Abstract
In this experimental study, we aimed to evaluate the antibacterial and anti-biofilm effects of photodynamic therapy with a photosensitizer in conjunction with Gold nanoparticles against Streptococcus mutans as an important cariogenic bacterial agent. This experimental in vitro study evaluated the antibacterial and anti-biofilm effect of five groups as followed against S. mutans: methylene blue (MB), Gold nanoparticles (AuNPs), methylene blue conjugated with Gold nanoparticles (MB–AuNPs), MB mediated photodynamic therapy (MB mediated PDT) and methylene blue conjugated with Gold nanoparticles mediated photodynamic therapy (MB–AuNPs mediated PDT). InGaAlP laser (Azor-2 K) with 25 mW total output, 660 nm wavelength and laser probe cross-section of 0.78 cm2 was used for methylene blue activation. Total dose of 19.23 J/cm2 for 10 min was irradiated to each group. Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and colony forming unit (CFU) were determined. Bacterial biofilm formation inhibition was assessed by crystal violet staining (The microtiter plate biofilm assay). The viability of S. mutans cells was assessed by MTT assay. MB mediated PDT and MB–AuNP mediated PDT were the most effective method for S. mutans biofilm inhibition (P < 0.05). MB alone, MB–AuNP alone and MB mediated PDT and MB–AuNP mediated PDT had the same effect against the planktonic phase of S. mutans (P > 0.05). Also they had similar pattern for bacterial growth inhibition and bactericidal effect (P > 0.05). Gold nano particle mediated photodynamic therapy represented antibacterial and antibiofilm activity against S. mutans; but this modality was not more effective than routine PDT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus mutans is a spherical gram-positive bacterium from the phylum firmicutes and the lactic acid bacteria group which plays an important role as a cariogenic bacterium [1,2,3,4]. Photodynamic therapy (PDT) is a safe treatment in which a photosensitizer is activated by a light with specific wave length. Singlet oxygen and free radicals are produced in the presence of oxygen and can damage cellular organelles [5]. PDT in dentistry is recommended for its antimicrobial effect, diagnosis and treatment of cancerous or dysplastic lesions. Antimicrobial effects of PDT have been demonstrated in previous studies [5]. PDT is safe and it is not mutagenic. PDT can inhibit biofilm formation and can cause decrease in microbial load [6, 7].
Different nanoparticles including gold nanoparticles, in previous studies have demonstrated antimicrobial properties [2, 8]. Colloidal gold nanoparticles (AuNPs) have proper bioconjugation surface for molecular probes and optical properties which make it a suitable choice for using it in nanomedicine. AuNP has been used as a carrier of drugs for selective killing of diseased cells and microbes [9, 10]. Cationic thiazine dyes such as methylene blue interact with AuNP strongly and increase ultraviolet (UV)-Visible absorption [11]. Nanoparticles in several studies have shown antibacterial properties [12,13,14,15]. Using both nano particles with photodynamic therapy may increase cell penetration and produce cytotoxic singlet oxygen strongly [16] and finally it seems to cause synergistic antibacterial effect.
To the best of our knowledge there is no study about the antibacterial effects of photodynamic therapy with gold nanoparticles or any other nanoparticles. There were some studies about the anticancer effect of photodynamic therapy with nanoparticles [16,17,18] In addition, some studies evaluated antibacterial properties of nanoparticles [13, 19]. Due to the properties of AuNPs and therapeutic effects of photodynamic therapy, we decided to evaluate the effect of photodynamic therapy with gold-nanoparticle on S. mutans as the most prevalent cariogenic bacteria.
Materials and methods
This experimental study has been approved by ethic committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1395.S916).
Synthesis of gold nanoparticles
A solution of 0.1 g gold nanoparticles (AuNP) dissolved in 50 mL water was prepared by Iranian Nanomaterial Pioneers Company. This solution was dark brown with more than 99.97% purity. Size of AuNPs was 28 nm with spherical morphology. Crystallographic structure of nanoparticles was cubic. True density and bulk density of this solution were 19.32 g/cm3 and 0.85 g/cm3, respectively.
Characterization of AuNP
Characterization of AuNP particles was determined by the company which the solution was prepared from. Transmission electron microscopy (TEM) image of colloidal solution of AuNPs was obtained at 100 kV with 180,000 magnitude (EM208, Philips). Image of scanning electron microscopy (SEM) of AuNPs solution was obtained with 70,000 magnitude (Leo 440i) The pattern of X-Ray powder diffraction (XRD) was recorded using EQUINOX (INEL, France) in the range of 30 to 120° in 2θ.
Bacterial strain
Standard strain of S. mutans ATCC25175 was used in this study. The culture of microorganism was prepared in Brain Heart Infusion (BHI) broth and incubated at 37 °C.
Photosensitizer and light source
The photosensitizer used in this study was Methylenee Blue (MB). A stock solution of 1 mg/mL was prepared with distilled water. A InGaAlp laser (Azor-2 k) low level laser with 25mW output power and 660 nm wavelength was used for irradiation. Calculation of effective radiant exposure of light was as fallow:
This irradiation area of laser beam was 0.78 cm2 (laser probe cross section). Continues irradiation with no distance from the plates, in 10 min can produce 19.23 J/cm2 dose.
Determination of minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) and colony formation unit (CFU)
Micro-dilution method was used for determination of MIC and MBC of MB, MB–AuNP conjugates with and without irradiation and AuNP against S. mutans in fresh BHI supplemented with 1% sucrose and fresh BHI alone.
Evaluation grous with fresh BHI alone
Group 1: MB.
Group 2: MB–AuNP.
Group 3: MB + Laser (MB mediated PDT).
Group 4: MB–AuNP + Laser (MB–AuNP mediated PDT).
Group 5: AuNP.
Evaluation grous with fresh BHI supplemented with 1% sucrose
Group 6: MB.
Group 7: MB–AuNP.
Group 8: MB + Laser (MB mediated PDT).
Group 9: MB–AuNP + Laser (MB–AuNP mediated PDT).
In two 96-wells microtiter plates, MB which was six fold serially diluted for four times (initial concentration 1 mg/mL) were added. In two series of MB serial dilution (one for S. mutans in BHI culture media alone and another for S. mutans in BHI supplemented culture media with sucrose), solution of AuNP with concentration of 0.2 mg/mL was added to each well for MB–AuNP conjugate. The standard count of bacterium (inoculum), equal to 0.5 McFarland turbidity, was prepared in PBS and added to all wells. Two wells in each plate were considered as positive and negative controls. One of the plates was considered for the experimental group without laser irradiation and the other one was considered for PDT study (activation of the methylene blue with laser).
In PDT study plate, after 10 min that MB was in contact with the inoculum, all wells were irradiated for 10 min, in dark with no distance between laser probe and plate. Dark environment can help to minimize the other confounding factors including environmental light which can affect the photosensitizer.
Both plates were incubated at 37 °C for 24 h under shake condition. Then the MIC, in which the lowest concentrations that inhibited visible bacterial growth was determined. MBC determination was assessed by culturing the test dilution on a blood agar plate incubated at 37 °C with 5% CO2 for 24 h. MBC was taken from the highest dilution at which no bacterial growth was seen. The number of colony forming unit (CFU) was calculated as well.
Biofilms formation assay by crystal violet staining
Biofilm formation of S. mutans biofilm treated with MB, MB–AuNP conjugate with and without irradiation and AuNP were assessed via micro-titer plate biofilm assay, as described previously (in 1% sucrose supplemented wells) and quantified by using crystal violet. For removing the not adhered bacteria, after incubation the medium was removed gently by washing three times with 200 µL PBS. Then 200 µL of 0.1% crystal violet was added to each well for biofilm staining. After incubating dye for 25 min, extra dye was removed by rinsing three times with 350 µL of sterile distilled water and immediately de-stained with 200 µL of 95% ethanol and remained for 15 min. Quantification of biofilm formation was then measured by optical density (O.D) of suspension at 490 nm, 570 nm and 630 nm [20] with an ELISA reader.
Interaction between MB and AuNPs causes a significant reduction in GNP absorption peak (534 nm) and change it to a new absorption peak (613–662 nm), measured by UV absorption spectroscopy. According to this reduction, a wide range of UV absorption for MB–GNP conjugation can be seen so in this study three different wave lengths have been considered for OD measurement [21].
MTT reduction assay
For the assessment of bacterial cell viability, the MTT assay was performed. A 5 mg/mL concentration of 3–4–5 diphenyl tetrazolium (MTT) dissolved in PBS was used for MTT assay. The solution was filter sterilized using 0.2 mm pore-size filter. As previously described in crystal violet assay, the adherent cells in treated and control S. mutans biofilm, were rinsed three times with 200 µL PBS to remove loosely adherent and planktonic cells. Afterward 100 µL of MTT solution was added to each well. Then the micro-titer plates were incubated at 37 °C for 2 h. Then 400 µL of acidic Isopropanol was added to each well. Colorimetric changes were measured using a microtiter plate ELISA reader at the wavelength of 530 nm.
Statistical analyses
Data analysis was performed using SPSS version 18. The mean value of MIC, MBC, CFU of duplicate evaluations were reported. Kruskal Wallis test was used for comparing the mean of MIC and MBC.
Results
Characterization of AuNP
TEM and SEM images (Fig. 1) of AuNPs show spherical shape and the 28 nm size of the particles. In Fig. 2 a typical power XRD pattern of AuNP is observed.
MIC, MBC and CFU
The minimum inhibitory (MIC) and bactericidal (MBC) concentrations and CFU count of groups 1–9 are reported in Table 1.
Group 4 (MB–AuNPs mediated PDT) demonstrated the lowest MIC and MBC. The CFU count was at lowest level for group 3 (MB mediated PDT) and group 4 (AuNPs-MB mediated PDT) but there was no statistical difference (P > 0.05). AuNP alone with a relative high concentration had inhibitory effect (250 µg/mL) but MBC and CFU could not be detectable even at the highest concentration tested.
No significant difference was detected between different concentrations of MB (P = 0.2) and between different studied groups (P = 0.5) for MIC and MBC.
Assessment of biofilm formation by S. mutans
Biofilm inhibitory effect of groups 1, 2, 3, 4 (MB and MB–AuNp with and without laser illumination) are demonstrated in Figs. 3, 4, 5. AuNP alone did not have anti-biofilm effect.
Applied three wave lengths (490 nm, 570 nm and 630 nm) in most of evaluations showed no significant difference between concentrations in evaluated groups was (P value > 0.05).
But, in 490 and 570 nm wave lengths, group 3 (MB mediated PDT) and 4 (MB–AuNP mediated PDT) were significantly more effective against biofilm formation in comparison to group 1 (MB alone) (P < 0.05). In addition, in 570 nm, group 2 (MB–AuNP) and in 630 nm, group 3 (MB mediated PDT) showed more effective than group 1 in biofilm inhibition (P < 0.05).
In all three wave-lengths, the concentration of 7.81 µg/mL of MB had the lowest biofilm inhibition than all other evaluated concentrations (P < 0.05).
Assessment of bacterial viability
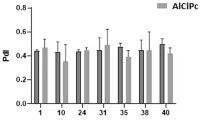
The viability of S. mutans during different antibacterial assays in this study is revealed in Fig. 6.
No difference was detected between different concentrations in evaluated groups (P > 0.05).
Discussion
A photosensitizer is capable to absorb light of specific wavelength and transform it into useful energy [22]. A proper photosensitizer should be low toxic toward mammalian cells. In order to fulfill this property, they should be high binding affinity for microorganisms (positively charged photosentisizer for appropriate adherence to negatively charged bacterial cell wall) [23], low binding affinity for mammalian cells and low chemical toxicity and mutagenicity [24].
PDT cause the bacterial cell wall damage, increasing cytoplasmic membrane permeability and breakage of DNA [25]. PDT can destroy carbohydrates, amino acids or phosphate transport through carrier proteins of cell membrane [26]. Intracellular enzymes will be knocked out and disturb ATP production and cellular metabolism [27] cyclic dependent kinase inhibitor and cell growth inhibition causes over expression of P21 which irritates mitochondria [28]. Biological chlorophores in cells and organisms excites via proper dose and wavelength of laser illuminance, and produce singlet oxygen and superoxide. These toxic products destroy microorganisms by DNA and cell membrane destruction [29].
The different nanoparticles including gold nanoparticles, in previous reports have revealed antimicrobial properties [2, 8] besides, Cationic thiazine dyes such as methylene blue interact with AuNP strongly and increase ultraviolet (UV)-Visible absorption [11]. Evaluation of the antibacterial and anti-biofilm effect of photodynamic effect of methylene blue with gold nanoparticles against S. mutans as the significant producer of cariogenic biofilm was the idea of the present study.
According to the results of the present study, all testing groups except for AuNP onle, had antibacterial and antibiofilm effect. AuNP alone with a very high concentration had inhibitory effect (more than twofold higher concentrations than the other groups) and MBC and CFU could not be measured.
The MIC, MBC and CFU counts of all evaluated groups (MB, MB–AuNPS, MB mediated PDT, MB–AuNP mediated PDT) for S. mutans cultured in BHI with and without sucrose supplementation, are in a similar pattern and there were no significant differences between them (P < 0.05). Biofilm assay confirmed the concentration dependency for better S. mutans biofilm inhibition. MB mediated PDT and MB–AuNP mediated PDT had the best inhibitory effect on S. mutans biofilm formation among all groups in all three applied wave lengths. In MTT assessment no statistical difference between all evaluated groups was detected.
There are a few studies that evaluated the effectiveness of nanoparticles mediated PDT including an in vitro antimicrobial and anti-biofilm study of PDT with AgNP, TBO and AgNP-TBO, used 9.1 J/cm2 irradiation dose with 630 nm wavelength for 70 s [19].
The MIC and MBC of TBO-AgNP mediated PDT (with an initial concentration of 1 mg/mL TBO and a constant concentration of 25 µg/mL AgNP) showed a twofold higher activity against S. mutans than TBO alone. In comparison, our results showed less MIC and MBC for MB–AuNP-mediated PDT than MB mediated PDT alone against S. mutans, however, these differences were not significant.
In Misba et al. study [19], crystal violet assay showed anti-biofilm effect in a concentration dependent manner. A more significant reduction of biofilm formation with TBO-AgNP mediated PDT was detected in comparison with TBO mediated PDT. Better effect of photodynamic therapy generally in comparison to use of methylene blue alone is in line with our results in the presenting study. In addition to the concentration dependent pattern, MB–AuNP-mediated PDT and MB-mediated PDT had a higher effectiveness in biofilm inhibition than MB and MB–AuNP alone. Misba et al. [19], observed a better effect when adding AgNP to TBO mediated PDT than TBO mediated PDT alone. This is not in accordance with the present study, which could not detect significant differences between the PDT groups, that is, no extra effect was obtained with the addition of AuNP to MB mediated PDT. Moreover, MB itself has antibacterial effect [30] also higher concentrations of AuNPs for preparing the MB and nanoparticle conjugation might have been needed for better a antibacterial effect. This may justify this controversy.
In addition, Misba evaluated the bactericidal activity by converting the value of XTT, which was found to be concentration dependent as well. TBO-AgNP mediated PDT had a noticeable bactericidal effect on S. mutans in comparison with TBO at the same concentrations. We used MTT assay for bacterial viability assessment and MB–AuNP mediated PDT had no superiority for bactericidal properties than MB mediated PDT and other groups at the same concentrations.
Other study declared that polymeric nanoparticles are potent to load MB and act as a carrier of MB for PDT system [31]. Khan et al. study showed that AuNPs (GNP) conjugates with photosensitizer (PS) in PDT exhibited synergistic antimicrobial activity against Candida albicans. The GNP-PS mediated PDT against Candida biofilm formation showed significant biofilm reduction and adverse effect against Candida albicans [32].
In Sherwani et al. study, the effect of GNP-PS based PDT on C. albicans was also evaluated, which confirmed the synergistic activity of GNP-PS conjugation against C. albicans [8].
Chemical structure of photosensitizer, incubation period of photosensitizer and bacterial cell, bacterial species, time of irradiation, photosensitizer concentration and laser energy dose would affect the PDT effectiveness [22, 25].
MB is a phenotiazinuim dye, which has a very low toxicity in dark, penetrates the bacterial cell membrane, affect the bacterium genome and kill them [30]. MB is hydrophilic with low molecular weight and positive charge. These properties allow MB to pass across purine-protein channel in the outer membrane of gram-negative bacteria [33, 34].
Bacterial colonies are more resistant when they accumulate as a biofilm, due to better communication between cells [35]. PDT has a direct effect on extracellular polysaccharides matrix of biofilm are susceptible to photo damage [36].
As explained previously, energy of light exposure is an important factor in PDT efficacy. Thus it has been indicated that light exposure with a high power density over a short period of time compared with low power density over a longer time even with the same energy density in both, may cause different antimicrobial effects [25].
Nowadays, metallic nanoparticles are mostly used for bio-conjugation, drug delivery and also diagnostic application [37, 38]. Metallic nanoparticles are large in surface area which affects its antimicrobial effect [39]. The antimicrobial activity of nanoparticles has an inverse relationship with nanoparticles’ size. A range size between 1 and10 nm have the best antibacterial activity. In comparison with conventional and narrow target antibiotics, bacteria are less likely to acquire resistance against metallic nanoparticles [40, 41].
Several studies indicated that positive charge on the metal ion is essential for antimicrobial activity. Bacterial cell membrane has negative charge. Thus an electrostatic attraction happens between the positive charge of metal ion and negative charge of cell membrane [42].
Gold nanoparticles (GNP) is known to have special chemical and physical and optical properties due to its remarkable surface plasmon resonance for imaging and fluorescence enhancing [43, 44] Molecular cytotoxicity mechanism of GNP is only disruption of protein conformation [6].
Interaction between MB and AuNPs causes a significant reduction in GNP absorption peak (534 nm) and change it to a new absorption peak (613, 662 nm), measured by UV absorption spectroscopy. According to this reduction, a wide range of UV absorption for MB–GNP conjugation was seen [20]. The interaction between the negative charge of GNP and positive charge of MB has been confirmed by TEM image [31]. A noticeable quenching of MB fluorescence in addition with GNP was reported. AuNPs interaction with MB in the form of a 2 ± 0.5 nm layer of MB around AuNPs causes the new peak of UV absorbance [31].
The mechanism of quenching the fluorophore by AuNPs are a radioactive energy transfers from fluorophore to GNP and collision dynamics between fluorophore and AuNPs [45, 46].
Pagonis et al., revealed that the permeability of cell walls for MB carrier increases by the concentration of nanoparticles on bacterial cell wall [47].
Photodynamic therapy can be used as a method for decreasing the oral cariogenic bacterial count especially in patients with rampant caries or in patients with greater susceptibility to dental caries including patients with history of xerostomia, chemo and radio therapy.
Although there is a good concept about nanoparticles efficacy, side effects must also be considered. for example, actively excreted form of copper and silver accumulation within normal body [48]. To understand the toxic kinetic properties of nanoparticles, information about their absorption, distribution, metabolism and excretion is necessary [49].
Performing the evaluation in laminar flow, which produce a sterile, isolated environment for such lab research, could assure the researcher about no external contamination. In this study the anti S. mutans effect of PDT mediated by different compositions of MB and MB–AuNPs, in several media (BHI and sucrose supplemented BHI) were assessed. To determine the effective component of these approaches, different groups were designed. The antibacterial effects of MB, MB–AuNP and AuNPs alone without any light activation were also analyzed. Higher concentrations of AuNPs for preparing the MB and nanoparticle conjugation should be assessed in further evaluations.
In our study, the cover of plates was not removed during laser application since multiple plate contamination had been occurred before in pilot studies. The presence of this translucent barrier could reduce the irradiation dose. According to some studies, Vahabi et al. [50], using shorter time and higher dose of irradiation can induce more prominent effect. Our laser device could not produce enough dose of irradiation in a short period. In six series of repeating the evaluation, there were somehow different and contradictory results; while we did our best effort to homogenize and statistically analyze the situation on each effort.
It can be suggested to evaluate different AuNP concentrations and different irradiation dose. To have more complete evaluation, it can be proposed to study the developed biofilm inhibition of S. mutans.
While there is a global interest to introduce nanotechnology as an effective modality for different medical interventions, treatments or preventive procedures, according to our results there was no significant superiority for nanoparticle association with PDT. Choosing a proper photosensitizer and light activation wavelength and irradiation dose can induce enough antibacterial effects.
Conclusion
Our study revealed MB-mediated PDT and MB–AuNP-mediated PDT were the most effective method for S. mutans biofilm inhibition. Increasing the concentrations of photosensitizer along with gold nanoparticles reduces the bacterial biofilm formation more effectively, however, side effects must also be an important concern. Different groups in this study had the same effect against the planktonic phase of S. mutans. MB alone, MB–AuNP alone and MB-mediated PDT and MB–AuNP-mediated PDT had similar pattern for bacterial inhibition and bactericidal effect. Based on this study, both MB-mediated PDT and MB–AuNP-mediated PDT had proper antibacterial biofilm formation activity.
Data availability
Not applicable.
References
Lavaee F, Modaresi F, Faez K, Esnaashari N (2019) Antimicrobial efficacy of two shrubs (punica granatum and camellia sinensis) and an herb (mentha piperita) on two human pathogens, Streptococcus mutans and Streptococcus sanguinis. Ambient Sci 6(1):31–36
Lavaee F, Faez K, Hadi N, Modaresi F (2016) Antimicrobial and antibiofilm activity of silver, titanium dioxide and iron nano particles. Am J Dent 29(6):315–320
Ghapanchi J, Moattari A, Lavaee F, Shakib M (2015) The antibacterial effect of four mouthw ashes against Streptococcus mutans and Escherichia coli. JPMA 65(350).
Grzech-Leśniak K, Nowicka J, Pajączkowska M, Matys J, Szymonowicz M, Kuropka P et al (2019) Effects of Nd: YAG laser irradiation on the growth of Candida albicans and Streptococcus mutans: in vitro study. Lasers Med Sci 34(1):129–137
Afroozi B, Zomorodian K, Lavaee F, Shahrabadi ZZ, Mardani M (2019) Comparison of the efficacy of indocyanine green-mediated photodynamic therapy and nystatin therapy in treatment of denture stomatitis. Photodiagnosis Photodyn Ther 27:193–197
Paschoal MA, Lin M, Santos-Pinto L, Duarte S (2015) Photodynamic antimicrobial chemotherapy on Streptococcus mutans using curcumin and toluidine blue activated by a novel LED device. Lasers Med Sci 30(2):885–890
Sampaio FJP, Pires-Santos GM, de Oliveira SC, Monteiro JS, Bagnato VS, Pinheiro AL (2016) Photodynamic antimicrobial chemotherapy (PACT) against oral microorganisms with the use of blue LED associated to curcumin. Proc of SPIE.
Sherwani MA, Tufail S, Khan AA, Owais M (2015) Gold nanoparticle-photosensitizer conjugate based photodynamic inactivation of biofilm producing cells: potential for treatment of C. albicans infection in BALB/c mice. PloS One 10(7):e0131684
Huang W-C, Tsai P-J, Chen Y-C (2007) Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomed 2(6)
Han G, Ghosh P, Rotello VM (2007) Functionalized gold nanoparticles for drug delivery. Nanomedicine 2(1):113–123
Narband N, Uppal M, Dunnill CW, Hyett G, Wilson M, Parkin IP (2009) The interaction between gold nanoparticles and cationic and anionic dyes: enhanced UV-visible absorption. Phys Chem Chem Phys 11(44):10513–10518
Aliasghari A, Rabbani Khorasgani M, Vaezifar S, Rahimi F, Younesi H, Khoroushi M (2016) Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: an in vitro study. Iran J Microbiol 8(2):93–100
Biradar D, Lingappa K, Dayanand A (2012) Antibacterial activity of nano goldparticles synthesized by Bacillus Sps. J Ecobiotechnol 4(1):43–45
Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Sakagami H, Morales-Luckie RA, Nakajima H (2015) Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appli Oral Sci 23(3):321–328
Ramazanzadeh B, Jahanbin A, Yaghoubi M, Shahtahmassbi N, Ghazvini K, Shakeri M et al (2015) Comparison of antibacterial effects of ZnO and CuO nanoparticles coated brackets against Streptococcus Mutas. J Dent 16(3):200–205
Jeong K, Park S, Lee YD, Kang CS, Kim HJ, Park H et al (2016) Size-engineered biocompatible polymeric nanophotosensitizer for locoregional photodynamic therapy of cancer. Colloids Surf, B 144:303–310
Kalluru P, Vankayala R, Chiang CS, Hwang KC (2016) Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 95:1–10
Miyoshi N, Kundu SK, Tuziuti T, Yasui K, Shimada I, Ito Y (2016) Combination of sonodynamic and photodynamic therapy against cancer would be effective through using a regulated size of nanoparticles. Nanosci Nanoeng 4(1):1–11
Zawrah M, El-Moez S, Center D (2011) Antimicrobial activities of gold nanoparticles against major foodborne pathogens. Life Sci J 8(4):37–44
Sharman WM, Allen CM, Van Lier JE (1999) Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today 4(11):507–517
Alves E, Costa L, Carvalho CM, Tomé JP, Faustino MA, Neves MG et al (2009) Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiolog 9(1):70
Soukos NS, Goodson JM (2000) Photodynamic therapy in the control of oral biofilms. Periodontol 55(1):143–166
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42(1):13–28
Paardekooper M, De Bruijne AW, Van Steveninck J, Van den Broek PJ (1993) Inhibition of transport systems in yeast by photodynamic treatment with toluidine blue. Biochim Biophys Acta Biomembr 1151(2):143–148
Paardekooper M, Bruune AWD, Steveninck JV, Van den Broek PJ (1995) Intracellular damage in yeast cells caused by photodynamic treatment with toluidine blue. Photochem Photobiol 61(1):84–89
Sasnauskiene A, Kadziauskas J, Vezelyte N, Jonusiene V, Kirveliene V (2009) Apoptosis, autophagy and cell cycle arrest following photodamage to mitochondrial interior. Apoptosis 14(3):276–286
Romanova N, Brovko LY, Moore L, Pometun E, Savitsky A, Ugarova N et al (2003) Assessment of photodynamic destruction of Escherichia coli O157: H7 and Listeria monocytogenes by using ATP bioluminescence. Appl Environ Microbiol 69(11):6393–6398
Misba L, Kulshrestha S, Khan AU (2016) Antibiofilm action of a toluidine blue O-silver nanoparticle conjugate on Streptococcus mutans: a mechanism of type I photodynamic therapy. Biofouling 32(3):313–328
Klepac-Ceraj V, Patel N, Song X, Holewa C, Patel C, Kent R et al (2011) Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers Surg Med 43(7):600–606
Wainwright M, Phoenix D, Gaskell M, Marshall B (1999) Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp. J Antimicrob Chemother 44(6):823–825
Khan S, Alam F, Azam A, Khan AU (2012) Gold nanoparticles enhance methylene blue–induced photodynamic therapy: a novel therapeutic approach to inhibit Candida albicans biofilm. Int J Nanomed 7:3245
Seong D-Y, Kim Y-J (2015) Enhanced photodynamic therapy efficacy of methylene blue-loaded calcium phosphate nanoparticles. J Photochem Photobiol B 146:34–43
Usacheva MN, Teichert MC, Biel MA (2003) The interaction of lipopolysaccharides with phenothiazine dyes. Lasers Surg Med 33(5):311–319
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358(9276):135–138
Konopka K, Goslinski T (2007) Photodynamic therapy in dentistry. J Dent Res 86(8):694–707
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346
Parak WJ, Gerion D, Pellegrino T, Zanchet D, Micheel C, Williams SC et al (2003) Biological applications of colloidal nanocrystals. Nanotechnology 14(7):R15
Holister P, Weener J, Vas CR, Harper T (2003) Nanoparticles Technology White papers no. 3. Cientifica 1–11
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT et al (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbio 73(6):1712–1720
Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ et al (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3(1):95–101
Tam F, Goodrich GP, Johnson BR, Halas NJ (2007) Plasmonic enhancement of molecular fluorescence. Nano Lett 7(2):496–501
Glomm WR (2005) Functionalized gold nanoparticles for applications in bionanotechnology. J Dispers Sci Technol 26(3):389–414
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P et al (2009) Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater 8(7):543–557
Dulkeith E, Morteani A, Niedereichholz T, Klar T, Feldmann J, Levi S et al (2002) Fluorescence quenching of dye molecules near gold nanoparticles: radiative and nonradiative effects. Phys Rev Lett 89(20):203002
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X et al (2010) Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod 36(2):322–328
Seetharam RN, Sridhar KR (2007) Nanotoxicity: threat posed by nanoparticles. Curr Sci 93(6):769–770
Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJ (2007) What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol Pharmacol 49(3):217–229
Vahabi S, Fekrazad R, Ayremlou S, Taheri S, Zangeneh N (2011) The effect of antimicrobial photodynamic therapy with radachlorin and toluidine blue on streptococcus mutans: an in vitro study. J Dent 8(2):48
Acknowledgements
The authors thank the Vice-Chancellor of Shiraz University of Medical Science for supporting this research (Grant# 11909). This manuscript is based on the thesis by Dr. Ghazaal Rafiee. The authors also thank to Dr.Mohammad Salehi of the Center of Research Improvement of the School of Dentistry for the statistical analysis.
Funding
The Vice-Chancellor of Shiraz University of Medical Science for supporting this research (Grant# 14183).
Author information
Authors and Affiliations
Contributions
FL conceived of or designed study, performed research, analyzed data, contributed new methods or models, wrote the paper. FM conceived of or designed study, performed research, analyzed data, contributed new methods or models, wrote the draft paper. SA conceived of or designed study, performed research, analyzed data, wrote the draft paper.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Ethical approval
The study has been approved by ethic committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1396.S727).
Code availability
Not applicable.
Consent to participate
Written consent form was taken before initiation of the study.
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lavaee, F., Motamedifar, M. & Rafiee, G. The effect of photodynamic therapy by gold nanoparticles on Streptococcus mutans and biofilm formation: an in vitro study. Lasers Med Sci 37, 1717–1725 (2022). https://doi.org/10.1007/s10103-021-03422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03422-x