Abstract
This study aimed to investigate the effects of photobiomodulation at a wavelength of 660 and 830 nm at different numbers of application points in the healing of open wounds in mice. In total, 120 mice were divided into 10 groups. The animals were submitted to cutaneous lesion of the open wound type (1.5 × 1.5 cm). Photobiomodulation at a wavelength of 660 and 830 nm and total energy of 3.6 J were used, applied at 1, 4, 5, and 9 points, for 14 days. The animals were subjected to analysis of the lesion area, skin temperature, and histological analysis. Macroscopic analysis results showed a difference (p < 0.05) between the irradiated groups and the sham group at 14 days PO. There was no statistical difference in skin temperature. Histological analysis findings showed better results for the epidermis thickness. Regarding the number of blood vessels, a difference was found between the 1- and 5-point 830-nm photobiomodulation groups and between the 4-point 660-nm group and the naive group. A significant difference in the number of fibroblasts was observed between the 830- and 660-nm photobiomodulation groups and the naive and sham groups. When comparing photobiomodulation wavelength, the 830-nm groups were more effective, and we emphasize the groups irradiated at 5 points, which showed an improvement in macroscopic analysis and epidermis thickness, an increase in the number of vessels, and a lower number of fibroblasts on the 14th day after skin injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin wounds are characterized as an anatomical change in skin integrity caused by cell rupture and occur due to multiple factors such as hypoxia, trauma, or pressure [1, 2]. Injuries to the integumentary system can be caused acutely, such as in operative wounds, traumatic injuries, and cut injuries, or late, highlighting pressure injuries and those caused intentionally such as grafts or skin flaps that are used in surgical procedures. Regardless of how they are caused, all of these injuries require proper management to minimize the risk of infections, tissue necrosis, and hypertrophic scars [3, 4]. Tissue healing can be impaired by local factors such as ischemia, infection, and elevated tissue pressure, or systemic factors such as immunosuppression, diabetes mellitus, hypothyroidism, and smoking [1].
Lesion treatments that affect the cutaneous tissue aim to reduce healing time and improve the appearance of the healing result. Among the different forms of treatment, we can mention wound debridement, use of dressings, medications [5, 6], nutritional supplementation for malnourished individuals, pressure relief with decubitus changes [7], vacuum therapy [8], extracorporeal shock waves [9], high-voltage electrical stimulation [10,11,12], therapeutic ultrasound [7], radio frequency [13, 14], and photobiomodulation [15, 16].
Regarding the various treatments for tissue injuries, photobiomodulation stands out as an ally in wound healing due to its photobiomodulator effect which accelerates the tissue repair process, causing a reduction of the inflammatory reaction and improved speed of the soft tissue repair process. Its irradiation of the injured tissues triggers a series of physiological effects due to the absorption of photon energy by photoreceptors. When the energy interacts with cells, it causes the activation of mitochondrial ATP due to absorption of light by cytochrome c oxidase, resulting in the photodissociation of nitric oxide and the proliferation of several cells, promoting anti-inflammatory effects and triggering increased proliferation–migration and cell differentiation, cytokine modulation, growth factor production, and deposition of extracellular matrix [17,18,19,20,21,22].
There is a variety of research on the healing of cutaneous lesions, with different parameters in the treatment for regeneration and viability, and without a consensus or therapeutic window described, in addition to a lack of studies or standardization of the parameters used in the different ways of applying stitches to injuries. Given the above, this study aimed to investigate the effect of photobiomodulation applied at different wavelengths and different numbers of points to cutaneous wounds in mice.
Materials and methods
This is an experimental study with animals, containing intervention groups and a control group (Fig. 1). It used 120 Swiss lineage male mice (40–45 g) with a mean age of 60 days, which were kept in the sectoral vivarium at the Araranguá Campus of the Federal University of Santa Catarina (UFSC).
The interventions were carried out in a room for animal experimentation at UFSC, following all the environmental precautions recommended by the Animal Use Ethics Commission (CEUA) and approved under number 4017201117; the ARRIVE checklist was used.
The experiments were performed during the clear cycle (from 7 am to 7 pm), and the animals were kept in the laboratory for acclimatization for at least 30 min before the evaluations were performed. All animals received tramadol analgesic every 8 h for 3 days [23, 24].
Surgical procedure
Mice were anesthetized with intraperitoneal (IP) injection of 100 mg/kg ketamine hydrochloride (Agener União®) associated with 10 mg/kg xylazine hydrochloride (Dopaser®) [25, 26]. Then, trichotomy was performed by manual traction of the hair on the back of the animals. They were then submitted to surgical incision: 1.5 × 1.5 cm of skin was surgically removed using a template developed for the experiment (Fig. 2a, b).
Intervention (photobiomodulation therapy)
The photobiomodulation therapy was performed at wavelengths of 830 nm (AsGaAl) and 660 nm (AlGaInP) using Ibramed® Medical Equipment (São Paulo, Brazil). The parameters used in this paper are shown in Table 1, and Fig. 2c demonstrates the localization of the application points.
Analysis of the samples
The animal analysis procedures were carried out in the sectoral bioterium and in the microscopy laboratory of the Center of Sciences, Technology, and Health at the Araranguá Campus of UFSC.
Skin lesions were assessed daily by macroscopic observation before the application of photobiomodulation. All animals were photographed with a Cyber-Shot DSC-P72 digital camera (5.1 megapixels, Zoom 3.2; Sony®, USA) kept at a constant distance of 20 cm; photographs were later analyzed using ImageJ® software. Analyses were performed immediately after surgery and on the 7th and 14th days after the surgical procedure.
Thermography is a technique that consists of observing temperature through high-resolution infrared technology. The evaluations were performed after irradiation of the photobiomodulation (PBM), in the following times: immediately after surgical incision, 7 and 14 days after the surgery4. We used a constant distance of 20 cm between the FLIR C2 camera and the animals’ dorsal region to record the temperature of the lesion region, which was later analyzed using FLIR Tools™ software.
After euthanizing the animals by anesthetic overdose on the 14th day after the surgical procedure, skin samples were removed and immersed in 10% formalin for 48 h. The samples were fixed, dehydrated, diaphanized, embedded in paraffin, and then cut by a microtome to obtain 5–6-μm-thick non-serial sections. We stained the skin samples with hematoxylin and eosin (HE) for histological evaluation by light microscopy.
A trinocular microscope and 14-megapixel digital camera, both from Global Optics, were used to acquire histological images.
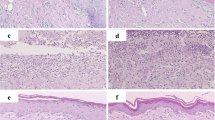
To determine the thickness (in micrometers) of the epidermis of each of the samples, quantitative analysis of the images of the histological sections was performed using ImageJ® software (Fig. 3a).
Illustration of histological evaluation. a Analysis of epithelial thickness, objective × 40. b Analysis of the number of vessels; the arrow indicates a blood vessel and the grids in the left corner demonstrate the form used for counting. c Counting of fibroblasts; the arrow indicates the location of a cell
The number of blood vessels was determined from the images of the samples which were standardized for counting a grid with 1.5 × 1.5 cm squares, totaling 100 squares in the lower quadrants (Fig. 3b).
The number of fibroblasts was determined using the ImageJ® cell counter tool which counts cells by hand marking (Fig. 3c).
Statistical analysis
We verified the normality of the data by the Shapiro–Wilk test, with the variables presenting normal distribution. We evaluated the lesion area and skin temperature with one-way ANOVA with repeated measures. Statistical analysis for histology was performed using one-way ANOVA and post hoc Tukey’s tests with GraphPad Prism 8.0 software.
Results
To obtain the data related to the present study, we used 125 male Swiss mice (40–45 g), mean age 60 days. During the procedure, some losses occurred due to autophagy (2 animals) and soon after anesthesia (3 animals). All 120 animals were distributed in 10 groups, 9 experimental groups and a naive group. The following results are described according to the analysis performed in this research.
Macroscopic observation of the wound area was performed; photos for evaluation were taken at three different times (immediately postoperative, and 7 and 14 days after the surgery) and area analysis was performed using ImageJ® software. Figure 4 shows the data regarding the wound area (cm2) at the three evaluation points, for the sham group and the groups irradiated with a laser at wavelengths of 660 and 830 nm. Analyses were performed with one-way ANOVA with repeated measures, p value < 0.05; in the first (immediately postoperative) and second (7 days) evaluations, no statistically significant differences were observed between the groups. In the third evaluation (14 days), the photobiomodulation groups were statistically different (p < 0.05) to the sham group, except for the 830-nm photobiomodulation group irradiated at 9 points.
Histological analysis was performed using a sample of cutaneous tissue taken from the dorsal region of the animals. The procedure was performed on the 14th day after euthanasia by using excess anesthetics. Microscopic analysis of skin thickness, permeated blood vessels, and the number of fibroblasts was performed. We used the one-way ANOVA statistical method to analyze the obtained data with p < 0.05.
Epidermis thickness in the group irradiated at 5 points at 830 nm was significantly different (p < 0.05) to that in the naive, sham, 1-point 660-nm, 5-point 660-nm, and 1-point 830-nm groups; the group irradiated at 4 points at 830 nm was significantly different to the 1-point 660-nm and 830-nm groups; and the group irradiated at 9 points at 830 nm was significantly different to the group irradiated at 1 point at 660 nm. All data are shown in Fig. 5.
Analysis of the number of permeated blood vessels was performed using a grid developed with 1.5 × 1.5 cm squares, containing 100 squares distributed in the lower quadrant of the image, totaling the number of vessels. Figure 6 shows a difference between the 1- and 5-point 830-nm groups and the 4-point 660-nm group vs the naive group.
Figure 7 demonstrates the histological analysis regarding the number of fibroblasts. A significant difference is observed between all the 830-nm groups (1, 4, 5, and 9 points of application) and the naive and sham groups; between the 1-, 4-, and 5-point 660-nm groups and the naive group and between the 1-, 4-, 5-, and 9-point 660-nm groups and the sham group. There is also a significant difference between the 1-point 660-nm group and the 4- and 5-point 660-nm groups.
Skin temperature analysis is shown in Fig. 8. There was no statistical difference (p > 0.05) between the groups evaluated.
Discussion
The present study aimed to investigate and compare the effect of laser PBM applied at different numbers of points and different wavelengths on healing of an open skin wound in mice. The animals described as sham are those that received the surgical intervention but were treated with placebo PBM (device off) for the time proposed for the animals that received the intervention (120 s). Naive animals, on the other hand, did not receive surgical intervention or any form of treatment, being necessary as controls for temperature and histological analysis.
Several studies on the healing of integumentary lesions performed with PBM at different wavelengths found positive effects of this treatment. Three of the studies investigated the effects of PBM in the red spectrum (635–670 nm) and found evidence of its effectiveness [27, 28] and effects similar to those of infrared wavelength (830 nm), except for an increase in the number of fibroblasts [29]. Most studies (six) found efficacy of healing for PBM at 810–870 nm, both in isolation and compared with infrared PBM [4, 30,31,32,33,34]. In line with most studies, we found superior PBM effects at 830-nm wavelength but, in some respects, we also observed positive results in the 660-nm groups.
Various authors have described that the dose–response of PBM is influenced by the intensity or time of exposure, and by parameters such as target tissue depth, attenuation, treatment interval, and wavelength. The present study sought to compare the effects on the tissue of two wavelengths and different numbers of points of application [35,36,37].
Based on this, we note that the results related to application at different numbers of points of the skin wound may have been influenced by the number of points and the division of energy deposited at each point. Several studies have used PBM in skin tissue injuries; most of these used an experimental skin flap model and demonstrated positive results for improving tissue viability. However, there is no consensus when analyzing the dose used that ranged from 3 to 144 J/cm2 or the number of points of application that varied from 1 to 54 points [38,39,40,41].
Based on that and the area of injury that we get with the experimental model, we chose to score the application at 1, 4, 5, and 9 points. Thus, we can verify that the parameters used can influence the result given that, in our study, we found that application at 4 and 5 points was more effective than that at 1 or 9 points. Where our study differs from those presented is that the others used several points with the sum of the parameters, at either different times or energy application, for example, and in our research, we made a control so that the final parameters were equal in all groups (total time, total energy, energy per point, fluence per point, and total fluence).
Regarding macroscopic analysis, we observed significant differences only in the groups in which 830-nm PBM was applied at 4 or 5 points, indicating that treatment in these groups was more effective. All groups presented a healing process in its natural course.
PBM therapy on the skin wounds can influence neoangiogenesis, epithelial and fibroblast proliferation, collagen synthesis and deposition, revascularization, and wound contraction, having a beneficial effect in accelerating skin wound healing. Corroborating our findings above, we obtained significant results for epithelium thickness, the 5-point 830-nm PBM group being different to the naive, sham, 1-point 830-nm, and 1-point 660-nm groups, and the 4-point 830-nm group being different to the 1-point 660-nm group [20, 42,43,44,45,46].
Leite et al. [47] investigated the effect of pulsed electric field and laser PBM on the viability of the TRAM flap in diabetic rats and found that PBM causes an increase in epidermis thickness; reduces necrosis area and leukocyte number; increases mast cells, vascular endothelial growth factor, and fibroblast growth factor; and enlarges the neoformed blood vessels. Their studies corroborate our findings, in which we observed an increase in epithelium thickness.
Regarding the number of vessels, there was a difference between the naive control and three of the treatment groups: the 660-nm group with 4 application points and the 830-nm groups with 1 and 5 points; the latter stands out, with a large increase in the number of vessels.
Melo et al. [48] aimed to evaluate the effect of low-power laser therapy at a wavelength of 904 nm on the healing of surgical wounds in rats. They found a reduced inflammatory response, better collagen fiber deposition, and an increase in the mean number of newly formed vessels.
Wagner et al. [49] evaluated the effects of PBM on the cytokine levels and angiogenesis during oral wound healing and concluded that cytokine modulation and increased angiogenesis are among the mechanisms of PBM that improve oral wound repair. Medeiros et al. [50] verified the effects of low-level laser therapy on matrix metalloproteinase (MMP-2) immunoexpression in wound healing and angiogenic processes and found that laser therapy improved wound healing, especially at 14 days, as evidenced by contraction of the wound, anti-inflammatory activity, neocollagenesis, and an increase in the number of vessels formed (neoangiogenesis). In our findings, we also found an increase in the number of blood vessels but in only three of the groups studied compared with the naive group.
Results regarding the number of fibroblasts show statistical differences for almost all treatment groups compared with the naive and sham groups, except when comparing the naive group with the 9-point 660-nm group. Fibroblasts are related to the production of collagen and extracellular matrix, which is an important component in wound healing.
The study by Golçalves et al. [51] aimed to compare the effects of low-level gallium–aluminum–arsenide laser therapy at 830 nm and healing ointment on cutaneous wound healing, in blood vessels and collagen maturation of skin wounds in Wistar rats. They found an increase in the number of blood vessels in the 830-nm PBM-treated group, in addition to a higher number of mature collagen fibers, but no difference was observed between the groups concerning fibroblasts.
Corroborating the present study, Sampaio et al. [52], Chaves et al. [53], and Solmaz, Ulgen, and Gulsoy [54] described that PBM increases fibroblast proliferation and new blood vessels, reduces inflammatory cells, stimulates angiogenesis and the formation of granulation tissue, and increases collagen synthesis and, consequently, healing of the wound.
Regarding temperature, we did not observe significant differences between the groups. However, it is possible to observe a lower temperature trend in the naive group which can be explained by the absence of the inflammatory process that occurs after an injury, it being influenced only by the variation of normal body temperature, followed by the treated groups and finally the sham group. Among researches that used thermographic evaluation, Christensen et al. [55] emphasized that thermography cannot be used to assess absolute temperature changes due to normal variations in skin temperature over time and is a complimentary assessment. Neves et al. [10] evaluated temperature in the flap and found a temperature increase on the 4th postoperative day in both groups evaluated (control and treatment with high-voltage electrical stimulation).
Dostalova and collaborators [56] used thermography after third molar extraction and found no significant changes in temperature. The study by Carvalho et al. [57] aimed to evaluate the anti-inflammatory potential of gallium arsenide (904 nm) in the healing of skin wounds by measuring the surface temperature of the skin wound and by histopathological examination; they observed an increase in the temperature of the treated group without confirming an anti-inflammatory action of PBM.
As we can see, those studies used thermography for evaluation and follow-up during the inflammatory phase of some lesions/procedures, but not during longer-term follow-up, as performed in this study. This would explain the results found in the study, with no significant difference between groups based on the healing of almost closed wounds, without the absence of inflammatory infiltrate.
Some limitations that should be considered are the non-quantification of myofibroblasts and collagen fibers and the lack of analysis of cytokines and important growth factors in the wound healing process, which could add relevant information to the study.
Conclusion
Based on the sample evaluated in this study, in the comparison of wavelengths, 830 nm was more effective when compared with the naive and sham groups and those irradiated at 660 nm. Macroscopic analysis results demonstrated a positive intervention result at both wavelengths, with reduction of wound area size compared with the control group, except in the 9-point 830-nm PBM group. When observing the epidermis thickness, there was a general statistical difference in the 830-nm PBM groups compared with the 1- or 5-point 660-nm PBM groups. Analysis of the number of permeated blood vessels showed a significant difference of the groups irradiated by 830-nm PBM (1 and 5 points) and PBM 660 nm (4 points) in relation to the naive group. Regarding quantification of fibroblasts, an increase was observed in the groups treated with 830-nm PBM in relation to the control groups (sham and naive) and between groups irradiated with 660-nm PBM. Temperature analysis results showed no significant difference between groups. Comparing the number of points, we highlight application at 4 and 5 points in the open injury, with emphasis on the group with 5 points of PBM application which showed an improvement in macroscopic analysis and epithelial thickness, an increase in the number of vessels, and fewer fibroblasts on the 14th day.
References
Campos ACL, Borges-Branco A, Groth AK (2007) Cicatrização de feridas. Arq Bras Cir Dig 20:51–58
Shah A, Amini-Nik S (2017) The role of phytochemicals in the inflammatory phase of wound healing. Int J Mol Sci. 18(5):1068. https://doi.org/10.3390/ijms18051068
Lanzafame RJ, Stadler I, Cunningham R, Muhlbauer A, Griggis J, Soltz R, Soltz BA (2013) Preliminary assessment of photoactivated antimicrobial collagen on bioburden in a murine pressure ulcer model. Photomed Laser Surg 31(11):539–546
das Neves LM, Leite GP, Marcolino AM, Pinfildi CE, Garcia SB, Araújo JE, Guirro ECO (2017) Laser photobiomodulation (830 and 660 nm) in mast cells, VEGF, FGF, and CD34 of the musculocutaneous flap in rats submitted to nicotine. Lasers Med Sci 32(2):335–341
Zielins ER, Brett EA, Luan A, Hu MS, Walmsley GG, Paik K, Senarath-Yapa K, Atashroo DA, Wearda T, Lorenz HP, Wan DC, Longaker MT (2015) Emerging drugs for the treatment of wound healing. Expert Opin Emerg Drugs 20(2):235–246
Norman G, Dumville JC, Moore ZE, Tanner J, Christie J, Goto S (2016) Antibiotics and antiseptics for pressure ulcers. Cochrane Database Syst Rev 4:Cd011586
Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA (2008) Treatment of pressure ulcers: a systematic review. Jama. 300(22):2647–2662
Han G, Ceilley R (2017) Chronic wound healing: a review of current management and treatments. Adv Ther 34(3):599–610
Zhang L, Weng C, Zhao Z, Fu X (2017) Extracorporeal shock wave therapy for chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen 25(4):697–706
Neves LM, Guirro EC, Albuquerque FL, Marcolino AM (2016) Effects of high-voltage electrical stimulation in improving the viability of musculocutaneous flaps in rats. Ann Plast Surg 77(4):e50–e54
Bora Karsli P, Gurcay E, Karaahmet OZ, Cakci A (2017) High-voltage electrical stimulation versus ultrasound in the treatment of pressure ulcers. Adv Skin Wound Care 30(12):565–570
Khouri C, Kotzki S, Roustit M, Blaise S, Gueyffier F, Cracowski JL (2017) Hierarchical evaluation of electrical stimulation protocols for chronic wound healing: an effect size meta-analysis. Wound Repair Regen 25(5):883–891
Chen B, Kao HK, Dong Z, Jiang Z, Guo L (2017) Complementary effects of negative-pressure wound therapy and pulsed radiofrequency energy on cutaneous wound healing in diabetic mice. Plast Reconstr Surg 139(1):105–117
Nicoletti G, Perugini P, Bellino S, Capra P, Malovini A, Jaber O, Tresoldi M, Faga A (2017) Scar remodeling with the association of monopolar capacitive radiofrequency, electric stimulation, and negative pressure. Photomed Laser Surg 35(5):246–258
Dungel P, Hartinger J, Chaudary S, Slezak P, Hofmann A, Hausner T, Strassl M, Wintner E, Redl H, Mittermayr R (2014) Low-level light therapy by LED of different wavelength induces angiogenesis and improves ischemic wound healing. Lasers Surg Med 46(10):773–780
Machado RS, Viana S, Sbruzzi G (2017) Low-level laser therapy in the treatment of pressure ulcers: systematic review. Lasers Med Sci 32(4):937–944
Karu TI, Pyatibrat LV, Afanasyeva NI (2005) Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med 36:307–314
De Oliveira RF, Oliveira DA, Monteiro W, Zangaro RA, Magini M, Soares CP (2008) Comparison between the effect of low-level laser therapy and low-intensity pulsed ultrasonic irradiation in vitro. Photomed Laser Surg 26(1):6–9
Lins R, Dantas E, Lucena K, Catão M, Granville-Garcia A, Carvalho Neto L (2010) Efeitos bioestimulantes do laser de baixa potência no processo de reparo. An Bras Dermatol. 85(6):849–55. https://doi.org/10.1590/s0365-05962010000600011
Andrade FS, Clark RM, Ferreira ML (2014) Effects of low-level laser therapy on wound healing. Rev Col Bras Cir 41(2):129–133
Sousa RC, Maia Filho AL, Nicolau RA, Mendes LM, Barros TL, Neves SM (2015) Action of AlGaInP laser and high-frequency generator in cutaneous wound healing. A comparative study. Acta Cir Bras 30(12):791–798
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):1–17. https://doi.org/10.1109/JSTQE.2016.2561201
Freitas APP, Antiorio ATB, Seabra DI (2017) Anestesia e analgesia em animais de laboratório. In: UNICAMP
Guia anestesia e analgesia em animais de laboratório. In: (CEUA), 2017
Schoell AR, Heyde BR, Weir DE, Chiang PC, Hu Y, Tung DK (2009) Euthanasia method for mice in rapid time-course pulmonary pharmacokinetic studies. J Am Assoc Lab Anim Sci 48(5):506–511
Manual de normas do Laboratório de Técnica Operatória e Cirurgia Experimental. In: UFSC, Santa Catarina, ed. 2013
Solmaz H, Dervisoglu S, Gulsoy M, Ulgen Y (2016) Laser biostimulation of wound healing: bioimpedance measurements support histology. Lasers Med Sci 31(8):1547–1554
Uzeda-E-Silva VD, Rodriguez TT, Rocha IA, Xavier FCA, Santos JN, Cury PR, Ramalho LMP (2016) Laser phototherapy improves early stage of cutaneous wound healing of rats under hyperlipidic diet. Lasers Med Sci 31(7):1363–1370
Chiarotto GB, Neves LM, Esquisatto MA, do Amaral ME, dos Santos GM, Mendonca FA (2014) Effects of laser irradiation (670-nm InGaP and 830-nm GaAlAs) on burn of second-degree in rats. Lasers Med Sci 29(5):1685–1693
Rocha Júnior AM, Oliveira RG, Farias RE, Andrade LCF, Aarestrup FM (2006) Modulação da proliferação fibroblástica e da resposta inflamatória pela terapia a laser de baixa intensidade no processo de reparo tecidual. An Bras Dermatol 81:150–156
das Neves LM, Marcolino AM, Prado RP, Ribeiro TS, Pinfildi CE, Thomazini JA (2011) Low-level laser therapy on the viability of skin flap in rats subjected to deleterious effect of nicotine. Photomed Laser Surg 29(8):581–587
Neves LMS, Marcolino AM, Prado RP, Thomazini JA (2011) Laser 830nm na viabilidade do retalho cutâneo de ratos submetidos à nicotina. Acta Ortop Bras 19(6):342–345
Gupta A, Dai T, Hamblin MR (2014) Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med Sci 29(1):257–265
Rathnakar B, Rao BS, Prabhu V, Chandra S, Rai S, Rao ACK, Sharma M, Gupta PK, Mahato KK (2016) Photo-biomodulatory response of low-power laser irradiation on burn tissue repair in mice. Lasers Med Sci 31(9):1741–1750
Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT (2001) Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system. J Clin Laser Med Surg 19(1):29–33
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94(2):199–212
Carroll J (2008) A 3D dose model for low level laser/led therapy biostimulation and bioinhibition. 3 Chap; pp. 327 - 443. In: Hamblin MR, Waynant RW, Anders J. Mechanisms for Low-Light Therapy III. Proc. SPIE 6846. https://doi.org/10.1117/12.771183
Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM (2005) Helium-neon laser in viability of random skin flap in rats. Lasers Surg Med 37(1):74–77
Prado RP, Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM (2009) Effect of application site of low-level laser therapy in random cutaneous flap viability in rats. Photomed Laser Surg 27(3):411–416
Pinfildi CE, Hochman BS, Nishioka MA, Sheliga TR, Neves MAI, Liebano RE, Ferreira LM (2013) What is better in TRAM flap survival: LLLT single or multi-irradiation? Lasers Med Sci 28(3):755–761. https://doi.org/10.1007/s10103-012-1130-3
Martignago CCS, Tim CR, Assis L, Neves LMG, Bossini PS, Renno AC, LIebano RE, Parizotto N (2018) Comparison of two different laser photobiomodulation protocols on the viability of random skin flap in rats. Lasers Med Sci 34:1041–1047. https://doi.org/10.1007/s10103-018-2694-3
Goncalves RV, Novaes RD, Matta SL, Benevides GP, Faria FR, Pinto MV (2010) Comparative study of the effects of gallium-aluminum-arsenide laser photobiomodulation and healing oil on skin wounds in Wistar rats: a histomorphometric study. Photomed Laser Surg 28(5):597–602
Goncalves RV, Novaes RD, Cupertino Mdo C, Moraes B, Leite JPV, Peluzio MCG, Pinto MVM, Patta LP (2013) Time-dependent effects of low-level laser therapy on the morphology and oxidative response in the skin wound healing in rats. Lasers Med Sci 28(2):383–390. https://doi.org/10.1007/s10103-012-1066-7
Carneiro C, Schleder JC, Fischer SV, Zedebski RAM, Verner AF, Lipinski L (2015) Efeito de lasers de baixa potência no reparo de lesões cutâneas. Publicatio uepg Ciências biológicas e da saúde (online) 21:109–115
Yadav A, Gupta A (2017) Noninvasive red and near-infrared wavelength-induced photobiomodulation: promoting impaired cutaneous wound healing. Photodermatol Photoimmunol Photomed 33(1):4–13
Gal P, Vidinsky B, Toporcer T, Mokrý M, Mozes S, Longauer F, Sabo J (2006) Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed Laser Surg 24(4):480–488
Leite GP, das Neves LM, Silva CA, Guirro RRJ, Souza TR, Souza AK, Garcia SB, Guirro ECO (2017) Photobiomodulation laser and pulsed electrical field increase the viability of the musculocutaneous flap in diabetic rats. Lasers Med Sci 32(3):641–648. https://doi.org/10.1007/s10103-017-2160-7
Melo VA, Anjos DCS, Albuquerque Júnior R, Melo DB, Carvalho FUR (2011) Effect of low level laser on sutured wound healing in rats. Acta Cir Bras 26:129–134. https://doi.org/10.1590/s0102-86502011000200010
Wagner VP, Curra M, Webber LP, Nor C, Matte U, Meurer L, Martins MD (2016) Photobiomodulation regulates cytokine release and new blood vessel formation during oral wound healing in rats. Lasers Med Sci 31(4):665–671. https://doi.org/10.1007/s10103-016-1904-0
Medeiros AC, Dantas-Filho AM (2017) Cicatrização das feridas cirúrgicas. J Surg Clin Res 7(2):87–102. https://doi.org/10.20398/jscr.v7i2.11438
Gonçalves RV, Mezêncio JMS, Benevides GP, Matta SLP, Neves CA, Sarandy MM, Vilela EF (2010) Effect of gallium-arsenide laser, gallium-aluminum-arsenide laser and healing ointment on cutaneous wound healing in Wistar rats. Braz J Med Biol Res 43:350–355. https://doi.org/10.1590/S0100-879X2010007500022
Sampaio SCPO, Monteiro JSC, Cangussu MCT, Santos GMP, Santos MAV, Santos JN, Pineiro ALB (2013) Effect of laser and LED phototherapies on the healing of cutaneous wound on healthy and iron-deficient Wistar rats and their impact on fibroblastic activity during wound healing. Lasers Med Sci 28(3):799–806. https://doi.org/10.1007/s10103-012-1161-9
Chaves ME, Araujo AR, Piancastelli AC, Pinotti M (2014) Effects of low-power light therapy on wound healing: LASER × LED. An Bras Dermatol 89(4):616–623
Solmaz H, Ulgen Y, Gulsoy M (2017) Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med Sci 32(4):903–910
Christensen J, Matzen LH, Vaeth M, Schou S, Wenzel A (2012) Thermography as a quantitative imaging method for assessing postoperative inflammation. Dentomaxillofac Radiol 41(6):494–499
Dostalova T, Kroulikova V, Podzimek S, Jelinkova H (2017) Low-level laser therapy after wisdom teeth surgery: evaluation of immunologic markers (secretory immunoglobulin a and lysozyme levels) and thermographic examination: placebo controlled study. Photomed Laser Surg 35(11):616–621
Carvalho A, Bizo MHA, Toma HS, Guedes KMR, Muraro LS, Musis C (2017) Effect of gallium arsenide low-level laser therapy on the inflammatory phase of skin wound healing in rats. Acta Vet Bras 11(4):226–230. https://doi.org/10.21708/avb.2017.11.4.7274
Funding
This research was funded by the Coordination for Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES). Financial support was received for the master’s student.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee (CEUA) under number 4017201117.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hendler, K.G., Canever, J.B., de Souza, L.G. et al. Comparison of photobiomodulation in the treatment of skin injury with an open wound in mice. Lasers Med Sci 36, 1845–1854 (2021). https://doi.org/10.1007/s10103-020-03216-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03216-7