Abstract
Objectives
The objective of this study was to evaluate the bleaching effectiveness, hydrogen peroxide diffusion (H2O2), and cytotoxicity of a bleaching gel with 35 % H2O2 either associated with ferrous sulfate (FeSO4) or not.
Materials and methods
Enamel/dentin discs adapted to artificial pulp chambers were placed in compartments containing a culture medium (Dulbecco's Modified Eagle's Medium (DMEM)) and distributed into the following groups: G1—no treatment (negative control), G2—10 % carbamide peroxide (one application for 4 h), G3—35 % H2O2 (three applications for 15 min), and G4—35 % H2O2 + 0.004 g FeSO4 (three applications for 15 min). After treatments, the extracts (DMEM + bleaching components that diffused across enamel and dentin) were applied on human dental pulp cells (HDPCs) and odontoblast-like cells (MDPC-23). Cell viability (MTT assay, Kruskal–Wallis and Mann–Whitney, α = 5 %), quantification of H2O2 diffusion, and color change of the enamel/dentin discs (Commission Internationale de I'Eclairage L*a*b* system) were assessed (analysis of variance and Tukey's tests, α = 5 %).
Results
For both cells, a significant reduction in cell viability was observed for G3 and G4 compared with G1 and G2. No statistical difference was observed between G3 and G4. The rate of H2O2 diffusion was significantly higher in G3 compared with that in G2 and G4. The ΔE value for G4 was statistically higher than that of the other groups.
Conclusions
Chemical activation of H2O2 by FeSO4 improves the bleaching effectiveness. However, this metal ion has no significant protective effect against pulp cell cytotoxicity.
Clinical relevance
Although the chemical activation of H2O2 by adding FeSO4 to the bleaching agent improved the bleaching effectiveness, this metal ion has no significant protective effect against pulp cell cytotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen peroxide (H2O2) is a reactive oxygen species widely used in dental bleaching, due to its capacity to dissociate into other reactive oxygen species (ROS) with a high level of oxidative activity, such as peri-hydroxyl ions (HO2 −), superoxide anions (O2 −), singlet oxygen (O2−), and hydroxyl ions (HO−) [1]. These ROS oxidize the chromophores present in the dentin structure, by breaking down the unsaturated bonds in their chains, and reducing tooth light absorption [2]. However, when bleaching gels with high concentrations of H2O2 are applied to the tooth surface, such as those proposed for the in-office bleaching technique, a large quantity of H2O2 quickly diffuses through enamel and dentin and causes intense pulpal damage and tooth sensitivity [3]. Thus, it is essential to reduce H2O2 penetration into the pulp chamber in order to achieve a safer and painless tooth bleaching treatment [4].
New alternatives for in-office bleaching have been recently evaluated [4–7], since this technique has wide clinical applicability, because it is performed under complete professional supervision [2]. Reduction in bleaching gel concentration [5–7] and reduction in contact time with the dental structure are two interesting options reported in the literature, regarding the minimization of pulp cell toxicity and clinical tooth sensitivity. These procedures decrease H2O2 diffusion through the tooth structure, thereby reducing the toxicity to pulp cells in vitro [2]. Clinically, both procedures may also decrease the incidence and intensity of tooth sensitivity [6, 7]. However, the bleaching effectiveness is harmed, and it is necessary to perform extra clinical sessions to obtain the desired esthetic outcome [4, 5]. In addition, bleaching gels (35 and 20 % H2O2) with high and stable pH (8.0–9.0) associated with calcium gluconate seem to be an interesting alternative. This is because these products result in effective tooth bleaching associated with low rates of tooth sensitivity [6], which are approximately three to four times lower than those previously reported in the literature for highly concentrated bleaching gels [8, 9].
Chemical activation of H2O2 by metal salts has been also proposed in the literature, with the goal of improving the clinical effectiveness of bleaching procedure [10, 11]. Moreover, Torres et al. [12] observed that the association of H2O2 with manganese gluconate or ferrous sulfate (FeSO4) resulted in an increase in bleaching effectiveness associated with a reduction in H2O2 diffusion through the tooth structure. According to the authors, metal ions boost H2O2 degradation, resulting in faster reactivity of the ROS with the dental structure, so that the quantity of unreacted free molecules that diffuse through the entire enamel and dentin structure is minimized [12]. Thus, it is believed that chemical activation of bleaching gels may reduce the oxidative damage to the pulp cells caused by H2O2 and, consequently, tooth sensitivity. Therefore, the aim of the present study was to evaluate whether the addition of FeSO4 to an in-office bleaching gel would improve its bleaching effectiveness and also interfere in the H2O2 diffusion through enamel/dentin, and its consequent toxicity to pulp cells. The null hypothesis of this study was that the chemical activation of H2O2 using FeSO4 would have no significant effect on bleaching effectiveness, H2O2 diffusion across enamel and dentin, as well as on pulp cell cytotoxicity.
Materials and methods
Cell culture
A human dental pulp cell (HDPC) primary culture was obtained from the enzymatic digestion of pulp tissue from the third molars donated by volunteers, after signing the term of free and informed consent (Proc. 13/11, Research Ethics Committee of the Araraquara School of Dentistry, SP, Brazil). The pulp tissue was aseptically removed and cut up with a scalpel blade to obtain small fragments. These were incubated at 37 °C and 5 % CO2 for 24 h, in a 25-cm2 culture flask (Corning Inc., Corning, NY, USA) containing Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10 % fetal bovine serum (FBS), 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine (Gibco, Grand Island, NY, USA), and 200 U/mL collagenase type II (Worthington Biochemical Corporation, Lakewood, NJ, USA). After this, the cells were trypsinized, cultivated, and subcultivated in cell culture flasks with a complete DMEM culture medium without collagenase. Cells between the fourth and sixth passages were used in the study. In addition, an immortalized odontoblast-like cell lineage (MDPC-23) was used, which was cultivated and subcultivated in DMEM with 10 % FBS every 3 days, in 75-cm2 flasks (Corning Inc.). To carry out the experimental procedure, the cells were separately seeded in 24-well plates at the density of 2 × 104 cells/cm2 for 48 h at 37 °C and 5 % CO2, and a pattern of 80 % confluence was obtained.
Trans-enamel and trans-dentinal cytotoxicity
A total of 36 enamel/dentin discs, measuring 5.6 mm in diameter and 3.5 mm thick, were obtained from the vestibular surface of bovine incisors, by the same method as described by Soares et al. [4]. The dentin surface of the discs was regularized with 400- and 600-grit abrasive papers and then treated with 0.5 N EDTA for 30 s for smear layer removal. The discs were fitted to artificial pulp chambers (APCs) [4], and the set was sterilized with ethylene oxide (ethylene oxide sterilization chamber, Acecil 1.900 lts, Campinas, SP, Brazil). The disc/APC sets were then placed in 24-well plates (Corning Inc.) containing 1 mL of DMEM (Gibco) without FBS (Gibco), in such a way that the dentin surface remained in intimate contact with DMEM. Bleaching was performed on the enamel surface according to the following groups (n = 9): G1—no treatment (negative control); G2—10 % carbamide peroxide (CP) (Whiteness Perfect, FGM, Joinville, SC, Brazil), one application for 4 h; G3–35 % H2O2 (Whiteness HP, FGM), three applications for 15 min; and G4—35 % H2O2 + FeSO4, three applications for 15 min. For G4, 0.004 g of FeSO4 was incorporated into one drop of thickener and, afterwards, mixed with three drops of liquid containing H2O2, immediately before gel application on the disc surfaces. The products, composition, and application regimens used in the bleached groups are summarized in Table 1. In a previously study, the trans-enamel and trans-dentinal cytotoxicity of Whiteness Perfect 10 % CP gel to MDPC-23 cells was evaluated, and no significant reduction in cell viability was observed [13]. Therefore, this product was considered the parameter of reference for noncytotoxic bleaching therapy. Immediately after the bleaching procedure, two aliquots of 400 μL of the culture medium in contact with dentin from each APC, which contained the components of the bleaching gel capable of performing trans-enamel and trans-dentinal diffusion (extract), were obtained, and each aliquot was applied to previously cultivated cells for 1 h. After this, cell viability was analyzed by means of methyl tetrazolium assay (MTT) (Sigma Chemical Co., St. Louis, MO, USA) [4], and the absorbance of formazan crystals was measured in an ELISA microplate reader at 570 nm (Tp Reader; Thermoplate, Nanshan District, Shenzhen, China). The negative control group (G1) was considered to present 100 % cell viability. Three independent experiments were performed.
Quantification of H2O2 diffusion
One aliquot of 100 μL of the remaining extract from six wells (n = 6) was transferred to tubes containing 900 μL of acetate buffer solution (2 mol/L, pH 4.5) immediately after the bleaching ended. This solution has the capacity to prevent the degradation of H2O2 up to the time of analyses. After this, one aliquot of 500 μL of the buffer solution containing the extract was transferred to tubes containing violet leukocrystal dye (0.5 mg/mL, Sigma Chemical Co.) and horseradish peroxidase enzyme (1 mg/mL, Sigma Chemical Co.). The final volume of the reaction was adjusted to 3 mL with deionized water, and the absorbance of the solutions was measured in an ELISA microplate reader at 600 nm. A standard curve of known H2O2 concentrations was used for the conversion of the optical density obtained in the samples into micrograms of H2O2, and the data were related to micrograms per milliliter of extract.
Bleaching efficacy
To analyze the effectiveness of the proposed bleaching protocols, the enamel/dentin discs were submitted to staining in a black tea solution (n = 6). The dentin surface of discs was treated with 35 % phosphoric acid (3M ESPE, St. Paul, MN, USA) for 60 s and then washed with distilled water for the same period. After this, the discs were incubated at 37 °C for 6 days in a standardized solution of black tea [4, 14]. After this period, the enamel was polished with a pumice stone solution and the discs were submerged in water for 7 days to eliminate the nonadhered pigments. Color analysis was performed in a portable ultraviolet–visible reflectance spectrophotometer (Color guide 45/0, BYK-Gardner GmbH Geretsried, Germany) at a wavelength ranging from 400 to 700 nm and standard D65 illumination. The specimens were adapted to a white silicone matrix, leaving only the enamel surface exposed. The portable spectrophotometer was positioned over the sample, three readouts were performed in each analysis, and the mean value was obtained. Color analysis was performed before and 24 h after the bleaching treatment. The enamel surface of the discs remained in contact with artificial saliva (3.9 % monobasic potassium phosphate, 3.6 % potassium chloride, 2 % sodium chloride, 2 % potassium chloride, 3.7 % magnesium chloride, 0.2 % Phenochem, 10 % Natrosol gel, distilled water qsp, pH = 7.0) [13], and dentin remained in a humid environment to prevent dehydration [4]. For color change analysis, the CIE L*a*b* model of colors, established by the “Commission Internationale de I'Eclairage—CIE” (International Commission on Illumination), was used. This system of color evaluation determines color in a quantitative manner by means of three parameters (L*, a*, and b*), where L* is the measure of luminosity of the object and is quantified on a scale in which black presents a value of L* equal to zero and totally reflected light a value of L* equal to 100, a* is the measure of the quantity of red (+a*) and green (−a*), and b* is the measure of the quantity of yellow (+b*) and blue (−b*). The color change (ΔE) was calculated by means of the following equation: ΔE* = [(ΔL)2 + (Δa)2 + (Δb)2]½, where ΔL = L*final − L*initial, Δa = a*final − a*initial, and Δb = b*final − b*initial.
Statistical analysis
Data was submitted to Levene's test to verify homoscedasticity. Thereafter, the cell viability data of the HDPC and MDPC-23 cells were submitted to the Kruskal–Wallis and Mann–Whitney tests, while the quantification data of H2O2 and of the elements that composed the color analysis (ΔL, Δa, Δb, and ΔE) were evaluated by one-way analysis of variance (ANOVA), complemented by the Tukey multiple comparison tests of pairs. All the tests were considered at a level of significance of 5 %.
Results
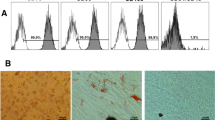
The cell viability results for the human pulp cells (HDPCs) and MDPC-23 are presented in Fig. 1. Considering G1 as presenting 100 % cell viability, the percentage of reduction in viability of the HDPC for groups G2, G3, and G4 was 24.6, 92.6, and 95.9 %, respectively. For MDPC-23 cells, the reduction in viability for groups G2, G3, and G4 was 18.8, 77.3, and 62.3 %, respectively. This reduction was significant for the groups treated with gel containing 35 % H2O2 in comparison with the negative control (G1) and the group bleached with 10 % CP (G2), irrespective of the association with FeSO4, for both studied cell lineages (p < 0.05). No significant difference was observed between G3 and G4 and between G1 and G2 for both cell lineages, when these groups were compared among them (p > 0.05). The data of H2O2 quantification in the extracts are represented in Fig. 2. No peroxide was detected in the negative control group, which was disregarded in the statistical analysis. The group bleached with 10 % CP presented the lowest H2O2 diffusion values (0.22 ± 0.21 μg/mL), followed by the group bleached with 35 % H2O2/FeSO4 gel (2.32 ± 0.56 μg/mL) and the group bleached with 35 % H2O2 (3.53 ± 0.73 μg/mL). All the groups presented a statistical difference when compared (p < 0.05). The results of ΔL, Δa, Δb, and ΔE for the samples submitted to bleaching are presented in Table 2. A significant increase was observed in the value of ΔL and ΔE, for all the bleached groups when compared with the negative control. A significant reduction of Δa and Δb in comparison with the negative control was observed only for the group bleached with 35 % H2O2/FeSO4 gel (G4). When the groups with and without FeSO4 were compared, a significant difference was observed for ΔE only.
a Human dental pulp cell (HDPC) and b odontoblast-like cell (MDPC-23) viability according to the experimental groups (n = 9). The values represent percentage of cell viability. The bottom and top lines of the boxes represent the percentiles 25 and 75, respectively. Therefore, the boxes represent 50 % of the data, while the bars represent the minimum and maximum values of each group. The median is represented by the horizontal line within each box. For each cell type, groups identified with the same letter do not differ statistically (Mann–Whitney, p > 0.05)
Discussion
In the presence of metal ions such as iron (Fe2+), the decomposition of H2O2 is catalyzed according to the Fenton reaction, generating the formation of Fe3+ and HO− [15]. Thus, one observes that the main effect of the addition of FeSO4 to the H2O2-based bleaching agents is to increase the formation of OH- ions, thereby activating the oxidative capacity of these dental products; this is because OH- is a free radical that presents intense reactivity, rapidly interacting with adjacent molecules [1]. Therefore, it is hypothesized that during the bleaching procedures, there is an increase in the formation of OH- on the tooth surface, resulting in a specific and fast reaction with the local chromophores, improving the bleaching effectiveness and reducing the quantity of unreacted H2O2 available for diffusion into the dental structure. This may explain the results of previous studies [10, 12], and data obtained in the present research, in which significant reduction in H2O2 diffusion was demonstrated when the bleaching gel was associated with FeSO4 in comparison with the gel in its pure form, in addition to a significant increase in the value of ΔE (around 30 %). When compared with the negative control, this group presented the best performance in the parameters related to color, such as a significant reduction in Δa and Δb, demonstrating a reduction in chroma, and the highest ΔL values, related to an increase in luminosity of the dental structure.
In the present study, the effect of FeSO4 on the trans-enamel and trans-dentinal cytotoxicity of the bleaching gel to two culture lineages obtained from dental pulp was also evaluated. The viability of MDPC-23 odontoblast-like cells bleached with 35 % H2O2/FeSO4 gel was 15 % higher than the viability observed for that bleached with 35 % H2O2 gel. For HDPCs, no protective effect was observed. However, for both cell lineages, no significant difference was observed when the group bleached with 35 % H2O2/FeSO4 was compared to that bleached with 35 % H2O2 gel. This discrepancy in the results of H2O2 diffusion and toxicity may be due to the fact that the test used detected only the presence of H2O2 in the extract and did not have the capacity to detect the presence of other ROS arising from its degradation [16]. As there is increased decomposition of H2O2 in the presence of FeSO4, one believes that the ROS formed may also have crossed the enamel/dentin disc to cause oxidation of cellular components. Therefore, in spite of this discrete reduction in toxicity, the bleaching gel with 35 % H2O2, irrespective of the association with FeSO4, promoted an intense reduction in viability of both the studied cell lineages when compared with the negative control and the group bleached for 4 h with 10 % PC gel, which is, at present, considered as the safest treatment modality for dental pulp [13, 17]. Other in vitro studies, using a methodology similar to that used in the present study, observed a reduction in cell metabolism of around 40 % when the products resulting from trans-enamel and trans-dentinal diffusion of a gel containing 35 % H2O2 were applied on MDPC-23 cells for 1 h [4, 18]. Trindade et al. [19] demonstrated a reduction of around 92 % in cell viability after 24-h contact of extracts with MDPC-23 cells, confirming that the products released by bleaching gels remain active for long periods and that cell toxicity is proportional to the time of contact of cells with these products.
As regards the 10 % CP gel evaluated in this study, no significant reduction in cell viability was observed when it was compared with the negative control group, as has also been reported in a previous study [13]. It may be suggested that this low cytotoxicity was due to the reduced diffusion of H2O2 through enamel and dentin found in this study. However, the 10 % CP gel used in the present investigation contained fluoride and potassium nitrate in its composition (Table 1). Pretreatment of enamel with fluoride has been indicated to prevent mineral loss during tooth-bleaching procedures, thereby reducing the H2O2 diffusion into the pulp chamber and consequently the occurrence of postoperative tooth sensitivity. In a recent study, the effect of fluoride on the trans-enamel and trans-dentinal cytotoxicity of a 16 % CP bleaching gel was assessed using the same methodology design used in the present investigation. The authors showed that 0.2 and 0.05 % fluoride application (1 min) immediately after the bleaching procedure decreased the enamel mineral loss. On the other hand, the fluoride pretreatment of enamel did not prevent the trans-enamel and trans-dentinal cytotoxic effects caused by the bleaching agent to the cultured MDPC-23 cells [17]. Furthermore, a clinical study observed no reduction in the prevalence of tooth sensitivity when fluoride was associated with at-home bleaching treatment [20]. With regard to potassium nitrate, clinical studies have demonstrated that the application of a desensitizer containing 5 % potassium nitrate and 2 % sodium fluoride did not influence the prevalence of tooth sensitivity immediately before tooth bleaching; however, the duration of sensitivity decreased in patients who used the desensitizer [8, 21]. It has been suggested that potassium ions can block the synapse between nerve cells, reducing the nerve excitation associated with pain [22]. Therefore, it may be suggested that the reduced tooth sensitivity duration reported in these previous in vivo studies was caused, at least in part, by a reduction in the transmission of pain mediated by potassium nitrate present in the desensitizer. Thus, one could expect that the low toxicity associated with the use of 10 % CP observed in this study was related to the low rates of H2O2 diffusion through enamel and dentin.
The greater susceptibility to H2O2 of HDPCs in comparison with the MDPC-23 lineage has been observed in other studies. Min et al. [23] related the occurrence of around 65 % reduction in the viability of HDPCs treated with 0.5 mM of H2O2 for 1 h, while Lee et al. [24] observed a reduction of around 35 % in MDPC-23 viability after 24 h of treatment with the same concentration of H2O2. The difference in response to H2O2 of distinct cell lineages has also been observed by Zhu et al. [25]. The same concentration of H2O2 (0.5 mM) resulted in almost total reduction in the viability of HDPCs and in an immortalized pre-osteoblast lineage (MC3T3-E1), after 24 h of contact. On the other hand, the absence of a significant reduction in cell viability was observed for primary culture lineages of human fibroblasts (HGF) and in an immortalized mouse fibroblast culture (L929). These results were directly related to the quantity of ROS produced by the cells (intracellular activity) after contact with H2O2. The HDPCs and MC3T3-E1 cultures produced a significantly larger quantity of ROS than the HGF and L929 after contact with H2O2. These data demonstrate that H2O2 induces an intense oxidative stress in human pulp cells.
In the face of intense oxidative stress, the intracellular protection mechanisms, such as the production of antioxidant enzymes, are incapable of completely eliminating the local ROS. As a consequence of this imbalance, cell membrane lesion occurs by lipid peroxidation, formation of protein aggregates, and the release of enzymes present in the lysosomes, generating a complete destructuring of cell function and induction of cell death by necrosis [1, 25]. Oxidative stress mediated by H2O2 arising from bleaching gels is also capable of activating proteolytic enzymes present in dentin, such as cathepsins and metalloproteinases, in addition to increasing the expression of these enzymes by the pulp cells, which promotes degradation of the extracellular matrix of the pulp tissue [26]. Consequently, depending on the intensity and amplitude of the cell damage and alterations in the extracellular matrix, the toxicity of the H2O2 present in high concentrations in in-office bleaching gels may cause effects ranging from pulp inflammatory reaction through to extensive areas of tissue necrosis [3].
In conclusion, the null hypothesis was partially rejected since a significant increase in bleaching effectiveness as well as reduction on H2O2 diffusion occurred when FeSO4 was added to the bleaching gel. However, this metallic ion did not significantly reduce the trans-enamel and trans-dentinal cytotoxicity of the bleaching gel to cultured pulp cells. In general, the data obtained in the present study must be interpreted with caution, seeing that an in vitro model was used to evaluate esthetic clinical procedures widely applied in vital teeth. Within this context, and knowing that under physiological conditions the vital tooth presents continuous exudation of fluid through the dentinal tubules, we may suggest that transdentinal diffusion of H2O2 may be reduced, limiting its deleterious effects on the pulp [5]. Thus, further studies in human teeth are needed to evaluate the capacity of transenamel and transdentinal diffusion of ROS released from in-office bleaching gels, to determine whether the concentration of these toxic agents that reach the pulp are capable of causing tissue damage, and finally to observe whether the addition of metal ions, such as FeSO4, to gels may interfere in the adverse effects caused by professional bleaching therapy.
Conclusion
According to the methodology used in the present study, it was concluded that the addition of FeSO4 to a bleaching gel with a high concentration of H2O2 improved the bleaching effectiveness of the dental product and reduced the H2O2 diffusion across enamel and dentin. Nevertheless, this chemical activation of bleaching gel did not significantly prevent the toxicity of the product to cultured pulp cells.
References
Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN (2007) Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta 1773:93–104. doi:10.1016/j.bbamcr.2006.08.039
Goldberg M, Grootveld M, Lynch E (2010) Undesirable and adverse effects of tooth-whitening products: a review. Clin Oral Investig 14:1–10. doi:10.1007/s00784-009-0302-4
De Souza Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:59–64. doi:10.1016/j.tripleo.2009.12.002
Soares DG, Ribeiro AP, da Silveira VF, Hebling J, de Souza Costa CA (2013) Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clin Oral Investig 17:1901–1909. doi:10.1007/s00784-012-0883-1
Soares DG, Basso FG, Pontes EC, Garcia LD, Hebling J, de Souza Costa CA (2013) Effective tooth-bleaching protocols capable of reducing H2O2 diffusion through enamel and dentine. J Dent. doi:10.1016/j.jdent.2013.09.001
Reis A, Kossatz S, Martins G, Loguercio A (2013) Efficacy of and effect on tooth sensitivity of in-office bleaching gel concentrations: a randomized clinical trial. Oper Dent 4:386–393. doi:10.2341/12-140-C
Moncada G, Sepúlveda D, Elphick K, Contente M, Estay J, Bahamondes V, Fernandez E, Oliveira O, Martin J (2013) Effects of light activation, agent concentration, and tooth thickness on dental sensitivity after bleaching. Oper Dent 38:467–476. doi:10.2341/12-335-C
Reis A, Dalanhol AP, Cunha TS, Kossatz S, Loguercio AD (2011) Assessment of tooth sensitivity using a desensitizer before light-activated bleaching. Oper Dent 36:12–17. doi:10.2341/10-148-CR
Reis A, Tay LY, Herrera DR, Kossatz S, Loguercio AD (2011) Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 36:590–596. doi:10.2341/10-173-C
Travassos AC, Rocha Gomes Torres C, Borges AB, Barcellos DC (2010) In vitro assessment of chemical activation efficiency during in-office dental bleaching. Oper Dent 35:287–294. doi:10.2341/09-256-L
Batista GR, Barcellos DC, Torres CR, Goto EH, Pucci CR, Borges AB (2011) The Influence of chemical activation on tooth bleaching using 10 % carbamide peroxide. Oper Dent 36:162–168. doi:10.2341/09-280-L
Torres CR, Wiegand A, Sener B, Attin T (2010) Influence of chemical activation of a 35 % hydrogen peroxide bleaching gel on its penetration and efficacy in vitro study. J Dent 38:838–846. doi:10.1016/j.jdent.2010.07.002
Soares DG, Ribeiro AP, Sacono NT, Coldebella CR, Hebling J, Costa CA (2011) Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast-like MDPC-23 cells. Int Endod J 44:116–125. doi:10.1111/j.1365-2591.2010.01810.x
Sulieman M, Addy M, Rees JS (2003) Development and evaluation of a method in vitro to study the effectiveness of tooth bleaching. J Dent 31:415–422. doi:10.1016/S0300-5712(03)00069-1
Bishop DF (1968) Hydrogen peroxide catalytic oxidation of refractory organics in municipal wastewaters. Ind Eng Chem Proc Des Dev 7:110–117
Mottola HA, Simpson BE, Gorin G (1970) Absorptiometric determination of hydrogen peroxide in submicrogram amounts with leuco crystal violet and peroxidase as catalyst. Anal Chem 42:410–411
Soares DG, Ribeiro APD, Sacono NT, Hebling J, de Souza Costa CA (2013) Effects of fluoride-treated enamel on the indirect cytotoxicity of a 16 % carbamide peroxide bleaching gel to pulp cells. Braz Dent J 24:121–127. doi:10.1590/0103-6440201302161
Dias Ribeiro AP, Sacono NT, Lessa FCR, Nogueira I, Coldebella CR, Hebling J, De Souza Costa CA (2009) Cytotoxic effect of a 35 % hydrogen peroxide bleaching gel on odontoblast-like MDPC-23 cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:458–464. doi:10.1016/j.tripleo.2009.05.006
Trindade FZ, Ribeiro AP, Sacono NT, Oliveira CF, Lessa FC, Hebling J, Costa CAS (2009) Trans-enamel and trans-dentinal cytotoxic effects of a 35 % H2O2 bleaching gel on cultured odontoblast cell lines after consecutive applications. Int Endod J 42:516–524. doi:10.1111/j.1365-2591.2009.01544.x
Armênio RV, Fitarelli F, Armênio MF, Demarco FF, Reis A, Loguercio AD (2008) The effect of fluoride gel use on bleaching sensitivity: a double-blind randomized controlled clinical trial. J Am Dent Assoc 139:592–597
Kose C, Reis A, Baratieri LN, Loguercio AD (2011) Clinical effects of at-home bleaching along with desensitizing agent application. Am J Dent 24:379–382
Markowitz K, Bilotto G (1991) Kim S (1991) Decreasing intradental nerve activity in the cat with potassium and divalent cations. Arch Oral Biol 36:1–7
Min KS, Lee HJ, Kim SH, Lee SK, Kim HR, Pae HO (2008) Hydrogen peroxide induces heme oxygenase-1 and dentin sialophosphoprotein m RNA in human pulp cells. J Endod 34:983–989. doi:10.1016/j.joen.2008.05.012
Lee DH, Lim BS, Lee YK, Yang HC (2006) Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol 22:39–46. doi:10.1007/s10565-006-0018-z
Zhu T, Lim BS, Park HC, Yang HC (2012) Effects of the iron-chelating agent deferoxamine on triethylene glycol dimethacrylate, 2-hydroxylethyl methacrylate, hydrogen peroxide-induce cytotoxicity. J Bioned Res B Appl Biomater 100:197–205. doi:10.1002/jbm.b.31939
Sato C, Rodrigues FA, Garcia DM, Vidal CM, Pashley DH, Tjaderhane L, Carrilho MR, Nascimento FD, Tersariol IL (2013) Tooth bleaching increases dentinal proteases activity. J Dent Res 92:187–192. doi:10.1177/0022034512470831
Acknowledgments
The authors thank the “Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP)” (grant # 2011/12938-8) and the “Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq” (grant # 301029/2010-1) for the financing granted.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira Duque, C.C., Soares, D.G., Basso, F.G. et al. Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin Oral Invest 18, 1631–1637 (2014). https://doi.org/10.1007/s00784-013-1147-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-013-1147-4