aggregation of the 81 LAB strains with S. aureus ATCC 29,213 was 23.90 ± 20.75%, ranging from 0 to 87.6% and for E. coli ATCC 25,922 was 20.64 ± 18.64%, ranging from 0 to 74.9%. Most of the isolates presented low co-aggregation with S. aureus (74.39%) and also with E. coli (80.49%), and less than 5% of the strains were able to co-aggregate to a high level with any of these pathogens. The same trend was observed for the cell surface hydrophobicity. The mean hydrophobicity of the isolates was 16.65 ± 18.04%. Cell surface hydrophobicity ranged from 0 to 96.36%, and most of the strains presented low hydrophobicity (85.37%), and only 9.76% showed high hydrophobicity. The cell surface properties showed no distribution tendency according to the origin of the strain (milk or intramammary swab).
Significant positive correlation was found between LAB autoaggregation and co-aggregation with E. coli ATCC 25,922 (Pearson coefficient = 0.3141; P = 0.0041), between co-aggregation with S. aureus ATCC 29,213 and co-aggregation with E. coli ATCC 25,922 (Pearson coefficient = 0.6281, P < 0.0001), and between cell surface hydrophobicity and co-aggregation with S. aureus ATCC 29,213 (Pearson’s coefficient = 0.2225; P = 0.0445).
Production of biofilm by the LAB strains
Only 17 of 81 isolates were able to produce EPS in the MRS medium with 8% sucrose instead of glucose, evidenced by the viscous colonies. Among EPS-producing LAB, there were 12 Streptococcus, 4 Weissella, and one Enterococcus. Interestingly, most EPS-producing LAB strains (70.6%) were obtained from intramammary swab samples.
Approximately half of the 81 strains (52.44%) did not produce biofilm in the polystyrene plate assay. Of them, 35.37% were poor biofilm producers, 4.88% were moderate biofilm producers, and 7.32% were high biofilm producers. Among the isolates that were active producers, only W. confusa GIR48L1* and W. confusa GIRO21L1* were also EPS producers. Remarkably, the highest proportion of non-producer biofilm strains was from intramammary swab samples, whereas the high and moderate producers were predominantly obtained from milk samples.
A significant positive correlation was found between the ability of each isolate to produce biofilm and its cell surface hydrophobicity (Pearson coefficient = 0.4360, P < 0.0001) and also its co-aggregation with E. coli (Pearson coefficient = 0.3636, P = 0.0008). These findings mean that the higher the hydrophobicity and co-aggregation with E. coli of a LAB strain, the higher its ability to produce biofilm. Results for the correction analyses between cell surface properties and biofilm and EPS production of 81 evaluated LAB are summarized in Table 1.
LAB antimicrobial activity
Only 21.95% of the strains did not produce H2O2, displaying white colonies in TMB-plus agar. Among the LAB isolates, 31.71% were weak H2O2 producers, 34.15% moderate producers, and 12.20% strong producers. Distribution of strains according to the degree of autoaggregation, co-aggregation with the pathogens, cell surface hydrophobicity, H2O2, EPS, and biofilm production are shown in Fig. 1.
Relative distribution (in %) of the 81 LAB strains by functional attributes of autoaggregation, co-aggregation with S. aureus ATCC 29,213, co-aggregation with E. coli ATCC 25,922, and cell surface hydrophobicity, ranked as low (0–33%), mid (34–66%), and high (67–100%) level for the assessed feature as established by Nader-Macías et al. 2008. H2O2 production evaluated in TMB-plus medium containing 3,3′,5,5′-TMB and peroxidase. EPS secretion and biofilm formation, according to criteria established by Milanov et al. 2010. Nil means not detected
Antagonism of the 81 LAB strains against bacterial pathogens associated with mastitis etiology was done by a double-layer diffusion method using 11 pathogenic bacteria species associated with cases of intramammary infection. Most of the strains showed antagonism against at least eight revealing pathogens (75%), with 35% of the LABs able to inhibit the growth of all the pathogens. Using the variable percentage of antagonism, defined as the number of pathogens that were inhibited by the LAB isolates divided by the total number of revealing pathogens and multiplied by 100, the mean percentage of antagonism of the 81 LAB was 87%.
LAB susceptibility to antibiotics
Concerning antibiotic susceptibility, it is recommended that microorganisms used as probiotics lack antimicrobial resistance genes on mobile genetic elements. Most strains were sensitive or moderately sensitive to the ampicillin, amoxicillin, ceftriaxone, erythromycin, and penicillin G, which are the drugs commonly used in intramammary infusions for the treatment of mastitis. The antibiotic microbial susceptibility of the 81 LAB strains is shown in Table S2.
Presence of virulence factor genes for mastitis
Among the LAB strains, 71 (87%) did not present any virulence factor–encoding gene for mastitis. Among the 10 strains that showed the presence of at least one gene, E. faecalis GIRO33L2* was the only one to present four genes encoding virulence factors (agg, gelE, efa, and cad) and most of the others had only one virulence gene. The description of the strains showing virulence genes is shown in Table S3.
The individual values for all probiotic characteristics in vitro tested are shown in Table S1, except the antibiotic susceptibility, antagonism to pathogens, and the presence of virulence factor genes for mastitis.
Probiotic criteria for selection of LAB strains to interact with mammary cells
According to the requirements proposed by EFSA (2005; 2007), Espeche et al. (2009), FAO/WHO (2002), Gaggìa et al. (2010), HC (2006), and Saarela et al. (2000), the LAB lineages to be selected for studies as probiotic candidates for the mammalian ecosystem should not be a pathogen associated with mastitis cases, to be isolated from healthy quarters, inhibit as many pathogens as possible in screening tests, have high or medium cell surface hydrophobicity and autoaggregation, and should not have virulence factors associated with mastitis. Added to this, the production of EPS or biofilm is desirable but not mandatory characteristics. The antimicrobial susceptibility should reflect only intrinsic, chromosomal-associated markers to rule out the risk of lateral gene transfer of them to commensal and pathogens present in the mammary gland (Calvinho et al. 2002). Therefore, among the 81 LAB strains, ten were selected for the binding assays in a co-culture with human MDA-MB-231 breast epithelial cells. These strains belong to different LAB genera and have different qualities among the several parameters desirable in probiotics (Table 2).
LAB adhesion and invasion to epithelial, human breast cancer MDA-MB-231 cell line
Adhesion of the ten LAB selected isolates was evaluated in a co-culture assay with MDA-MB-231 cells and was expressed as CFU/mL of cell lysate recovered from each well. Among ten LABs, the population of adhered bacteria ranged from 0 to 2.75 × 106 CFU/mL of cell lysate with an average of 7.57 × 106 ± 7.27 × 105 CFU/mL of cell lysate. In comparison to the total of LAB added to the cell monolayer (107 CFU), approximately 102 CFU of LAB were unable to adhere to MDA-MB-231 cells. Two isolates (WciGIRO27L2 *—W. cibaria GIRO27L2 *; StrelutGIR25S5—S. lutetiensis GIR25S5) showed significantly lower levels of adhesion (P < 0.05) compared to the others. StrelutGIR25S5 isolate, in addition to having the lowest adhesion capacity, showed difficulty in growing under aerobic conditions and was therefore excluded from the other co-cultivation tests (data not shown).
Among the remaining nine LABs, the internalized population bacteria ranged from 0 to 5.00 × 104 CFU/mL cell lysate with an average of 3.72 × 103 ± 7.01 × 103 CFU/mL cell lysate. In comparison to the total of LAB added to the cell monolayer (107 CFU), approximately 104 CFU of LAB were unable to enter the MDA-MB-231 cells. Comparing the levels of adherence and internalization, there was an average reduction of 8.37 × 105 ± 7.15 × 105 CFU/mL of cell lysate in the total of adhered bacteria that were not able to enter the mammary epithelial cells. Interestingly, nine LABs showed two significantly different behaviors (P < 0.05) with respect to the internalization capacity, which seems to be lineage-dependent. Five isolates (PstiHOL36L1*—P. stilesii HOL36L1*; LactGIRO4S8*—L. lactis GIRO4S8*; WparGIR46L4*—W. paramesenteroides GIR46L4*; WcoGIRO48L1*—W. confusa GIR48L1*; and StrelutHOL36l2*—S. lutetiensis HOL36L2*) with an internalization average of 6.59 × 103 ± 8.66 × 103 CFU/mL of cell lysate were significantly more efficient in invading MDA-MB-231 cells than the others (WcoGIRO21L1*—W. confusa GIRO21L1*; WciGIRO27L2*—W. cibaria GIRO27L2*; LplanGUZ3L2*—L. plantarum GUZ3L2*; and LparaGIR53L1*—L. paracasei GIR53L1*) with internalization average of 1.29 × 102 ± 9.54 × 10 CFU/mL of cell lysate. LAB adhesion and invasion to MDA-MB-231 cell line are shown in Fig. 2, except for the isolate StrelutGIR25S5 that was excluded from invasion assays.
Adhesion of LAB strain to MDA-MB-231 cells/well at a 100:1 ROI for 1 h and invasion after 2 h. Data are the mean and error deviation of adhered bacteria, shown as LAB CFU/mL cell lysate. Each assay was done in quadruplicate. Differences between the groups were evaluated using one-way ANOVA followed by Tukey’s post-test. Statistically significant (P < 0.05) differences adhered and invaded counts are shown in the figure with a and b, respectively. Strain codes: Pediococcus stilesii HOL36L1* (PstiHOL36L1*); Lactococcus lactis GIRO4S8* (LactGIRO4S8*); Weissella paramesenteroides GIR46L4* (WparGIR46L4*); Weissella confusa GIR48L1* (WcoGIRO48L1*); Weissella confusa GIRO21L1* (WcoGIRO21L1*); Weissella cibaria GIRO27L2* (WciGIRO27L2*); Lactobacillus plantarum GUZ3L2* (LplanGUZ3L2*); Lactobacillus paracasei GIR53L1* (LparaGIR53L1*); Streptococcus lutetiensis HOL36L2* (StrelutHOL36L2*)
LAB interference on the S. aureus ATCC 29,213 adhesion and invasion of human MDA-MB-231 cell line
Evaluation of the LAB interference under the adhesion and invasion of S. aureus ATCC 29,213 was carried out in simultaneous co-culture assays in MDA-MB-231 cells and was expressed as a pathogen CFU/mL of cell lysate recovered from each well. S. aureus ATCC 29,213 showed adherence average levels of 6.59 × 104 ± 1.56 × 104 CFU/mL of cell lysate in a control well. When evaluating the effect of nine LABs tested on interfering with S. aureus ATCC 29,213 adhesion, no statistically significant differences (P > 0.05) were detected in relation to the control, without LAB. S. aureus ATCC 29,213 presented invasion average levels of 1.49 × 104 ± 5.20 × 103 CFU/mL of cell lysate in control wells. However, some of the nine LABs evaluated were able to interfere in S. aureus ATCC 29,213 invasion, impairing the entry of pathogen in cell. LactGIRO4S8* (L. lactis GIRO4S8*), WparGIR46L4* (W. paramesenteroides GIR46L4*), and LparaGIR53L1* (L. paracasei GIR53L1*) were able to significantly reduce (P < 0.05) the invasion of the pathogen compared to the control, without LAB. The most effective isolate in reducing the invasion of S. aureus ATCC 29,213 was LparaGIR53L1*, which showed an average reduction of 88.10 ± 4.03% in the invasion of this pathogen. The data from LAB interference on the S. aureus ATCC 29,213 adhesion and invasion of MDA-MB-231 cell line are shown in Fig. 3.
Adhesion of S. aureus ATCC 29,213 to MDA-MB-231 cells at 100:1 ROI for 1 h and invasion after 2 h. Data are the mean and error deviation of adhered or internalized bacteria, shown as log10 of S. aureus CFU/mL cell lysate. Each assay was done in quadruplicate. Differences between the groups were evaluated using one-way ANOVA followed by Tukey’s post-test. No significant differences (P > 0.05) were found in the effect of LAB on the adhesion of S. aureus ATCC 29,213. Statistically significant differences in invaded counts compared to the control without LAB are shown in the figure with * (P ≤ 0.05). Strain codes: Pediococcus stilesii HOL36L1* (PstiHOL36L1*); Lactococcus lactis GIRO4S8* (LactGIRO4S8*); Weissella paramesenteroides GIR46L4* (WparGIR46L4*); Weissella confusa GIR48L1* (WcoGIRO48L1*); Weissella confusa GIRO21L1* (WcoGIRO21L1*); Weissella cibaria GIRO27L2* (WciGIRO27L2*); Lactobacillus plantarum GUZ3L2* (LplanGUZ3L2*); Lactobacillus paracasei GIR53L1* (LparaGIR53L1*); Streptococcus lutetiensis HOL36L2* (StrelutHOL36L2*)
Discussion
A human is the unique mammal species that continue to ingest milk after weaning, and they use milk from other animals as food, having to create them for this purpose. Currently, dairy farming represents an important sector of the economy of countries, and mastitis is the main pathology that affects farmers and generates the most considerable losses to this sector (Capper and Bauman 2013). Subclinical mastitis is the absence of clinical signs of infection in animals presenting SCC > 200,000 cells/mL (NMC 2001). A high incidence of this disease is reported in Brazil, with rates varying from 11.9 to 58.8% of infected cows (Pereira et al. 2001). In general, the different cow breeds studied here had subclinical mastitis rates between 16.7 and 44.9%, and the lowest incidence was seen in the Guzerat race. This breed is resistant to infection by endo- and ectoparasites and tolerant to thermal stress (Penna et al. 2014), which explains the low rate of infected animals and a mean SCC of 100,000 cells/mL.
Besides SCC, the microbial load is an essential characteristic of animal health, milk quality, and technological capacity. In this work, bacterial counts on MRS agar ranged from 0 to 4.75 log10 CFU/mL of milk and swab. Samples from 14 animals (12.17%) showed no bacterial growth in this medium. Similarly, total bacterial counts in the bovine milk ranged from 3.4 to 6.2 log10 CFU/mL of milk collected in Italy, Argentina, Germany, and Austria (Espeche et al. 2012; Franciosi et al. 2009; Fricker et al. 2011). Although MRS medium is used for LAB isolation, bacterial counts obtained in it were not named here as LAB counts, as described by other authors (Espeche et al. 2012; Ouadghiri et al. 2008), since different types of bacteria can ferment the carbohydrates present in their formulation and grow in MRS (De Man et al. 1960).
LAB isolates obtained in this work were mainly cocci. This cocci dominance over the rods in the milk is well documented in the literature (Wouter et al. 2002). In general, the types and quantities of LAB isolated in this work agree with previously reported data for bovine milk (Quigley et al. 2013). To reduce the number of bacterial strains by clustering the same isolates, a rep-PCR (GTG)5 fingerprinting was done, discarding 122 (39%) isolates that represented a replicate of another one obtained from the same animal. This methodology was used in several studies of LAB from bovine milk samples and has been a usual approach (Bouchard et al. 2015; Franciosi et al. 2009; Ouadghiri et al. 2008).
Pediococci are associated with the surface of vegetables, mainly maize and sugarcane. During the ensiling process, these bacteria are incorporated and influence the fermentation process (Cai et al. 1999). In this sense, P. pentosaceus and P. acidilactici are considered dominant members of the microbiota of forage silage. Some studies have reported the isolation of Pediococcus spp. from milk and derivatives (Franciosi et al. 2009; Pogacic et al. 2013). In this work, 69 isolates were identified as P. pentosaceus, but 98.5% of them from a single farm, all from swab samples. Considering that the swab may isolate members of the microbiota from the roof channel and considering that corn and sugarcane silage were used in this farm, it is possible to speculate that the isolates of P. pentosaceus represent environmental contamination of the udder with microorganisms present in the silage and are not members of the microbiota residing in this ecosystem.
Autoaggregation is the ability of bacteria to form aggregates with others of the same strain in suspension (Espeche et al. 2012). Half of the isolates obtained here presented low autoaggregation and cell surface hydrophobicity. Most of the isolates from milk samples (89.2%) had low autoaggregation and cellular hydrophobicity values, and none showed high values of these characteristics among LABs isolated from two studies in Argentina (Espeche et al. 2009; 2012). In turn, co-aggregation is the ability of bacteria to form aggregates with other genetically different bacteria in suspension (Ekmekci et al. 2009). In the present study, almost 80% of LAB strains showed low co-aggregation capacity with pathogens, a trend reported by other authors (Espeche et al. 2009; 2012). It appears that low autoaggregation, co-aggregation with pathogens, and hydrophobicity are typical characteristics of the cell surface of microorganisms of various bovine niches, such as the gastrointestinal tract, vaginal mucosa, milk, and the roof canal (Bouchard et al. 2015; Nader-Macías et al. 2008).
Correlation between individual values of co-aggregation, autoaggregation, and hydrophobicity indicated an overlap of surface molecules involved in these characteristics. The hydrophobic nature of the outer surface of microorganisms facilitates the adhesion of bacteria to the host and confers competitive advantage during colonization (Vinderola and Reinherimer 2003). Espeche et al. (2012) reported a low Pearson coefficient value between cell hydrophobicity and LAB autoaggregation, showing that these characteristics are not correlated. However, LAB strains having a low cell hydrophobicity may still form hydrophilic autoaggregates, since they may have glycoproteins or glycosylated peptides on their surface (Otero et al. 2006). A significant correlation between the co-aggregation of LAB and S. aureus with the LAB cell hydrophobicity indicates that hydrophobic molecules are involved in LAB binding with this pathogen (Espeche et al. 2012). Opposing, the correlation between the co-aggregation of LAB and E. coli with the LAB autoaggregation suggests that in this co-aggregation, the presence of polysaccharides, lipoteichoic acid, and other hydrophilic substances is relevant (Reniero et al. 1991). Interestingly, the correlation between LAB co-aggregation capacity with both pathogens indicates that even involving different molecules, some significant level of molecular overlap must exist, but more detailed studies are needed to clarify these findings.
Among the 81 LAB strains, seventeen produced EPS. Other studies have demonstrated that LAB isolated from milk does not present EPS production in vitro screening tests (Espeche et al. 2012; Franciosi et al. 2009). The EPS is an essential determinant in the adherence of bacteria to host cells or inert surfaces (Milanov et al. 2010) and provides higher protection against opsonization and phagocytosis (Arslan and Özkardes 2007). Therefore, it is not surprising that most of the EPS isolates originated from swab samples, which collected bacteria that were adhered to the mucosa of the cow ceiling channel. Biofilm is defined as a sessile microbial community characterized by cells that are irreversibly attached to a substrate, interface, or each other, by a matrix of polymeric extracellular substances that they have produced (Donland and Costerton 2002). Among the 81 LAB isolates, seven were classified as active biofilm producers, and half of all the strains did not show the capacity to produce it. This finding is the first work reported in the literature that evaluates the biofilm production potential of LABs isolated from the mammary gland.
An exciting result of this work was that most of the LAB that presented a strong or moderate capacity of biofilm production originated from milk samples. Also, 53% of EPS-producing isolates did not produce biofilm and, at the same time, between LABs that are strong or moderate biofilm producers, only 24% produced EPS. During biofilm formation, the intercellular space of the microbial aggregates is filled by extracellular polymeric substances, such as EPS, which form the biofilm matrix (Vu et al. 2009). Therefore, EPS production should be directly associated with biofilm production. However, this trend was not verified in this study. The positive result for the production of EPS does not necessarily imply that isolate is a major producer of biofilm, in a test using polystyrene plates, as also evidenced by the publications of Milanov et al. (2010), Oliveira et al. (2006), and Vaseduvan et al. (2003). Other studies indicate that the expression of certain types of protein (such as ESP and BAP proteins) on the surface of bacteria seems to have an extremely significant and probably more important effect than the production of EPS in the formation of biofilm on inert surfaces such as polystyrene (Cucarella et al. 2001; Toledo-Arana et al. 2001).
The significant positive correlation between the individual LAB capacity to produce biofilm on polystyrene plates and cell surface hydrophobicity and the negative relationship between LAB-E. coli co-aggregation was seen in this work. This finding indicated that the hydrophobic nature of the LAB cell surface contributes to biofilm production. Clinical isolates of Pseudomonas spp. with a hydrophobic cell surface showed a higher rate of biofilm formation and accumulation of biomass (Drenkard and Ausubel 2002). Regardless of the association between biofilm production in polystyrene and EPS secretion, LAB exhibiting at least one of these characteristics may be necessary for its survival in hostile environments and colonization of mucosal surfaces (Milanov et al. 2010).
Antimicrobial susceptibility is an important criterion for the selection of probiotic strains due to the risk of lateral genetic transfer of resistance markers to commensal and pathogenic members of the host indigenous microbiota. Although the LAB is resistant to some antibiotics (Mathur and Singh 2005), strains that are found in foods are typically susceptible to main antibiotics of clinical use such as ampicillin, penicillin, and gentamicin (Herreros et al. 2005). Some resistance patterns appear to be lineage-specific or species-specific, but not an appropriate standard for LAB classification has been proposed (Silva et al. 2013). LAB has chromosome resistance to bacitracin, cephalothin, ciprofloxacin, oxacillin, amikacin, kanamycin, gentamycin, metronidazole, nitrofurantoin, norfloxacin, streptomycin, and vancomycin (Danielsen and Wind 2003). In general, this intrinsic resistance profile was seen in the LAB of this work.
Production of antimicrobial compounds such as bacteriocins, organic acids, and H2O2 explains the antagonism of LAB against some pathogens (Fernández et al. 2008). Most LAB isolates in this work were H2O2 producers, different from other studies that showed low prevalence or even absence of H2O2-producing LAB in this niche in cattle (Bouchard et al. 2015; Espeche et al. 2009). In the vaginal tract of adult women, the production of H2O2 by LAB isolates is associated with healthy women. Therefore, this evidence indicates that LABs present in healthy mammary and vaginal mucosa producing H2O2 can produce a protective effect against the establishment of infections (Patterson et al. 2008). Most of the LAB strains evaluated in this study (75%) presented antagonism against at least eight mastitis pathogens. In another study, only 21% of LAB isolates obtained from the cow udder were able to inhibit more than three pathogens associated with the etiology of mastitis (Espeche et al. 2009).
According to criteria proposed by EFSA (2005; 2007), Espeche et al. (2009), FAO/WHO (2002), Gaggìa et al. (2010), HC (2006), and Saarela et al. (2000), we selected potentially probiotic LABs to evaluate their adhesion capacity to mammary epithelial cells. Therefore, L. garvieae isolates have been ruled out since they have been associated with cases of mastitis and endocarditis (Collins et al. 1983; Zuily et al. 2011), as well as S. bovis, which is considered the etiological agent of breast infections. Enterococcus isolates were also discarded due to heated discussion about their safe use as probiotics. Given these facts, ten LAB isolates that met the safety requirements for use in mastitis bacteriotherapy were selected and evaluated in co-culture assays with MDA-MB-231 breast epithelial cells.
The results showed that among the ten LAB strains, eight had suitable adhesion levels in MDA-MB-231 breast epithelial cells, with a mean adhesion of 7.57 × 106 CFU/mL of cell lysate. The strains WciGIRO27L2* and StrelutGIR25S5 had significantly lower adherence compared to the others. Similar adhesion levels were found in co-cultivation trials of L. brevis 1613, 1595, and 1597 Lactococcus lactis V7 on MAC-T mammary epithelial cells (Assis et al. 2015; Bouchard et al. 2015). Lactococcus lactis CRL 1655 and Lactobacillus perolens CRL 1724 were observed adhered to bovine theta channel epithelial cells (BTCEC) on examination by optical microscopy (Gram stain) (Pellegrino et al. 2019). The integrity of the monolayers and the viability of the MDA-MB-231 cells were not affected by the incubation with the LAB, without changes in their morphology, and no significant differences in their viability as detected in MTT assay (data not shown). This finding is consistent with other reports of the absence of deleterious effects of L. casei strains on cell viability (Bouchard et al. 2013) and L. perolens on membrane morphology of mammary epithelial cells (Frola et al. 2012).
Internalization or invasion capacity of LAB in eukaryotic cells is still not well documented (Guimaraes et al. 2006), different from the adhesion ability (Bouchard et al. 2013). Invasion assays show that the nine LABs tested were able to enter the MDA-MB-231 mammary epithelial cells, with an average invasion rate of 3.72 × 103 CFU/mL cell lysate, and that two significantly different internalization profiles (P < 0.05) were found, apparently lineage-dependent. L. lactis V7 had an invasion rate of 4.90 log10 of CFU (Assis et al. 2015) of the magnitude of the isolates that more efficiently invaded MDA-MB-231 cells reported here. Bouchard et al. (2015) showed that the capacity for internalization was more strongly affected by the type of bacterial lineage than the capacity for adherence, even reporting values of up to 3 log10 CFU of difference between the strains evaluated.
A high adhesion capacity in eukaryotic cells demonstrates the ability of a LAB to compete with pathogens by the colonization of host tissues (Bouchard et al. 2015). This capacity is important in the mammalian ecosystem since intense milk flow occurs during the lactation period (Tamilselvam et al. 2006). In fact, different proteins involved in adherence to mucus, fibronectin, and collagen present in the S-layer or involved in the biosynthesis of polysaccharides seem to be determinants in the adhesion of LAB to epithelial cells (Lebeer et al. 2010; Sengupta et al. 2013; Turpin et al. 2012). The focus of LAB internalization studies in eukaryotic cells assesses their role as vehicles for intracellular delivery of molecules of interest (Innocentin et al. 2009). The presence of sequences with conserved domains homologous to the internalin-J of L. monocytogenes in the genome of five invasive LABs of breast cells points to a possible role of this type of molecule in the internalization of LAB (Bouchard et al. 2015). In addition, fibronectin-binding proteins have been identified in the genome of Lactobacillus species, which suggests that they may also be involved in the ability to adhere and internalize LABs in mammary gland cells (Castaldo et al. 2009; Lorca et al. 2002). However, internalization capacity of LABs must be analyzed with caution since the risks and effects of invasion in other tissues of the host are not yet well known.
Staphylococcus aureus and other mastitis-causing bacteria have a high capacity for adhesion and invasion to mammary epithelial cells in co-culture assays (Assis et al. 2015; Bouchard et al. 2013). In the nine LAB interference tests of S. aureus ATCC 29,213 invasion, were found strains here that significantly reduced (P < 0.05) these parameters. L. casei 667 and L. lactis V7 were able to reduce the adhesion of S. aureus by 40% and 75%, respectively, in addition to both reducing the invasion of different S. aureus pathogenic strains by 45 to 88% in MAC-T cells (Assis et al. 2015; Bouchard et al. 2013). It is worth noting at this point that these authors did not publish the counts (expressed here in CFU/mL of cell lysate) obtained in the adhesion inhibition and pathogen invasion experiments, expressing their data only as a percentage of adhesion or invasion. Data from counts in CFU/mL of cell lysate recovered from each well appear to be a more robust way of analyzing the phenomenon of inhibiting pathogen adhesion or invasion in co-cultured assays. For this reason, the data on the reduction of S. aureus ATCC 29,213 invasion by the LABs reported here are statistically robust and are worthy of a better evaluation in in vivo models.
One of the main ways in which pathogens can evade the immune response is through invasion into the host cells, a well-characterized mechanism for S. aureus in mastitis (Almeida et al. 1996). Therefore, adhesion and invasion of host cells by pathogens are critical steps for the development of mastitis and so evaluation of the inhibition of these processes by the administration of potentially probiotic bacteria is essential (Bouchard et al. 2013). Few studies have been reported investigating the ability of LAB to interfere with the invasion of pathogenic bacteria in host cells (Assis et al. 2015; Bouchard et al. 2013; Campana et al. 2012). The inhibition of pathogen adhesion and invasion seen in this and other studies can be attributed to the competitive exclusion mechanism, described as an important beneficial effect conferred by probiotics to the host (Bouchard et al. 2013). In fact, the ability to inhibit pathogen adhesion and invasion is a criterion often used in the selection of LAB candidates for bacteriotherapy against intestinal pathogens, such as L. monocytogenes (Garriga et al. 2014; Lavilla-Lerma et al. 20132014). Even though the probiotic strains evaluated here come from cattle, in this first screening, human mammary epithelial cells MDA-MB-231 were used due to their ease of cultivation. However, future studies on bovine mammary epithelial cells are needed. The findings reported here justify future work using the Lactococcus lactis GIRO4S8* (LactGIRO4S8*), Weissella paramesenteroides GIR46L4* (WparGIR46L4*), and Lactobacillus paracasei GIR53L1* (LparaGIR53L1*) strains in in vitro and in vivo models to better assess their probiotic potential in the therapy and/or prevention of bovine mastitis. In the future, these isolates may be used in the formulation of probiotic products to be administered to dairy cows in external and/or intramammary formulations in the prevention and treatment of cases of mastitis (Paduch et al. 2020).
In conclusion, this work identified and characterized the probiotic potential of 81 LABs from cow mammalian ecosystem sampling milk and gland duct of 115 Gyr, Guzerat, Holstein, and Girolando 1/2 cows in southeastern of Brazil. From this collection, 10 LAB strains with different promising probiotic characteristics were selected for adhesion with breast epithelial cells. In these assays, 8 LAB isolates showed satisfactory adhesion results in mammary epithelial cells. Obviously, these isolates require further investigation into their protective, immunomodulating, and therapeutic effects in the mammalian ecosystem. However, the findings reported here are of relative importance, as they open space for an accurate evaluation of the use of these potentially probiotic LAB, as well as their feasibility for prevention or as an adjuvant in the intramammary treatment of mastitis under field conditions.
Data availability
Please contact author for data requests.
References
Abdullah SA, Osman MM (2010) Isolation and identification of lactic acid bacteria from raw cow milk, white cheese and rob in Sudan. Pak J Nutr 9:1203–1206. https://doi.org/10.3923/pjn.2010.1203.1206
Akers RM, Nickerson SC (2011) Mastitis and its impact on structure and function in the ruminant mammary gland. J Mammary Gland Biol Neoplasia 16:275–289. https://doi.org/10.1007/s10911-011-9231-3
Almeida RA, Matthews KR, Cifrian E, Guidry AJ, Oliver SP (1996) Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci 79:1021–1026. https://doi.org/10.3168/jds.S0022-0302(96)76454-8
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Arroyo R, Martín V, Maldonado A, Jiménez E, Fernández L, Rodríguez JM (2010) Treatment of infectious mastitis during lactation: antibiotics versus oral administration of lactobacilli isolated from breast milk. Clin Infect Dis 50(12):1551–1558. https://doi.org/10.1086/652763
Arslan S, Özkardes F (2007) Slime production and antibiotics susceptibility in staphylococci isolated from clinical samples. Mem Inst Oswaldo Cruz 102(1):29–33. https://doi.org/10.1590/S0074-02762007000100004
Assis BS, Germon P, Silva AM, Even S, Nicoli JR, Le Loir Y (2015) Lactococcus lactis V7 inhibits the cell invasion of bovine mammary epithelial cells by Escherichia coli and Staphylococcus aureus. Benef Microbes 6(6):879–886. https://doi.org/10.3920/BM2015.0019
Beecher C, Daly M, Berry DP, Klostermann K, Flynn J, Meaney W, Hill C, Mccarthy TV, Ross RP, Giblin L (2009) Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1 b and IL-8 gene expression. J Dairy Res 76:340–348. https://doi.org/10.1017/S0022029909004154
Begum MR, Anaruzzaman M, Khan MSI, Yousuf M (2014) Factors affecting the milk production of dairy cattle in northern rural areas of Bangladesh. Int J Agric Innov Res 4(2):41–45. https://doi.org/10.3329/ijarit.v4i2.22646
Bouchard DS, Rault L, Berkova N, Le Loir Y, Even S (2013) Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei. Appl Environ Microbiol 79(3):877–885. https://doi.org/10.1128/AEM.03323-12
Bouchard DS, Seridan B, Saraoui T, Rault L, Germon P, Gonzalez-Moreno C, Nader-Macias FM, Baud D, François P, Chuat V, Chain F, Langella P, Nicoli J, Le Loir Y, Even S (2015) Lactic acid bacteria isolated from bovine mammary microbiota: potential allies against bovine mastitis. PLoS ONE 10:12. https://doi.org/10.1371/journal.pone.0144831
Cai Y, Kumai S, Ogawa M, Benno Y, Nakase T (1999) Characterization and identification of Pediococcus species isolated from forage crops and their application for silage preparation. Appl Environ Microbiol 65(7):2901–2906. https://doi.org/10.1128/AEM.65.7.2901-2906
Calvinho LF, Toselli FG, Weimann WR, Canavesio VR, Neder VE, Iguzquiza IA (2002) Antimicrobial sensitivity of coagulase-positive staphylococcal strains isolated from bovine mastitis in the central dairy catchment area of Argentina. Rev Argent Microbiol 34:171–175
Campana R, Federici S, Ciandrini E, Baffone W (2012) Antagonistic activity of Lactobacillus acidophilus ATCC 4356 on the growth and adhesion/invasion characteristics of human Campylobacter jejuni. Curr Microbiol 64:371–378. https://doi.org/10.1007/s00284-012-0080-0
Capper JL, Bauman DE (2013) The role of productivity in improving the environmental sustainability of ruminant production systems. Annu Rev Anim Biosci 1(1):469–489. https://doi.org/10.1146/annurev-animal-031412-103727
Castaldo C, Vastano V, Siciliano RA, Candela M, Vici M, Muscariello L, Marasco R, Sacco M (2009) Surface displaced alfa-enolase of Lactobacillus plantarum is a fibronectin binding protein. Microb Cell Fact 8:14. https://doi.org/10.1186/1475-2859-8-14
Charteris WP, Kelly PM, Morelli L, Collin JR (1998) Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot 61(12):1636–1643
Chen H, Wanga S, Chena M (2008) Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol 25:492–501. https://doi.org/10.1016/j.fm.2008.01.003
Collins MD, Farrow JA, Phillips BA, Kandler O (1983) Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J Gen Microbiol 129:3427–3431. https://doi.org/10.1099/00221287-129-11-3427
Contreras GA, Rodríguez JM (2011) Mastitis: comparative etiology and epidemiology. J Mammary Gland Biol Neoplasia 16:339–356. https://doi.org/10.1007/s10911-011-9234-0
Crispie F, Alonso-Gómez M, Óloughlin C, Klostermann K, Flynn J, Arkins S, Meaney W, Ross RP, Hill C (2008) Intramammary infusion of a live culture for treatment of bovine mastitis: effect of live lactococci on the mammary immune response. J Dairy Res 75:374–384. https://doi.org/10.1017/S0022029908003385
Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penades JR (2001) Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol 183(9):2888–2896. https://doi.org/10.1128/JB.183.9.2888-2896.2001
Dalton JC (2006) Antibiotic residue prevention in milk and dairy beef. Western Dairy News 6:79
Danielsen M, Wind A (2003) Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol 82(1):1–11. https://doi.org/10.1016/s0168-1605(02)00254-4
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Microbiol 23(1):130–135. https://doi.org/10.1111/j.1365-2672.1960.tb00188.x
Del Re B, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31:438–442. https://doi.org/10.1046/j.1365-2672.2000.00845.x
Donland RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193. https://doi.org/10.1128/cmr.15.2.167-193.2002
Drenkard E, Ausubel FM (2002) Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416(6882):740–743. https://doi.org/10.1038/416740a
Ducatelle R, Eeckhaut V, Haesebrouck F, Van Immerseel F (2015) A review on prebiotics and probiotics for the control of dysbiosis: present status and future perspectives. Animal 9(1):43–48. https://doi.org/10.1017/S1751731114002584
Ekmekci H, Aslim B, Ozturk S (2009) Characterization of vaginal lactobacilli coaggregation ability with Escherichia coli. Microbiol Immunol 53(2):59–65. https://doi.org/10.1111/j.1348-0421.2009.00115.x
Espeche MC, Otero MC, Sesma F, Nader-Macias MEF (2009) Screening of surface properties and antagonistic substance s production by lactic acid bacteria isolated from the mammary gland of healthy and mastitic cows. Vet Microbiol 135:346–357. https://doi.org/10.1016/j.vetmic.2008.09.078
Espeche MC, Pellegrino M, Frola I, Larriestra A, Bogni C, Nader-Macías MEF (2012) Lactic acid bacteria from raw milk as potentially beneficial strains to prevent bovine mastitis. Anaerobe 18:103–109. https://doi.org/10.1016/j.anaerobe.2012.01.002
European Food Safety Authority (EFSA) (2005) Opinion of the scientific committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J 226:1–12
European Food Safety Authority (EFSA) (2007) Introduction of a qualified presumption of safety (QPS) approach for assessment of selected microorganisms referred to EF SA Opinion of the Scientific Committee (Question No EFSA - Q- 2005–293) Adopted on 19 November 2007. EFSA J 58:1–16
Falentin H, Rault L, Nicolas A, Bouchard DS, Lassalas J, Lamberton P, Aubry JM, Marnet PG, Le Loir Y, Even S (2016) Bovine teat microbiome analysis revealed reduced alpha diversity and significant changes in taxonomic profiles in quarters with a history of mastitis. Front Microbiol 7:480. https://doi.org/10.3389/fmicb.2016.00480
Fernández L, Delgado S, Herrero H, Maldonado A, Rodríguez JM (2008) The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. J Hum Lact 24:311–316. https://doi.org/10.1177/0890334408317435
Food and Agricultural Organization of the United Nations/ World Health Organization (FAO/WHO) (2002) Report of a Joint FAO/WHO expert consultation on guidelines for the evaluation of probiotics in food. FAO/WHO of the United Nations, London, Ontario, Canada.
Franciosi E, Settanni L, Cavazza A, Poznanski E (2009) Biodiversity and technological potential of wild lactic acid bactéria from raw cow’s milk. Int Dairy J 19:3–11. https://doi.org/10.1016/j.idairyj.2008.07.008
Fricker M, Skånseng B, Rudi K, Stessl B, Ehling-Schulz M (2011) Shift from farm to dairy tank milk microbiota revealed by a polyphasic approach is independent of geographical origin. Int J Food Microbiol 145:24–33. https://doi.org/10.1016/j.ijfoodmicro.2010.08.025
Frola ID, Pellegrino MS, Espeche MC, Giraudo JA, Nader-Macias ME, Bogni CI (2012) Effects of intramammary inoculation of Lactobacillus perolens CRL1724 in lactating cows’ udders. J Dairy Res 79(1):84–92. https://doi.org/10.1017/S0022029911000835
Gaggìa F, Mattarelli P, Biavati B (2010) Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol 141:15–28. https://doi.org/10.1016/j.ijfoodmicro.2010.02.031
Garriga M, Rubio R, Aymerich T, Ruas-Madiedo P (2014) Potentially probiotic and bioprotective lactic acid bacteria starter cultures antagonise the Listeria monocytogenes adhesion to HT29 colonocyte-like cells. Benef Microbes 8:1–7. https://doi.org/10.3920/BM2014.0056
Gevers D, Huys G, Swings J (2001) Applicability of rep-PCR fingerprinting for identification of Lactobacillus species. FEMS Microbiol Lett 205(1):31–36. https://doi.org/10.1111/j.1574-6968.2001.tb10921.x
Guimaraes VD, Innocentin S, Lefevre F, Azevedo V, Wal JM, Langella P, Chatel JM (2006) Use of native lactococci as vehicles for delivery of DNA into mammalian epithelial cells. Appl Environ Microbiol 72:7091–7097. https://doi.org/10.1128/AEM.01325-06
Harmon RJ (1994) Physiology of mastitis and factors affecting somatic cell counts. J Dairy Sci 77:2103–2112. https://doi.org/10.3168/jds.S0022-0302(94)77153-8
Health Canada (HC) (2006) Evidence for safety and efficacy of finished natural health products. [online]. Ottawa (ON): Natural Health Products Directorate, Health Canada. Available at: http://www.hc-sc.gc.ca/dhpmps/prodnatur/legislation/docs/efe-paie_e.html.
Heikkilä MP, Saris PEJ (2003) Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol 95:471–478. https://doi.org/10.1046/j.1365-2672.2003.02002.x
Heikkilä AM, Nousiainen JI, Pyörälä S (2012) Costs of clinical mastitis with special reference to premature culling. J Dairy Sci 95(1):139–150. https://doi.org/10.3168/jds.2011-4321
Herreros M, Sandoval H, Gonzalez L, Castro J, Fresno J, Tornadijo M (2005) Antimicrobial activity and antibiotic resistance of lactic acid bacteria isolated from Armada cheese a Spanish goats’ milk cheese. Food Microbiol 22:455–459. https://doi.org/10.1016/j.fm.2004.11.007
Hunt KM, Foster JA, Forney LJ, Schütte UME, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA (2011) Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 6(6):e21313. https://doi.org/10.1371/journal.pone.0021313
Huovinen P (2001) Bacteriotherapy: the time has come. BMJ 323:353–354. https://doi.org/10.1136/bmj.323.7309.353
Innocentin S, Guimarães V, Miyoshi A, Azevedo V, Langella P, Chatel JM, Lefèvre F (2009) Lactococcus lactis expressing either Staphylococcus aureus fibronectin-binding protein A or Listeria monocytogenes internalin A can efficiently internalize and deliver DNA in human epithelial cells. Appl Environ Microbiol 75(14):4870–4878. https://doi.org/10.1128/AEM.00825-09
International Dairy Federation (IDF) (2013) The economic importance of dairying. Available at: www.fil-idf.org accessed in February 2013.
Jiménez E, Fernández L, Maldonado A, Martín R, Olivares M, Xaus J, Rodríguez JM (2008) Oral administration of strains Lactobacillus isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl Environ Microbiol 74(15):4650–4655. https://doi.org/10.1128/AEM.02599-07
Juárez Tomás MS, Otero MC, Ocaña V, Nader-Macías MEF (2004) Production of antimicrobial substances by lactic acid bacteria I: determination of hydrogen peroxide. In: Spencer, R. J.; Ragout de Spencer, A. (ed.). Methods in molecular biology. Totowa: Humana Press. 337, e46.
Klostermann K, Crispie F, Flynn J, Ross RP, Hill C, Meaney W (2008) Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: comparison with antibiotic treatment in field trials. J Dairy Res 75:365–373. https://doi.org/10.1017/S0022029908003373
Kos B, Susković J, Vuković S, Simpraga M, Frece J, Matosić S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987. https://doi.org/10.1046/j.1365-2672.2003.01915.x
Lavilla-lerma L, Pérez-Pulido R, Martínez-Bueno M, Maqueda M, Valdivia E (2013) Characterization of functional, safety, and gut survival related characteristics of Lactobacillus strains isolated from farmhouse goat’s milk cheeses. Int J Food Microbiol 163(2–3):136–145. https://doi.org/10.1016/j.ijfoodmicro.2013.02.015
Lebeer S, Vanderleyden J, De Keersmaecker SC (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8(3):171–184. https://doi.org/10.1038/nrmicro2297
Lorca G, Torino MI, Font D, Ljungh VAA (2002) Lactobacilli express cell surface proteins which mediate binding of immobilized collagen and fibronectin. FEMS Microbiol Lett 206:31–37. https://doi.org/10.1111/j.1574-6968.2002.tb10982.x
Maidak BL, Cole JR, Lilburn TG, Parker CTJ, Saxman PR, Farris RJ, Garrity GM, Olsen GJ, Schmidt TM, Tiedje JM (2000) The RDP (Ribosomal Database Project) continues. Nucleic Acids Res 28:173–174. https://doi.org/10.1093/nar/28.1.173
Martín R, Olivares M, Perez M, Xaus J, Torre C, Fernandez L, Rodríguez JM (2010) Identification and evaluation of the probiotic potential of lactobacilli isolated from canine milk. Vet J 185(2):193–198. https://doi.org/10.1016/j.tvjl.2009.04.014
Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria – a review. Int J Food Microbiol 105:281–295. https://doi.org/10.1016/j.ijfoodmicro.2005.03.008
Mentem JFM (2001) Aditivos alternativos na produção de aves: probióticos e prebióticos. In: Anais da 38º Reunião Anual da Sociedade Brasileira de Zootecnia Piracicaba, SP: SBZ, 141–157.
Milanov D, Lazić S, Vidić B, Petrović J, Bugarski D, Šeguljev Z (2010) Slime production and biofilm forming ability by Staphylococcus aureus bovine mastitis isolates. Acta Vet Brno 60(2–3):217–226. https://doi.org/10.2298/AVB1003217M
Nader-Macías ME, Otero MC, Espeche MC, Maldonado NC (2008) Advances in the design of probiotic products for the prevention of major diseases in dairy cattle. J Ind Microbiol Biotechnol 35(11):1387–1395. https://doi.org/10.1007/s10295-008-0438-2
National Mastitis Council (NMC) (2001) Guidelines on normal and abnormal raw milk based on somatic cell counts and signs of clinical mastitis. Available at: http://www.nmconline.org/wp-content/uploads/2016/09/Guidelines-on-Normal-and-Abnormal-Raw-Milk.pdf.
Oikonomou G, Machado VS, Santisteban C, Schukken YH, Bicalho RC (2012) Microbial diversity of bovine mastitic milk as described by pyrosequencing of metagenomics 16S rDNA. PLoS ONE 7(10):e47671. https://doi.org/10.1371/journal.pone.0047671
Oliveira M, Bexiga R, Nunes SF, Carneiro C, Cavaco LM, Bernardo F, Vilela CL (2006) Biofilm-forming ability profiling of Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol 118:133–140. https://doi.org/10.1016/j.vetmic.2006.07.008
Otero MC, Morelli L, Nader-Macias ME (2006) Probiotic properties of bovine vaginal lactic acid bacteria to prevent metritis. Lett Appl Microbiol 43:91–97. https://doi.org/10.1111/j.1472-765X.2006.01914.x
Ouadghiri M, Vancanneyt M, Vandamme P, Naser S, Gevers D, Lefebvre K, Swings J, Amar M (2008) Identification of lactic acid bacteria in Moroccan raw milk and traditionally fermented skimmed milk ‘lben.’ J Appl Microbiol 106(2):486–495. https://doi.org/10.1111/j.1365-2672.2008.04016.x
Quigley L, O’Sullivan O, Stan Ton C, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD (2013) The complex microbiota of raw milk. FEMS Microbiol Rev 37:664–698. https://doi.org/10.1111/1574-6976.12030
Paduch JH, Lücking J, Mansion-de Vries E, Zinke C, Wente N, Krömker V (2020) Prevention of intramammary infections by prepartum external application of a teat dip containing lactic acid bacteria with antimicrobial properties in dairy heifers. Pathogens 9:288. https://doi.org/10.3390/pathogens9040288
Patterson JL, Girerd PH, Karjane NW, Jefferson KK (2008) Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol 197:e170. https://doi.org/10.1016/j.ajog.2007.02.027
Pellegrino MS, Frola ID, Natanael B, Gobelli D, Nader-Macias MEF, Bogni CI (2019) In vitro characterization of lactic acid bacteria isolated from bovine milk as potential probiotic strains to prevent bovine mastitis. Probiotics Antimicrob Proteins 11:74–84. https://doi.org/10.1007/s12602-017-9383-6
Penna VM, Peixoto MGCD, Verneque RS (2014) O programa de melhoramento da aptidão leiteira da raça Guzerá. Available at: http://www.guzeraibituruna. com.br/textooprograma.html acessed at 18 de out. 2014.
Pereira AR, Machado PF, Sarrìes GA (2001) Contagem de células somáticas e características produtivas de vacas da raça holandesa em lactação. Sci Agric 58(4):649–654. https://doi.org/10.1590/S0103-90162001000400001
Pérez-Ibarreche M, Castellano P, Vignolo G (2014) Evaluation of anti-Listeria meat borne Lactobacillus for biofilm formation on selected abiotic surfaces. Meat Sci 96(1):295–303. https://doi.org/10.1016/j.meatsci.2013.07.010
Pogacic T, Mancini A, Santarelli M, Bottari B, Lazz C, Neviani E, Gatti M (2013) Diversity and dynamics of lactic acid bacteria strain during aging of a long ripened hard cheese produced from raw milk and undefined natural starter. Food Microbiol 36:207–215. https://doi.org/10.1016/j.fm.2013.05.009
Reniero R, Cocconcelli PS, Bottazzi V, Morelli L (1991) High frequency conjugation in lactobacillus mediated by an aggregation promoting factor. J Gen Microbiol 138:763–768. https://doi.org/10.1099/00221287-138-4-763
Ryan MP, Meaney WJ, Ross RP, Hill C (1998) Evaluation of lacticin 3147 and a teat seal containing this bacteriocin for inhibition of mastitis pathogens. Appl Environ Microbiol 64:2287–2290
Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T (2000) Probiotic bacteria: safety, functional and technological properties. J Biotechnol 84:197–215. https://doi.org/10.1016/s0168-1656(00)00375-8
Sandes SH, Alvin LB, Silva BC, Zanirati DF, Jung LR, Nicoli JR, Neumann E, Nunes AC (2014) Lactobacillus species identification by amplified ribosomal 16S–23S rRNA restriction fragment length polymorphism analysis. Benef Microbes 5(4):471–481. https://doi.org/10.3920/BM2013.0092
Schmitz S, Suchodolski J (2016) Understanding the canine intestinal microbiota and its modification by pro-, pre- and synbiotics – what is the evidence? Vet Med Sci 2(2):71–94. https://doi.org/10.1002/vms3.17
Sengupta R, Altermann E, Anderson RC, Mcnabb WC, Moughan PJ, Roy NC (2013) The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediators Inflamm 2013:237921. https://doi.org/10.1155/2013/237921
Silva BC, Jung LR, Sandes SC, Alvim LB, Bomfim MR, Nicoli JR, Neumann E, Nunes AC (2013) In vitro assessment of functional properties of lactic acid bacteria isolated from fecal microbiota of healthy dogs for potential use as probiotics. Benef Microbes 4:267–275. https://doi.org/10.3920/BM2012.0048
Silva BC, Sandes SH, Alvim LB, Bomfim MR, Nicoli JR, Neumann E, Nunes AC (2017) Selection of a candidate probiotic strain of Pediococcus pentosaceus from the fecal microbiota of horses by in vitro testing and health claims in a mouse model of Salmonella infection. J Appl Microbiol 122(1):225–238. https://doi.org/10.1111/jam.13339
Strauss E (2000) Fighting bacterial fire with bacterial fight. Science 290:2231–2233. https://doi.org/10.1126/science.290.5500.2231a
Tamilselvam B, Almeida RA, Dunlap JR, Oliver SP (2006) Streptococcus uberis internalizes and persists in bovine mammary epithelial cells. Microb Pathog 40:279–285. https://doi.org/10.1016/j.micpath.2006.02.006
Thaker H, Brahmbhatt M, Nayak J, Thaker HC (2013) Isolation and identification of Staphylococcus aureus from milk and milk products and their drug resistance patterns in Anand, Gujarat. Vet World 6:10–13. https://doi.org/10.5455/vetworld.2013.10-13
Toledo-Arana A, Valle J, Solano C, Arrizubieta MJ, Cucarella C, Lamata M, Amorena B, Leiva J, Penades JR, Lasa I (2001) The enterococcal surface protein, esp, is involved in Enterococcus faecalis biofilm formation. Appl Environ Microbiol 67(10):4538–4545. https://doi.org/10.1128/aem.67.10.4538-4545.2001
Turpin W, Humblot C, Noordine ML, Thomasm M, Guyotm JP (2012) Lactobacillaceae and cell adhesion: genomic and functional screening. PLoS ONE 7(5):e38034. https://doi.org/10.1371/journal.pone.0038034
Van den Elsen LW, Poyntz HC, Weyrich LS, Young W, Forbes-Blom EE (2017) Embracing the gut microbiota: the new frontier for inflammatory and infectious diseases. Clin Transl Immunology 6(1):e.125. https://doi.org/10.1038/cti.2016.91
Van Der Meulen R, Grosu-Tudor S, Mozzi F, Vaningelgem F, Zamfir M, Font De Valdez G, De Vuyst L (2007) Screening of lactic acid bacteria isolates from dairy and cereal products for exopolysaccharide production and genes involved. Int J Food Microbiol 118:250–258. https://doi.org/10.1016/j.ijfoodmicro.2007.07.014
Vaseduvan P, Nair MKM, Annamalai T, Venkitanarayanan KS (2003) Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet Microbiol 92:179–185. https://doi.org/10.1016/s0378-1135(02)00360-7
Verstegen MWA, Williams BA (2002) Alternatives to the use of antibiotics as growth promoters for monogastric animals. Anim Biotechnol 13:113–127. https://doi.org/10.1081/ABIO-120005774
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative in vitro study of probiotic characteristics and biological barriers resistance. Food Res Int 36:895–904. https://doi.org/10.1016/S0963-9969(03)00098-X
Vu B, Chen M, Crawford RJ, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14:2535–2554. https://doi.org/10.3390/molecules14072535
Waldherr FW, Doll VM, Meissner D, Vogel RF (2010) Identification and characterization of a glucan-producing enzyme from Lactobacillus hilgardii TMW 1.828 involved in granule formation of water kefir. Food Microbiol 27:672–678. https://doi.org/10.1016/j.fm.2010.03.013
Walker WA, Iyengar RS (2015) Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res 77(1–2):220–228. https://doi.org/10.1038/pr.2014.160
Watts JL (1988) Etiological agents of bovine mastitis. Vet Microbiol 16:41–66. https://doi.org/10.1016/0378-1135(88)90126-5
Wouters JTM, Ayad EH, Hugenholtz J, Smit G (2002) Microbes from raw milk for fermented dairy products. Int Dairy J 12:91–109. https://doi.org/10.1016/S0958-6946(01)00151-0
Zuily S, Mami Z, Meune C (2011) Lactococcus garvieae endocarditis. Arch Cardiovasc Dis 104:138–139. https://doi.org/10.1016/j.acvd.2010.05.005
Acknowledgements
Pathogens used in antagonism in vitro assays were gently given by Dr. Nivaldo da Silva (UFMG) and CNPGL-EMBRAPA.
Funding
This study was funded by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq/UFMG). FAPEMIG, CNPq, and PRPq/UFMG provided resources for the collection of biological samples, laboratory analysis, and structure for data analysis and publication writing.
Author information
Authors and Affiliations
Contributions
Conceptualization: Raphael S. Steinberg and Álvaro C. Nunes. Field sample collection: Raphael S. Steinberg, Lilian C. Silva e Silva, Ronaldo Braga Reis, and Adriano F. Bicalho. Funding acquisition: Marcelo R.e de Souza, Jacques R. Nicoli, Elisabeth Neumann, and Álvaro C. Nunes. Investigation: Raphael S. Steinberg, L. Cristina Silva e Silva, Marcelo R. de Souza, João P. S. Nunes, Adriana A. M. Dias, Jacques R, Nicoli, Elisabeth Neumann, and Álvaro C. Nunes. Data curation: Raphael S. Steinberg and Álvaro C. Nunes. Formal analysis: Raphael S. Steinberg and Álvaro C. Nunes. Project administration: Raphael S. Steinberg, Jacques R. Nicoli, Elisabeth Neumann, and Álvaro C. Nunes. Writing—original draft: Raphael S. Steinberg. Writing—review and editing: Jacques R. Nicoli, Elisabeth Neumann, and Álvaro C. Nunes.
Author information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies performed by any of the authors with humans or animals participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Steinberg, R.S., Silva, L.C.S.e., de Souza, M.R. et al. Prospecting of potentially probiotic lactic acid bacteria from bovine mammary ecosystem: imminent partners from bacteriotherapy against bovine mastitis. Int Microbiol 25, 189–206 (2022). https://doi.org/10.1007/s10123-021-00209-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-021-00209-6