Abstract
It is a given in biology that structure and function go hand-in-hand. At the level of the mammary alveoli, copious milk production depends on the proliferation of mammary epithelial cells and the biochemical and structural differentiation of these cells after parturition. For example, data from quantitative structural studies demonstrate that differences in milk production between beef and dairy cows correspond with a relative failure of alveolar cell differentiation in cattle not specifically selected for milk yield. It is likely, but not proven, that production differences within or between dairy breeds are also determined by differences in the capacity of alveolar cells to differentiate or to maintain an adequate state of differentiation. These observations strongly support the belief that insults from mastitis that lead to losses in mammary function are directly related to disruption of alveolar cell integrity, sloughing of cells, induced apoptosis, and increased appearance of poorly-differentiated cells. Ironically, reduced milk production in cases of subclinical mastitis, is also associated with increases in milk somatic cell count. Thus the elevated neutrophil migration evoked to fight inflammation can inadvertently rendered alveolar epithelial cells non-secretory. A challenge to future researchers will be to devise mastitis treatments and therapies that prevent and/or repair damage to alveolar structure and maximize subsequent secretory cell differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The purpose of our paper is to consider relationships between structure and function of the bovine mammary gland, and in particular, the impact of mastitis. For the dairy industry, mastitis is the most costly common disease, and the economic loss due to mastitis in dairy cattle is estimated at $185/cow/year annually in the US [1]. This totals more than 2 billion dollars annually in the US alone. Losses are in the form of discarded abnormal milk from clinically infected quarters and as the result of antibiotic therapy, cull cow replacement costs, extra labor to handle mastitic cows, antibiotic and other treatment costs, veterinary services, and most importantly, reduced milk production in subclinically infected cows, which contributes to two-thirds of mastitis losses. In fact, a single quarter infected throughout lactation may reduce milk production of a cow by 10 to 12%.
Disease status is usually monitored via changes in the so-called somatic cell count of milk (MSCC). In milk from healthy uninfected glands, the cell count is typically <200,000 cells per ml. Most of these cells are lost epithelial cells, macrophages, and neutrophils. With the onset of inflammation, dramatic increases in MSCC are accounted for by increases in neutrophils. For most dairies, mastitis monitoring depends on evaluation of apparent udder health at the time of milking i.e. appearance of the udder and foremilk, periodic (typically monthly) measurement of MSCC in milk samples collected as part of production evaluation programs (milk yield, as well as concentrations of protein and fat), and in some situations measurement of milk electrical conductivity and lactose concentration. The MSCC is valuable because increases in MSCC are typically the result of an influx of neutrophils into the milk from the blood stream. The relationship between MSCC and milk production loss was established by a many workers in the 1970s and 80s as illustrated in the Jones et al. study [2] (Table 1).
The function of the neutrophil influx via chemotaxis is to combat inflammation. There is evidence however, that this defense response can also impair and disrupt mammary function [3–5]. Figure 1 illustrates the dramatic change in milk production, MSCC and passage of the milk proteins (α-lactalbumin) and casein into blood serum resulting from the intramammary infusion of sterile bacterial endotoxin [6]. Indeed, concentrations of α-lactalbumin in the blood serum of lactating cows are highly correlated with MSCC [6] and correspond with changing mammary development and function [7]. To establish relationships between mammary structure and function, we will discuss studies that quantitatively describe links between alveolar cell differentiation and milk production. Our assumption is that intramammary infections that impair alveolar development or reduce alveolar cell differentiation correspondingly reduce capacity of the secretory epithelium to synthesis and secrete milk. We begin with a description of intramammary infection.
The upper panel shows changes in milk somatic cell count following intramammary infusion of sterile endotoxin. The middle panel shows the corresponding changes in milk production. The lower panel shows changes in serum concentrations of α-lactalbumin and casein before and after infusion. Adapted from McFadden et al. [7]
Bacterial Invasion
Intramammary infection results once bacteria pass through the teat duct of a mammary quarter, multiply in the teat and gland cisterns, and progress dorsally to the milk-producing tissues. These microorganisms breach the teat duct in several ways during the milking process as well as during the intermilking period. For example, during machine milking, microorganisms may be propelled into or through the teat duct into the teat cistern via milking machine-induced droplet impacts, which can occur toward the end of milking when the fluid volume in the udder decreases.
Also, during milking, the lower teat skin surfaces are exposed to any contagious bacteria that might remain in teat cup liners from cows previously milked cows. Immediately after the teat cups are removed from an udder, these contaminating bacteria remain on the distal teat surface, pool in milk residues, and concentrate at the teat opening due to gravity, remaining in an opportunistic position to cause mastitis. Microorganisms can also be introduced through the teat duct when animals are being treated by the intramammary infusion process. Also, between milkings, microorganisms may pass through the teat duct by multiplying inside the ductal lumen, or by physical movement resulting from pressure placed on the teat end as the cow moves about. Lastly, potential for bacterial invasion is greatly increased by bacteria that reside in or colonize the teat skin, teat orifice, and teat duct, such as Staphylococcus aureus and the coagulase-negative staphylococci.
Establishment of Infection
Once the organisms breach the teat duct and the cisternal spaces of the udder, adherence of bacteria to tissues lining the interior of the mammary gland may affect their ability to remain inside the gland, especially during lactation when the contents of the udder are periodically flushed during each milking; up to 4 times a day, or more with robotic milking. Streptococcus agalactiae and S. aureus adhere well to tissues lining the milk collecting spaces. Escherichia coli do not adhere but multiply rapidly in quarters with low MSCC. Bacteria initially affect tissues lining the large milk-collecting ducts and cisterns by inflicting damage to small areas of tissue. Then the microorganisms enter small ducts and alveolar areas of the ventral portions of the gland, probably by multiplication and via milk currents produced by cow movement.
Interaction of bacteria with milk leukocytes affects the establishment of infection. In milk of uninfected, healthy mammary glands, macrophages are the predominant leukocyte type, and serve as nonspecific sentinels for the detection of invading pathogens. After detection of bacteria, macrophages release chemoattractants that recruit polymorphonuclear neutrophilic leukocytes (PMN) from the vasculature into the area of infection. The PMN extravasate in large numbers, and initially accumulate around alveoli, with the goal of migrating across the alveolar, ductal, and cistrnal lumina to contact, engulf, and kill the invading pathogens. Inflammation that ensues in response to bacterial presence is initiated by the release of interferons, interleukins, and tumor necrosis factor (TNF-α).
Structure and Function Relationships
It seems intuitive to reason that, in the lactating animal, that milk production depends on the number of active milk secreting alveolar cells in the mammary gland and the corresponding degree to which these cells are fully differentiated. Certainly mastitis can impact secretory cell differentiation and alveolar structure. But is there direct evident to link such changes with function? Since mastitis can and does occur throughout the lactation cycle, disease impacts on both mammary development and function occur. By way of background, alveolar epithelial cells require both the structural and biochemical elements to allow for the copious milk secretion after parturition. Lactogenesis is largely driven by the removal of inhibitory progesterone in coordination with stimulatory increases in secretion of prolactin, glucocorticoids, growth hormone, and estradiol [8–11]. Thus, the primary endocrine and growth factor drivers of mammogenesis, lactogenesis, and galactopoiesis [12] are well defined.
Despite this knowledge base, irrespective of insults from mastitis, there is little quantitative information to determine if differences in milk production either between animals within a breed or between breeds are due to differences in the number of alveolar cells in the mammary gland or the functionality of the cells. Nor do we have a good grasp on precise mechanisms responsible for the typical lactation curve i.e. increasing to peak production (typically 60 to 90 days postpartum in dairy cows) that is followed by a gradual decline. For example, there is some evidence that cows treated with recombinant bovine somatotropin exhibit increased milk production in part because these animals have more persistent lactations. This may reflect changes in rates of cell turnover or apoptosis, but this explanation is far from certain [13].
While milk production capacity is usually not a focus for mammary biologists working in the breast cancer area, it is central to those interested in mammary development and function in agricultural research centers and in universities and colleges with dairy science and animal science departments. Successful lactations are also central to the reproductive performance of nearly all mammals [14], with the minor exceptions of animals that are bottle fed rather than suckled. To evaluate the role of differentiation status on milk production, Akers et al. [15] utilized mammary tissues collected from beef and dairy heifers that were milked twice daily in a standard milking parlor. Groups of beef (Hereford) and dairy (Holstein) heifers were slaughtered at 150, 180, and 260 days of gestation and at day 49 of lactation. The goal of the study was to address the following questions. Has the structure or function of the bovine mammary gland been impacted by long-term selection for increased milk production? Can the dramatic improvements in milk production in dairy cattle apparent in the past 50 years be explained by changes in the mammary gland? Based on milk production and composition, measurement of rates of casein and α-lactalbumin synthesis and radiolabeled acetate incorporation into fatty acids in mammary explants and mammary DNA and RNA [16] it was estimated that 22% and 55% of the 5.8-fold greater milk production in Holstein heifers were due to differences in cell mass and biochemical function, respectively.
A specific objective of the structure study [15] was to determine if milk production differences were related to cytological differentiation of the secretory epithelium. Cells from lactating animals were characterized as (E1) poorly differentiated, (E2) intermediately differentiated or (E3) fully differentiated as described previously [17–19]. Briefly, E1 cells would be similar to the alveolar cells typical of the later portion of gestation. There is a nearly full complement of alveolar epithelial cells but the cells have few organelles (often irregular nuclei, scattered mitochondria, minimal RER and Golgi, few lipid droplets or secretory vesicles). E3 cells exhibit a rounded, basally displaced nucleus, lacy appearing apical region (indicating many secretory vesicles and fat droplets), and much smaller nuclear to cytoplasmic ratio than E1 rated cells. E2 cells would have some of these characteristics but to a lesser degree than E3.
Results of this analysis (Table 2) showed that while about half of the alveolar cells in both breeds were intermediately differentiated (E2), the proportions of fully (E3) and poorly differentiated (E1) cells were essentially reversed between breeds. More than half of the epithelial cells in Holstein heifers were classified as fully differentiated. Consequently, nearly all the epithelial cells of the Holsteins were either fully or intermediately differentiated. For the beef heifers, more than 40% of the cells had little evidence of secretory activity. These poorly differentiated cells were correspondingly rare in dairy heifers. These data support the idea that much of the difference in milk production between beef and dairy animals depends on not only increased parenchymal mass in dairy animals but to a greater degree, increased mammary cell function. Is it possible that selection for increased milk production has allowed for the maximization of differentiation signals during the critical periparturient period? It is unclear if similar effects on cell differentiation are apparent when Holsteins selected for milk production are compared with Holsteins not selected (or heavily selected) for milk production. However, Filep and Akers [20] demonstrated that mammary explants from Holstein bulls selected for high milk production produced more casein and showed droplet containing epithelial cells when explants were incubated in the presence of prolactin compared with bulls from a control genetic line.
Data from Nickerson and Akers [19] show that treatments that interfere with the usual course of lactogenesis around the time of parturition have negative impacts that carry on into the subsequent lactation. Specifically, they showed that intramammary infusions of colchicine in the period before calving markedly reduced subsequent milk production. During the third week of lactation, milk production was 3.5-fold lower (60 vs. 17 kg) in colchicine-treated compared with placebo-treated udder halves. This difference corresponded with dramatic reductions in the rates of protein and fatty acid synthesis and utilization of acetate for energy (CO2 generation) in mammary tissue slices [21]. But in particular mammary tissue samples showed that fully differentiated alveolar cells increased from 45% to 81% of cells between days 5 and 21 postpartum in control glands but fully differentiated alveolar cells were rare (4 and 5% of alveolar cells on days 5 and 21, respectively) in treated glands. Indeed, in treated glands, nearly half of the alveolar cells remained undifferentiated on day 21 compared with only 7% in control glands. Differences in cytological based evaluations of differentiation were confirmed with ultrastructural analysis. These results suggest that mastitis may be especially harmful when it occurs in the periparturient period when alveolar cell proliferation and secretory cell differentiation is particularly active.
These data provide good examples linking structure and function at the level of alveolar cell cytology. Namely characteristics of well differentiated cells evident at the light microscopic level correspond with copious milk secretion. Thus these lines of evidence support the idea that mastitis cases that interfere with either alveolar development or alveolar cell differentiation will correspondingly reduce function. Further, although lost milk production due to mastitis is usually associated with damage or destruction of alveolar epithelial cells, mastitis effects that create blockages of mammary ducts or impairment of milk letdown also reduce milk production. Similarly, systemic effects due to mastitis (or other diseases for that matter) that alter feed intake, digestion, or blood flow to the mammary gland reduce milk production.
Histological Response of Mammary Tissues to Presence to Infection
As stated previously, the presence of microbial infection in the mammary gland of the dairy cow has been shown to decrease milk yield, but how is this reduction in milk synthesis brought about? Paradoxically, it is believed that the host immune response and toxins produced by mastitis-causing bacteria are the causal mechanisms that have deleterious effects on mammary tissue. The mammary tissue response to mastitis in dairy cows as a result of both natural and experimental infections has been quantified using histological and cytological techniques. In general, quarters with natural infections demonstrate reduced ability of secretory tissue to synthesis and secrete milk; namely, decreased percentages of tissue areas occupied by alveolar epithelium and lumina, and increased interalveolar stromal areas. Helmbolt et al. [22] reviewed the progress made on the histopathology of bovine mastitis from the late 1800 s through 1950 and summarized pathogenesis as follows. After penetration of bacteria into the quarter, neutrophils migrated from the blood into the interalveolar stroma. The secretory epithelium became vacuolated resulting from build-up of milk constituents, and then sloughed off the basement membrane as the stoma thickened due to multiplication of fibroblasts. With advancing involution, mononuclear leukocytes infiltrated the tissue to clean up tissue debris and then disappeared as the glandular tissue assumed a non-secretory or undifferentiated state.
The histopathological differences between acute and chronic mastitis were reviewed by Spencer [23], who found that in acute infections, medium and small ducts became occluded with fibrin, leukocytes, and bacteria. Epithelial cells were vacuolated and alveolar lumina became distended due to milk stasis but later decreased in size due to interstitial edema. As a result of duct blockage, bacteria multiplied and spread to produce microscopic foci of infection resulting in involution and necrosis. Pathology of chronic mastitis was observed as scattered foci of infection due to duct blockage of small ducts. Areas of infection were kept localized by infiltrating phagocytes.
A more detailed study of the progressive pathology of streptococcal and staphylococcal mastitis was made by Pattison [24], who found that after bacterial penetration, multiplication, and invasion of the mammary ductal system, neutrophils infiltrated the infected tissues. Bacteria were observed within the ductal lumina and within neutrophils. As infection progressed, macrophages were more numerous and the ductal epithelium became hypertrophied. Fibroblast activity was stimulated, and the subsequent increase in stromal area and concurrent damage to the alveolar epithelium led to depressed secretory activity, milk stasis, and involution. In many cases, large ducts became thickened and cornified, and in smaller ducts, the epithelium and connective tissue cells had multiplied and invaginated into the alveolar and ductal lumina, which completely blocked milk flow. In later stages of infection, hypertrophied ducts and intraductal invaginations broke down and were passed from milk as clots. Damaged areas of the affected quarter were nonfunctional due to fibrosis or involution. Eventually, ducts regained a normal epithelial lining and became patent again. Pathological changes due to staphylococcal and streptococcal mastitis were similar; however, changes in staphylococcal mastitis were more severe since staphylococci were more resistant to phagocytosis and released more powerful toxins. Defense mechanisms generated in response to staphylococci were more intense, and neutrophils and macrophages obliterated the secretory structure in attempts to wall off infection.
Chandler and Reid [25] made the first ultrastructural observations on the pathology of naturally occurring staphylococcal and streptococcal bovine mastitis. Histological study of S. aureus mastitis revealed numerous neutrophils in alveoli and ducts. Several luminal areas contained large epithelial-like cells and hyaline-like material, as well as corpora amylacea. Cocci were only rarely seen. Complete loss of secretory structure was observed in some areas due to cytolysis, and fibrosis in connective tissues was frequently observed. Such changes represented reduced secretory potential, regional milk stasis, and inflammation. Electron microscopic observations showed reduced activity of the mammary epithelium as evidenced by reductions in cytoplasmic areas occupied by rough endoplasmic reticulum and in size and content of Golgi components. The chromatin was condensed in some epithelial nuclei, but junctional complexes and basement membranes appeared intact. However, intercellular spaces between basal portions of alveolar cells showed swelling at times. Alveolar luminal contents consisted of amorphous material derived from milk components, few casein micelles, very few lipid droplets, and numerous neutrophils. Neutrophils were also observed in the stroma beneath the epithelial cells. In some alveolar epithelia, complete degeneration of organelles was observed, leading to sloughing of cells from the basal lamina and subsequent fibrin deposition. Milk protein particles were sometimes observed in the connective tissue adjacent to the alveolar epithelium.
In addition to natural exposure studies, experimental challenge studies have been conducted to better assess mammary tissue damage at specific times after an infection is initiated. For example, in an attempt to develop a model for studying S. aureus mastitis and to describe cytologic changes at 12 and 24 h after challenge, Heald [26] inoculated bacteria (500–5,000 cfu) using a hypodermic needle directly into the mammary parenchyma. Inoculated tissues showed swelling and cytolysis of the mammary epithelia, an increase in the interalveolar stromal area, a reduction in luminal areas, and the presence of cellular debris in alveolar lumens. At 12 h after injection, the percentage of secretory epithelial area was reduced by half and the percentage of involuted epithelial areas doubled. By 24 h, the effects were more dramatic, with an increase in stromal area, a decrease in secretory epithelial area, a decrease in open luminal area, and an increase in debris-filled luminal area. The presence of S. aureus was associated with increases in stromal areas and involuting epithelial areas. Thus, by 24 h, the secretory parenchyma had, in part, been replaced by an epithelial population without secretory characteristics along with a decrease in alveolar luminal areas and an increase in stroma, all of which was interpreted as a decrease in accumulated secretory product in lumina, an increase in cellular debris in milk, and swelling of stromal areas. The challenged cows eventually recovered and returned to normal milk production. The author (Heald [26]) attributed this recovery to 1) the possible redevelopment of secretory potential in damaged alveoli and/or 2) compensatory hypertrophy of remaining healthy tissue or 3) both of these possibilities. More recent research Capuco [27] would suggest that stem cells associated with the mammary tissue, which are responsible for growth and maintenance of the mammary epithelium, may differentiate into milk-producing cells a part of the recovery process
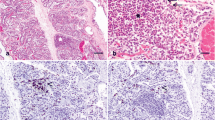
Subsequently, Nickerson [28] and Nickerson and Heald [29] induced S. aureus mastitis in dairy cattle by intracisternal inoculation of 240 cfu of bacteria. At 2 and 10 days after initial infection, mammary tissue from 2 zones in infected quarters was morphometrically compared with similar zones in contralateral control quarters. Zone 1 (deep parenchyma) was approximately 5–10 cm dorsal to the gland cistern, whereas Zone 2 (cisternal parenchyma) was approximately 1 cm dorsal to the gland cistern. In comparison with control tissue, infected tissue exhibited more interalveolar stromal area, reduced alveolar luminal area, more damaged and involuted alveolar epithelial area (poor state differentiation), and more neutrophil infiltration (Fig. 2).
a. Uninfected control mammary parenchymal tissue exhibiting large luminal area, minimal cellular debris, and limited stromal area (625X); b. Portions of an alveolus from control tissue characterized by polarized secretory cells with a large cytoplasmic:nuclear ratio and round medially to basally located nuclei (2500X); c. Tissue from an infected gland characterized by large stromal areas and small luminal areas, portions of which contain cellular debris (625X); d. Portions of an alveolus from infected tissue characterized by irregular epithelium, some of which has degenerated and sloughed into the alveolar lumen that contains some cellular debris (2500X); e. Tissue from a nonsecretory area of an infected gland characterized by a large stromal area and a limited, open luminal area (625X); f. Portions of an alveolus from an infected area of the gland undergoing involution, characterized by non-secretory alveolar epithelium. Polymorphonuclear leukocytes (PMN) and macrophages are observed in the lumen; a few PMN were observed within the alveolar epithelial lining (2500X). Abbreviations: D—Cellular debris, E—Alveolar epithelium, L—Lumen, M—Macrophage, N—PMN, S—Stroma. Nickerson, 1980 [28]
Much of the luminal areas were filled with cellular debris and leukocytes. These changes appeared more advanced at 10 days than at 2 days after infection was induced, indicating that by day 10, glandular parenchyma was losing its secretory potential. Quarter zones differed greatly in the responses to infection. Parenchyma taken from a zone of an infected quarter close to the gland cistern appeared to undergo involution more rapidly than a zone further from the gland cistern. Involuted parenchyma displayed reduced secretory activity, diminished luminal areas, and abundant connective tissue.
Ultrastructural damage to infected tissue included epithelial cells that were very loosely adhered to the basal lamina and swollen cytoplasm as well as swollen mitochondria. The rough endoplasmic reticulum assumed a vesicular form instead of flattened parallel cisternae, and Golgi components were indistinct, sometimes appearing in the form of scattered, flattened stacks of membranes. Cells classified as nonsecretory that were common in infected tissues displayed a paucity of organelles, e.g., few Golgi components and secretory vesicles, reduced rough endoplasmic reticulum, and few lipid droplets. See Fig. 3.
a. Alveolar epithelium from uninfected control tissue characterized by well polarized cells with a supranuclear Golgi apparatus and paranuclear to basally located rough endoplasmic reticulum (4200X); b. Epithelium from a nonsecretory area of infected tissue characterized by a large cytoplasmic:nuclear ratio, a few cisternae of rough endoplasmic reticulum, and limited Golgi components (4300X); c. Damaged epithelium from infected tissue characterized by swollen cytoplasm with swollen mitochondria, vesicular rough endoplasmic reticulum, indistinct cell borders, and flattened stacks of Golgi membranes (4800X); d. Remains of a damaged alveolus from infected tissue consisting of a bare basal lamina with portions of adhering secretory cell cytoplasm; note swelling of the interalveolar stroma (7400X). Abbreviations: Bl—basal lamina, Ga—Golgi apparatus, L—lumen, Ld—lipid droplet, M—mitochondria, My—myoepithelial cell, N—nucleus, R—rough endoplasmic reticulum. Nickerson, 1980 [28]
Thus, challenging the bovine mammary gland with a relatively mild bacterial inoculum revealed drastic histological changes, which varied depending on location within the quarter. As the mammary alveoli became affected by bacterial toxins or byproducts of inflammation, they underwent lytic and involutionary changes at 2 and 10 days of infection. Secretory potential decreased and stromal tissue displaced inactive alveoli as disrupted cells sloughed into alveolar lumina. Neutrophils infiltrated alveoli in attempts to eliminate bacteria as well as milk constituents. By day 10, the damage originally inflicted upon the secretory tissue had not been reversed or repaired, and had slightly increased as evidenced by an increase in the percentage area occupied by damaged epithelial cells. Additionally, the area occupied by nonsecretory tissue was increased on day 10 compared to day 2. Thus, infection continued to cause further destruction of epithelial cells resulting in involution through day 10. It remains to be determined if damaged tissue repairs itself, if involuted tissue redevelops secretory potential, or if there is compensatory hypertrophy of the remaining healthy tissue.
Cytologic Response to Intramammary Infection In Vivo
The migration of leukocytes, namely PMN, across the alveolar epithelial lining is thought to cause mechanical and/or chemical damage to the milk secretory cells as well as to the ductal cells. One of the first in vivo investigations to study PMN migration in mammary tissues was conducted by Harmon and Heald [30] incident to a study on experimentally-induced S. aureus mastitis in lactating dairy cows. They used both light and electron microscopic methods and observed PMN in various stages of margination (accumulation and adhesion to blood vessel endothelium at the site of injury) in capillary lumina and migration of cells into the perivascular space. These leukocytes encountered up to 6 structural barriers while crossing the vascular system and into alveolar lumina. The first was the capillary endothelial cell (1) that surrounded the vessel lumen, followed by the basal lamina (2) that enveloped the endothelium. The PMN were able to breach these barriers by squeezing through small gaps in these structures. Then, the PMN had to traverse a periendothelium (3) that often surrounded the basal lamina. The PMN then migrated through the connective tissue (4) before encountering the basal lamina (5) that enveloped each milk-producing alveolus. The last structural barrier was the alveolar epithelial cell lining (6) through which PMN must traverse to migrate into the alveolar lumen.
Harmon and Heald [30] observed that in tissue areas infected with S. aureus showing little evidence of epithelial damage, PMN had accumulated in the perivascular connective tissue space after 24 h and had aligned themselves against the basal lamina of alveoli, but not in the alveolar lumen. This suggested that PMN did not pass freely into all alveoli, but accumulated around these structures. Other areas of infected tissue showed progressive degeneration of alveolar epithelial tissue (autophagic vacuoles, vesiculation of endoplasmic reticulum, and cellular debris in the luminal milk) along with accumulation of PMN in alveolar lumina. The PMN that had traversed the alveolar basal lamina were observed between the basal ends of adjacent epithelial cells having intact tight junctions but showing signs of damage, and appeared to be migrating toward the alveolar lumen. These cells were never seen traversing the junctional complexes between the epithelial cells, and it was suggested that this process, if it occurs, must be rapid and allow for immediate reclosure. However, alveoli with a portion of the epithelium sloughed off the basement membrane and into the lumen were frequently seen, and PMN appeared to be entering alveolar lumina in large numbers at these damaged sites. Here in the lumens, PMN were observed with phagocytosed S. aureus in various stages of digestion. Marked PMN infiltration in tissues lining the teat and gland cisterns was also observed in addition to infiltration into the mammary parenchymal areas. The authors suggested that the cisternal areas in the udder should be considered to be the first sites of PMN migration after bacteria breach the teat canal during the initial stages of infection.
Thus, findings of this study suggested two mechanisms by which leukocytes migrated across the alveolar epithelial layer and into milk to phagocytose S. aureus. One mechanism was via migrating through gaps or holes remaining in the epithelial lining as a result of lysis or degeneration of individual milk-producing alveolar cells. The other mechanism was through sloughing of portions of the epithelial lining. When traversing the epithelium, many PMN were often observed arranged end-to-end as if following one another, suggesting that the presence of some weakness or gap in the tissue barriers provided for PMN migration en masse. Likewise, Seelig and Beer [31] suggested that gaps in the alveolar epithelium in the lactating mammary gland of the rat allowed leukocytes to enter into alveolar lumina. Similarly, Frost et al. [32] observed that PMN migrated through such epithelial lesions in the teat and gland cisterns of cows, and not through intact epithelial tissues.
This initial work by Harmon and Heald [30] was subsequently supported by Nickerson and Heald [33]. After experimentally infecting cows with S. aureus for 2 and 10 days, a study of leukocyte infiltration from blood capillaries into mammary parenchymal tissues was made. At 2 days, PMN were widespread in tissues exposed to S. aureus and observed to penetrate the alveolar epithelium where many of the phagocytes became lodged and appeared to degenerate. Intact PMN were observed in spaces of the alveolar lining that had been previously occupied by epithelial cells that had sloughed off the basal lamina or that had been dislodged by these leukocytes. See Fig. 4.
a. Neutrophils and lymphocyte in large gap between myoepithelium and secretory epithelium of an alveolus (4900X); b. Mononuclear and polymorphonuclear phagocytes filling lumina and original epithelial lining of alveolus (4900X). Abbreviations: Ep—epithelium, L—alveolar lumen, Ly—lymphocyte, Ma—macrophage, My—myoepithelial cell, N—neutrophil. Adapted from Nickerson and Heald, 1982 [29]
As the infection progressed, alveolar luminal areas became populated with PMN, and as they degenerated, more monocytes and macrophages appeared in the lumens. These mononuclear cells were observed more frequently penetrating the secretory parenchyma and entering alveolar lumina at 10 days following infusion of bacteria. As with PMN, the monocytes appeared to migrate through damaged epithelia or entered lumina as the epithelium sloughed off the basement membrane.
Lymphocytes infiltrating the mammary tissues were more numerous at 10 days than at 2 days following infection, where they were observed wedged between the basal ends of alveolar epithelial cells and adjacent to myoepithelial cells, the contraction of which may play a role in leukocyte movement across epithelial linings. In areas where swelling and damage to the epithelium was more evident, lymphocytes were observed displacing the basal secretory cell cytoplasm or they were seen in open spaces between, or basal to, adjacent secretory cells. The occurrence and size of such spaces increased as damage to epithelial cells became greater, and such spaces frequently contained monocytes, neutrophils, and epithelial cell debris as well as lymphocytes. It was suggested that this cellular immune response may have contributed to the sloughing or degeneration of the secretory cell layer. Lymphocytes were also observed free among cellular constituents of damaged epithelia as well as in collapsed alveolar lumina. The damage caused by cytoplasmic swelling in apparently intact epithelia with invaginated lymphocytes suggested that the lymphocytes had actually caused the damage. All leukocytes preferentially infiltrated the zone of the infected quarter closest to the route of bacterial entry.
Leukocyte migration has also been studied in teat cisternal tissues of bovine mammary quarters experimentally challenged with S. aureus for 24 h as well as those with chronic, long-term infections [34]. After crossing the vascular system and into the connective tissue adjacent to the 2-celled epithelial linings of the distal teat cistern, PMN were observed to penetrate the epithelial basal lamina and migrate between basal epithelial cells to gain access to the luminal cell layer. Holes or gaps in the lamina penetrated by neutrophils were approximately half the diameter of the cell. Because no tissue damage was observed in the basal cell layer, it is doubtful that weakness in the basal lamina or epithelial degeneration preexisted to accommodate neutrophils. Once within the basal lamina, neutrophils appeared to migrate freely between the double-layered epithelium.
Some neutrophils as well as other leukocytes (macrophages and lymphocytes), accumulated below the luminal epithelial cells and were frequently observed in spaces adjacent to degenerated epithelial cells where they projected through degenerate epithelial cell cytoplasm into the teat cisternal lumen. Other neutrophils were positioned among apparently intact healthy luminal cells, several having free access to the teat cistern lumen. In areas where metaplasia of the cisternal epithelium was observed, neutrophils were observed below the loosely-anchored luminal cell layer that had undergone a squamous transformation. A few neutrophils were in a state of lysis at intraepithelial sites. There were no differences in mechanisms of neutrophil infiltration and migration between tissue from quarters with chronic infections and those collected from quarters infused with S. aureus 24 h before collection. However, the degree of neutrophil infiltration in infused quarters was much more pronounced, and cells appeared to be concentrated in the epithelial linings between the 2 cell layers of the teat cistern.
Possible modes of migration through these luminal cells and into milk included: 1) projection through individual, degenerate luminal cells in milk-producing parenchymal tissues as suggested by Harmon and Heald [30] in milk-producing parenchymal tissues; 2) penetration between intact epithelia via tight junctions; and 3) passage into milk as luminal cells desquamated in areas of epithelial metaplasia under which neutrophils accumulated. In the first instance, the cause of degeneration of individual luminal cells, which allows neutrophil migration is largely unknown. However, the spaces or areas of swelling in the epithelial layer occupied by neutrophils and other leukocytes, such as lymphocytes, may be caused by factors released by these leukocytes, and contribute to lysis of the adjacent epithelial cells. The frequent observation of these associations indicates some type of influence of leukocytes on intact epithelial cells. See Fig. 5.
Electron micrograph of leukocytes in the epithelial lining illustrating the progression from a. Leukocyte accumulation below intact luminal epithelial cell layer (3200X); b. Formation of space below degenerating luminal cell (DE) (3900X); c. Bulging and further lysis of luminal cell (3200X); and d. Projection through degenerated epithelial cell cytoplasm (3200X). Adapted from Nickerson and Pankey (1984) [34]
Direct penetration of neutrophils into necrotizing epithelia adjacent to lymphocytes might imply that the lymphocytes were the cause of the damage [33, 35]. Similarly, it was observed that individual hepatocyte degeneration in the liver resulted from penetration of cytotoxic lymphocytes through the parenchyma in chronic liver disease [36]. The second mode of migration (penetration between tight junctions) was suggested based on light and electron morphologic study, but conclusive ultrastructural evidence by electron microscopy was not found (Nickerson and Heald, [29]). See Fig. 6.
a. Electron micrograph of neutrophil (N) approaching teat cistern luminal surface between intact epithelial cells (4550X). b. Light micrograph of neutrophiI (arrow) at luminal surface between intact epithelial cells (1125X). c. Electron micrograph of neutrophil (N)similar to that in Fig. 6b (8600X). d. Light micrograph illustrating neutrophils in various stages of migration through the metaplastic epithelium from the basal cell layer (arrow 1) to the luminal surface (arrow 2) (950X). Adapted from Nickerson and Pankey, (1984) [34]
McKenzie and Anderson [37] and Harmon and Heald [30] also failed to observe neutrophils traversing tight junctions between intact alveolar cells and suggest that if this did occur, the process must be very rapid and allow for quick reclosure of the junctions. This may occur in conjunction with myoepithelial cell contraction during milk let-down. The third mode of penetration, wherein neutrophils were present in sufficient numbers to cause separation of the luminal cell layer, probably led to sloughing of this layer and subsequent release of the neutrophils (Fig. 6d). This same observation was made in mammary parenchymal areas infected with S. aureus in which a massive neutrophil migration contributed to sloughing of the secretory cell layer [29].
In the study by Nickerson and Heald [29], the first method (projection through individual, degenerate luminal cells) appeared to be the predominant mechanism of neutrophil migration. These data suggested that elevated numbers of neutrophils in distal teat end epithelium and in cisternal milk may be instrumental in the initial events that prevent establishment of infection in the bovine mammary gland.
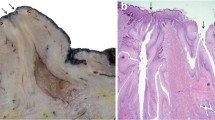
In a subsequent study, Nickerson and Pankey [38] evaluated neutrophil migration in teat duct tissues of cows chronically infected with S. aureus. After entering the subepithelial dermal tissues from capillaries, neutrophils penetrated the basal lamina and migrated between cells of the stratum germinitivum of the teat duct epidermis. These leukocytes then penetrated the stratum spinosum and stratum granulosum by traversing intercellular bridges and migrating between the cells comprising these layers toward the teat duct lumen. As the neutrophils approached the luminal surface of the stratified squamous epithelium of the teat duct, they often accumulated beneath the outermost luminal cell layer in massive numbers, sometimes separated from the teat duct lumen by only a narrow rim of squamous cell cytoplasm, which appeared to impede cellular migration. See Fig. 7.
a. Accumulation of neutrophils at the epithelial surface below teat duct lumen (L) (3960X); b. Neutrophils migrating under a thin rim of squamous cell cytoplasm adjacent to teat duct lumen (L) (2785X). Adapted from Nickerson and Pankey, 1985 [38]
Other neutrophils were observed below breaks in the stretched cytoplasm of the squamous epithelial cells. In some areas, neutrophils were observed in close association with degenerating epithelial cells as well as with apparently healthy epithelial cells containing internalized staphylococci. On a few occasions, neutrophils were seen penetrating junctional complexes between luminal squamous cells, a phenomenon not observed in epithelial linings of the mammary parenchyma [29] or in those of the teat cistern [34]. See Fig. 8.
a. Neutrophil adjacent to a break in the luminal cell surface (5135X); b. Neutrophils adjacent to a degenerate (D) surface cell (2785X); c. Neutrophil migrating through degenerate (D) surface cell cytoplasm (5960X); d. Neutrophil in close association with squamous cell containing internalized cocci. Dividing cells are observed at arrows (2785X); e. Luminal surface illustrating neutrophil penetrating between surface epithelia (arrow) into teat duct lumen (4870X); f. Serial section of neutrophil in Fig. 8f illustrating penetration of the tight junction between cells (17835X). Adapted from Nickerson and Pankey, 1985 [38]
This investigation concluded that neutrophil migration into the teat duct lumen appeared to be accomplished by one or more of several mechanisms. The first was through neutrophil pressure exerted on the thinly-stretched cytoplasm of a squamous epithelial cell that was blocking its path, followed by subsequent rupture and release of the neutrophil. These squamous cells had transformed into scale-like keratin, and may have been less structurally sound than living cells. However, the authors cautioned that ultrastructural evidence for this theory was hampered by possible artifacts of tissue fixation and processing, causing breaks in the squamous lining. Alternatively, in areas of marked neutrophil accumulations, cell numbers may have been sufficient to separate the superficial epithelia layer, causing it to slough off, and release neutrophils. Another mode of migration was by way of lysis of individual surface epithelial cells and subsequent neutrophil migration as observed in the teat cistern [34] and alveolar epithelium [29]. A final possibility for neutrophil migration was by breaching cell junctions between adjacent luminal epithelial cells, leaving these luminal cells intact.
In a study using sheep, Akers and Thompson [3] studied the effect of induced leukocyte migration on mammary cell morphology and milk synthesis following intramammary infusion of sterile oyster glycogen in lactating ewes. Infusion of oyster glycogen following each of six consecutive milkings had no direct effect on mammary tissue utilization of acetate, but did increase MSCC (6-fold) and increased percent fat and protein but decreased milk lactose concentration. In addition, the quantity of damaged or nonsecretory mammary epithelial cells was increased. Infused glands had increased concentrations of neutrophils (3-fold) and plasma cells (1.3-fold) in the subepithelial stroma, and of neutrophils (2.7-fold) within the epithelial lining of alveoli. Infusion of oyster glycogen every 3 d for 30 d elicited similar changes in MSCC count and mammary histology. These authors suggested that loss of functional mammary cells associated with leukocytosis may explain lost milk production associated with increased MSCC.
Cytologic Response to Intramammary Infection In Vitro
Lin et al. [35] investigated the process of bovine neutrophil diapedesis or migration across bovine mammary gland epithelium using an in vitro model. They used the bovine mammary epithelial cell line MAC-T, grown on collagen-coated filters, which formed a confluent monolayer with characteristic tight junctions, basal-apical polarity, and functional cell barriers. Neutrophils, which were added on the apical surface of the monolayer, were stimulated to migrate across the epithelium by the addition of 107 cfu of S. aureus to the basal compartment of the culture chamber. Light and transmission electron microscopy revealed the following series of events for neutrophil transmigration. Initially there was an accumulation of neutrophils on the surface of the epithelial monolayer, followed by projection of pseudopodia into intercellular junctions and movement of neutrophils between adjacent epithelial cells. This was followed by reapproximation of the lateral epithelial cell membranes and reformation of the apical tight junctions after neutrophils crossed the epithelium. Morphologically, epithelial cell damage caused by neutrophil diapedesis was not evident. This in vitro model provided a two-dimensional epithelial sheet by which neutrophil diapedesis could be qualitatively studied under defined conditions. Results suggested a major mode by which bovine neutrophils may migrate across the alveolar epithelia into milk during mastitis.
Capuco et al. [4] also studied the effects of neutrophils on mammary tissue of lactating cows in vitro. The leukocytes were isolated from mammary glands of nulliparous heifers given an injection of 5 micrograms of E. coli endotoxin, and mammary tissue explants were obtained from uninfected quarters of 5 lactating Holstein cows. Mammary explants were treated by addition of lysed neutrophils (105, 106, 107/ml) or intact neutrophils (105, 107/ml), which were allowed to phagocytose opsonized zymosan. Controls included cultures of mammary tissue alone, neutrophils alone, and mammary tissue plus zymosan. Cultures were incubated at 37 C for 3, 8, or 24 h. Tissue from 1 randomly selected culture/treatment was weighed and processed for microscopy. Tissue from remaining cultures was incubated with [3H]amino acids or [14C]acetate to determine rates of protein and fatty acid synthesis. Media from all cultures were assayed for activity of the lysosomal enzyme, N-acetyl-beta-D-glucosaminidase. An increase in the activity of this enzyme was detected in the medium of explant cultures treated with 107 phagocytosing neutrophils/ml at 3 and 8 h and with 107 intact or lysed neutrophils/ml at 8 h. Treatment did not inhibit the rates of protein or fatty acid synthesis. Microscopic examination indicated that epithelial cell damage resulted from treatment with 106 and 107 intact, lysed, or phagocytosing neutrophils/ml. Greatest morphologic damage resulted from treatment with phagocytosing neutrophils. It was concluded that, even in the absence of mammary epithelial damage caused by neutrophil migration across the alveolar lining and into the lumen, neutrophil phagocytic function may itself damage the epithelial cells.
To differentiate the mammary alveolar cell response to bacterial products of infection between intact tissue in vivo and isolated alveoli in vitro, Heald and Harmon [39] cultured isolated rat mammary alveoli with increasing concentrations of cell-free staphylotoxin containing α- and þ-hemolysins for 90 min. The use of the isolated alveoli allowed the examination of the effect of the toxin on milk secretory epithelial cells without the complications of an intact system, thereby excluding the bacteria themselves, the indirect effects of a toxin-induced inflammatory response, leukocytosis and accompanying tissue migration, and the associated ischemia or a decrease in blood supply due to constriction of the vascular system. With increasing concentrations of toxin (0, 10, 100, 1,000 μg/ml), decreasing rates of protein synthesis and secretion by alveoli were observed, along with decreasing areas of epithelial cell cytoplasm occupied by rough endoplasmic reticulum, Golgi dictyosomal membranes, and Golgi secretory vesicles. Such changes demonstrated a decrease in milk synthetic and secretory activities. The cytoplasmic to nuclear ratio increased with increasing toxin concentrations, and was most likely due to swollen areas of cytoplasm as well as vacuolation of the epithelial cells. In this in vitro study, coagulative necrosis or disintegration of the alveolar epithelium and sloughing into lumina was rarely observed, even at the highest dose of toxin. This suggests that, although cell-free toxin had significant detrimental effects on alveolar epithelium at the ultrastructural and biochemical levels, this effect was not as destructive as that observed when influenced by the inflammatory response and ischemic necrosis in vivo. Thus, components of the inflammatory reaction, e.g., leukocytes, as well as vascular destruction may be more harmful to mammary tissue than the bacterial toxins themselves. However, after incubating mouse mammary tissue with staphylotoxin for 2 h, Harmon and Heald [39] demonstrated epithelial lysis with as little as 0.1 μg/ml of toxin. Although this was an in vitro model, authors suggested that tissue damage may have been caused by degenerate neutrophils, which may have been present between alveolar epithelial cells and in lumina. Upon lysis, these phagocytes could have released their lysosomal contents of hydrolytic enzymes, causing subsequent secretory cell lysis.
Conclusions
For the dairy industry despite progress in mastitis control associated with milking management and hygiene, mastitis remains as a primary cause of less than optimal milk production and premature culling of cows. Quantitative morphology studies have emphasized the significance of development of well differentiated alveolar epithelial cells to meet full milk production potential as well as the negative impacts of bacteria, their toxins and cellular immune responses in structural disruption and destruction of mammary alveolar cells following mastitis. Advanced cellular, genomic, and imaging techniques offer new tools to better understand and possibly mitigate the severity of mastitis cases and in particular repair or prevent appearance of nonsecretory (undifferentiated) secretory epithelial cells which occur following mastitis.
Abbreviations
- MSCC:
-
milk somatic cell count
- DHI:
-
Dairy Herd Improvement Association
- PMN:
-
polymorphonuclear leucocyte
- TNF-α:
-
tumor necrosis factor
References
Bramley AJ, Cullor JS, Erskine RJ, Fox LK, Harmon RJ, Hogan JS, et al. The mastitis problem. Current concepts of bovine mastitis. 4th ed. Madison: National Mastitis Council; 1996.
Jones GM, Pearson RE, Clabaugh GA, Heald CW. Relationships between somatic cell counts and milk production. J Dairy Sci. 1984;67:1823–31.
Akers RM, Thompson W. Effect of induced leucocyte migration on mammary cell morphology and milk component biosynthesis. J Dairy Sci. 1987;70:1685–95.
Capuco AV, Paape MJ, Nickerson SC. In vitro study of polymorphonuclear leukocyte damage to mammary tissue of lactating cows. Am J Vet Res. 1986;47:633–68.
Zhao X, Lacasse P. Mammary damage during mastitis: causes and control. J Anim Sci. 2008;86:57–68.
McFadden TB, Akers RM, Capuco AV. Relationships of milk proteins in blood with somatic cell counts in milk of dairy cows. J Dairy Sci. 1988;71:826–34.
McFadden TB, Akers RM, Kazmer GW. Alpha-lactalbumin in bovine serum: relationships with udder development and function. J Dairy Sci. 1987;70:259–64.
Akers RM. Lactation and the mammary gland. Iowa State Press Blackwell Publishing Company; 2002. 278 pages.
Akers RM. Major advances associated with hormones and growth factor regulation of mammary growth and lactation in dairy cows. J Dairy Sci. 2006;89:1222–34.
Tucker HA. Physiological control of mammary growth, lactogenesis, and lactation. J Dairy Sci. 1981;64:1403–21.
Tucker HA. Hormones, mammary growth, and lactation: a 41 year perspective. J Dairy Sci. 2000;70:1958–66.
Capuco AV, Akers RM. Lactation | galactopoiesis, effects of hormones and growth factors. In: Fuquay JW, Fox PF, McSweeney PLH, editors. Encyclopedia of dairy sciences, vol. 2. 2nd ed. San Diego: Academic; 2011. p. 26–31.
Capuco, AV, Wood DL, Baldwin R, McLeod K, Paape MJ. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relationship to milk production and the effect of bST. J Dairy Sci. 2001;2177–87.
Capuco AV, Akers RM. The origin and evolution of lactation. J Biology. 2009;8:1–4.
Akers RM, Capuco AV, Keys JE. Cellular differentiation in mammary tissue of beef and dairy heifers. Livest Sci. 2006;105:44–9.
Keys JE, Capuco AV, Akers RM, Djiane J. Comparative study of mammary gland development and differentiation between beef and dairy heifers. Domest Anim Endocrinol. 1989;6:311–9.
Akers RM, Bauman DE, Goodman GT, Capuco AV, Tucker HA. Prolactin regulation of cytological differentiation of mammary epithelial cells in periparturient cows. Endocrinology. 1981;109:31–40.
Capuco AV, Akers RM, Smith JJ. Mammary growth in Holstein cows during the dry period: quantitation of nucleic acids and histology. J Dairy Sci. 1997;80:477–87.
Nickerson SC, Akers RM. Effect prepartum blockade of microtubule formation on ultrastructural differentiation of the mammary epithelium in Holstein heifers. Int J Biochem. 1983;15:777–88.
Filep R, Akers RM. Casein secretion and cytological differentiation in mammary tissue from bulls of high or low genetic merit. J Dairy Sci. 2000;83:2261–8.
Akers RM, Nickerson SC. Effect prepartum blockade of microtubule formation on milk production and biochemical differentiation of the mammary epithelium in Holstein heifers. Int J Biochem. 1983;15:771–5.
Helmbolt CF, Jungherr EL, Plaistridge WN. The histopathology of bovine mastitis. Storrs Agric Exp Stat Bull. 1953;505–95.
Spencer GR. The significance of hypersensitivity in bovine mastitis as determined by a study of its pathogenesis. Thesis. Univ. of Wisconsin. Madison, WI; 1949.
Pattison IH. The progressive pathology of bacterial mastitis. Vet Rec. 1958;70:114–7.
Chandler RL, Reid IM. Ultrastructural and associated observations in clinical cases of mastitis in cattle. J Comp Path. 1973;83:233–41.
Heald CW. Morphometric study of experimentally induced Staphylococcus bovis mastitis in the cow. Am J Vet Res. 1979;40:1294–8.
Capuco AV. Identification of putative bovine mammary epithelial stem cells by their retention of labeled DNA strands. Exp Biol Med. 2007;232:1381–90.
Nickerson SC. Histological response of the bovine mammary gland to experimental S. aureus infection. Ph.D. Thesis, Virginia Tech, Blacksburg, VA; 1980.
Nickerson SC, Heald CW. Histopathologic response of the bovine mammary gland to experimentally induced Staphylococcus aureus infection. Am J Vet Res. 1981;42:1351–5.
Harmon RJ, Heald CW. Migration of polymorphonuclear leukocytes into the bovine mammary gland during experimentally induced Staphylococcus aureus mastitis. Am J Vet Res. 1982;43:992–8.
Seelig LL, Beer AE. Transepithelial migration of leukocytes in the mammary gland of lactating rats. Biol Reprod. 1978;18:736–44.
Frost AJ, Hill AW, Brooker BE. The early pathogenesis of bovine mastitis due to Escherichia coli. Proc R Soc Lond B Biol Sci. 1980;209:431–9.
Nickerson SC, Heald CW. Cells in local reaction to experimental Staphylococcus aureus infection in bovine mammary gland. J Dairy Sci. 1982;65:105–16.
Nickerson SC, Pankey JW. Neutrophil migration through teat end tissues of bovine mammary quarters experimentally challenged with Staphylococcus aureus. J Dairy Sci. 1984;67:826–34.
Lin Y, Xia L, Turner JD, Zhao X. Morphologic observation of neutrophil diapedesis across bovine mammary gland epithelium in vitro. Am J Vet Res. 1995;56:203–7.
Kawanishi H. Morphologic association of lymphocytes with hepatocytes in chronic liver disease. Arch Pathol Lab Med. 1977;101:286–91.
McKenzie WN, Anderson RR. Endotoxin Induced migration of leukocytes from blood to milk. J Dairy Sci. 1981;64:227–35.
Nickerson SC, Pankey JW. Electron microscopic study of leucocytic infiltration of the mammary teat duct during infection with Staphylococcus aureus. Res Vet Sci. 1985;38:167–73.
Heald CW, Harmon RJ. Staphylotoxin induced cytolysis of mouse mammary alveoli at 2, 4, and 6 hours in vitro. JDS 1980; 63, Supplement 1, abstract no. 150
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akers, R.M., Nickerson, S.C. Mastitis and its Impact on Structure and Function in the Ruminant Mammary Gland. J Mammary Gland Biol Neoplasia 16, 275–289 (2011). https://doi.org/10.1007/s10911-011-9231-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-011-9231-3