Abstract
The aim of this study was to assess the effects of IL-6 and IL-8 cytokines on human gingival fibroblasts (HGF) cultured in a 3-D model and the possible photobiomodulation (PBM) of such effects by low-level laser therapy. In complete culture medium (DMEM), HGF from a healthy patient were seeded in a type I collagen matrix inserted into 24-well plates. After 5 days of incubation, the cytokines were added or not to serum-free DMEM, which was applied to the cell-enriched matrices. Then, PBM was performed: three consecutive irradiations using LaserTable diode device (780 nm, 0.025 W) at 0.5 J/cm2 were delivered or not to the cells. Twenty-four hours after the last irradiation, cell viability and morphology, gene expression, and synthesis of inflammatory cytokines and growth factors were assessed. The histological evaluation demonstrated that, for all groups, matrices presented homogeneous distribution of cells with elongated morphology. However, numerous cytokine-exposed cells were rounded. IL-6 and IL-8 decreased cell viability, synthesis of VEGF, and gene expression of collagen type I. PBM enhanced cell density in the matrices and stimulated VEGF expression, even after IL-6 challenge. Reduced TNF-α synthesis occurred in those cells subjected to PBM. In conclusion, PBM can penetrate collagen matrix and stimulate HGF, highlighting the relevance of this research model for further phototherapy studies and in vitro biomodulation of the healing process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis (OM) is the most common adverse effect of oncological treatments and is characterized by painful ulcerative and erosive lesions of the oral mucosa. This pathology affects approximately 70% of patients undergoing chemotherapy and/or radiotherapy and severely interferes with the quality of life of such patients [1, 2]. Proinflammatory cytokines, such as interleukins (IL)-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), play an important role in the occurrence of OM lesions [3,4,5]. The presence of high concentrations of these cytokines in the oral environment is related to the greater severity of ulcerative lesions and delayed tissue healing [6]. This is because such cytokines at high concentrations decrease the proliferation and migration of oral mucosal cells [7,8,9,10].
Taking these facts into consideration, one may conclude that the modulation of local tissue inflammation reduces the local concentration of inflammatory cytokines, improving oral mucosal healing [7, 9]. It has been shown that photobiomodulation (PBM) by low-level laser therapy regulates the expression and synthesis of inflammatory cytokines and growth factors, which have positive effects on cellular pathways such as proliferation, migration, and extracellular matrix synthesis [11, 12], favoring the healing of oral mucosal lesions [12,13,14,15].
The cellular and molecular effects of PBM have been widely assessed, and most of the data currently available are supported mainly by laboratory studies performed in monolayer cell-culture models [11,12,13,14,15]. Since cells cultured in a monolayer exhibit morphological, phenotypical, and functional limitations, it seems that the results obtained in this model do not fully duplicate PBM effects and may not even take its effects for granted [16, 17]. To overcome these limitations, while maintaining the benefits of in vitro studies, such as a controlled and standardized environment, three-dimensional (3-D) cell culture models were developed. The 3-D cell culture is a suitable research model, since it maintains the morphology and phenotypes of cells similar to those observed for in vivo conditions [16,17,18,19,20,21]. Thus, we may consider that 3-D cell culture in collagen matrices is adequate to mimic cellular connective tissue, and this laboratory model has been used to culture gingival fibroblasts [17]. In addition, the 3-D culture model has been used specifically for PBM studies, in which different parameters of light irradiation are delivered to oral mucosal cells [16, 17].
The aim of the present study was to assess the effects of IL-6 and IL-8 cytokines on human gingival fibroblasts (HGF) in a 3-D collagen culture model and the possible PBM of cytokines’ negative effects by the application of specific low-level laser therapy. The study hypothesis is that PBM is able to adequately penetrate the 3-D cell culture model and stimulate HGF, minimizing inflammatory cytokines’ negative effects on these cells and improving tissue repair–related functions.
Materials and methods
Gingival fibroblast isolation
This investigation was developed with the use of primary cell culture of HGF [17]. To achieve this cell culture, a tissue fragment was collected from the gingival papilla of a young male patient subjected to a third molar extraction procedure, in accordance with a protocol previously approved by the Ethics Committee (CAAE: 74823317.0.0000.5416) and to published literature [10, 12, 16, 17, 22]. Following collection, gingival tissue was placed in complete culture medium DMEM (Dulbecco’s modified Eagle medium, Gibco, Carlsbad, CA, USA) containing 1% of antibiotic and antimycotic solution (Gibco Antibiotic-Antimycotic – 10,000 units/mL of penicillin, 10,000 μg/mL of streptomycin, and 25 μg/mL of Gibco Amphotericin B; Gibco, Carlsbad, CA, USA), without fetal bovine serum (FBS – Gibco, Carlsbad, CA, USA). Then, the tissue fragment was physically dissociated by means of a scalpel and then subjected to enzymatic dissociation with collagenase type 1 (Worthington Biochemical Corporation, Lakewood, NJ, USA), 3 mg/mL concentration, in serum-free DMEM at 37 °C and 5% CO2. After 24 h, cells were collected, centrifuged, and transferred to a new cell culture flask (75 cm2 – Corning, New York, NY, USA) containing complete DMEM + 10% FBS.
3-D cell culture of gingival fibroblasts
A three-dimensional cell culture model was obtained from HGF seeded in a collagen type I matrix (BD Collagen Type I, BD Biosciences, Franklin Lakes, NJ, USA), simulating the exposure of the oral connective tissue [17]. For this purpose, an acellular collagen matrix was prepared by collagen type I mixed to a final concentration of 0.77 mg/mL in DMEM + 10% FBS. A 250-μL quantity of this solution was applied in wells of 24-well plates (TPP – Techno Plastic Products, Trasadingen, SH, Switzerland) and incubated at room temperature for 30 min to allow collagen to stabilize. Then, a new collagen solution was obtained by type I collagen (BD Collagen Type I, BD Biosciences, Franklin Lakes, NJ, USA) mixed at 0.77 mg/mL final concentration in DMEM + 10% FBS and HGF (2 × 105 cells/mL). For each sample, 1 mL of this solution was applied at the top of the first matrix, and samples were maintained for 1 h at room temperature, followed by 1-h incubation at 37 °C and 5% CO2.
After preparation of the matrices, 1 mL of DMEM + 10% FBS was added to each sample and incubated for an additional 5 days. The culture medium was replaced every 48 h. The experiment was conducted using two 24-well plates: one plate was not submitted to irradiation and the other plate was irradiated. The experiment was performed on two different occasions (duplicates) and at the end of the second occasion, each experimental group presented 6 samples and four 24-well plates were used.
Cytokine exposure
At 4-day incubation, the 3-D cell culture was exposed or not to interleukins IL-6 and IL-8 (Sigma-Aldrich, St. Louis, MO, USA) at 10 ng/mL [10, 13] in serum-free DMEM for 24 h.
In vitro PBM
After the cells were exposed to the interleukins, the 3-D cell cultures were irradiated by means of a laser diode device (LaserTable, 780 nm, 0.025 W) [33] with specific irradiation parameters [34] (Table 1) as described in previous studies [12,13,14,15,16,17, 22]. This investigation was carried out using a specific laser diode prototype (LaserTable) for cell irradiation. This device was developed and standardized to provide uniform irradiation of a 24-well cell culture plate. To provide this uniformity, considering the characteristics of a laser beam, a pair of lenses was inserted, and plate support is fixed at a 2.5-cm distance of the diode. LaserTable features 12 laser diodes that are activated simultaneously enabling standardization for parallel infrared cell irradiation. The 12 diodes are positioned in a way that each well of the cell culture plate is individually irradiated (2 cm2), in this 2.5-cm distance [33].
The energy dose used in this study was 0.5 J/cm2 (40 s of irradiation time, 0.025 W emitted power, and 2 cm2 target area). The cells were irradiated three consecutive times with 24-h intervals [13, 14, 17]. Twenty-four hours after the last irradiation, cells were assessed for their viability (alamarBlue assay – Invitrogen, Carlsbad, CA, USA); gene expression of collagen type I and VEGF (real-time PCR); synthesis of VEGF, TNF-α, and IL-1β (ELISA); and the morphology of matrix and cells (light microscopy).
In control groups, 3-D cell cultures were established as reported for the experimental groups but were not exposed to laser irradiation.
Cell viability
Viability of the cells exposed or not to interleukins and PBM was evaluated by the alamarBlue assay (Invitrogen, Carlsbad, CA, USA) [17]. This test demonstrated the respiratory activity of cultured cells by the production of a fluorescent dye according to the respiratory activity rates of these cells. After PBM, a free-serum culture medium containing 10% of the alamarBlue was added to the samples and incubated for 4 h at 37 °C and 5% of CO2. Then, cell viability was determined by the evaluation of fluorescence intensity (Synergy H1 microplate reader, BioTek Instruments, Winooski, VT, USA; excitation of 530 nm and emission of 590 nm).
Gene expression of collagen type I and VEGF
Gene expression of collagen type I and VEGF was performed by real-time PCR [17]. Total RNA was isolated by filtration with the use of the RNAqueous Kit (Ambion by Life Technologies, Carlsbad, CA, USA). cDNA of each sample was obtained by means of a High Capacity cDNA Reverse Transcriptions Kit (Applied Biosystems by Thermo Fisher Scientific, Foster City, CA, USA) and a thermocycler (iCycler, Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s instructions.
For the qPCR reaction, TaqMan Universal PCR Master Mix and TaqMan assays (Applied Biosystems by Thermo Fisher Scientific, Foster City, CA, USA) were used. Reactions were realized by StepOnePlus equipment (Applied Biosystems by Thermo Fisher Scientific, Foster City, CA, USA) and analyzed in StepOne Software 2.1 (Applied Biosystems by Thermo Fisher Scientific, Foster City, CA, USA), by the standard curve method, with BActin as the endogen control.
Synthesis of VEGF, TNF-α, and IL-1β
Synthesis of soluble VEGF, TNF-α, and IL-1β was performed by ELISA assay [12, 13], with standardized kits (R&D Systems, Minneapolis, MN, USA). Plates were prepared by incubation with specific primary antibodies overnight, followed by the addition of the culture medium that was collected from each sample. Then, samples were treated with secondary antibodies and streptavidin (R&D Systems, Minneapolis, MN, USA). Results were analyzed by spectrophotometry at 455 nm (Synergy H1 microplate reader, BioTek Instruments, Winooski, VT, USA).
Matrix and cell morphology
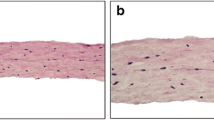
Morphology of the 3-D cell culture model was evaluated by an optical microscope (Olympus BX51, Olympus America Inc., Center Valley, PA, USA). For this purpose, the samples were fixed in 10% formalin and processed according to a standard histological protocol [17]. Then, the sections were stained by hematoxylin and eosin (H&E, Merck Millipore, Burlington, MA, USA) and analyzed at × 100 and × 200 magnification. The photomicrographs obtained were analyzed by ImageJ software (Wayne Rasband, National Institute of Mental Health, NIH, Bethesda, MD, USA).
Statistical analysis
Data for cell viability, gene expression, and protein synthesis did not show normal distribution (Shapiro-Wilk); therefore, non-parametric statistical Kruskal-Wallis and Mann-Whitney tests were selected for analysis of these data. All statistical inferences were performed at a 5% significance level.
Results
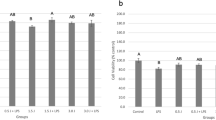
The viability of HGF exposed to IL-8 decreased 20% compared with the control group (p < 0.05); however, the group exposed to IL-8 and PBM showed an increase of 10% compared with the group exposed only to IL-8, but this result was not statistically significant. The viability of this primary cell culture subjected to PBM was similar to the control group (Fig. 1).
Viability of human gingival fibroblasts (HGF) seeded onto a 3-D cell culture model and exposed or not to IL-6/IL-8 and L (low-level laser therapy – PBM, 0.5 J/cm2). Boxplots indicate median values as 25% and the 95th percentile. Groups identified by different letters show a statistically significant difference (Kruskal-Wallis and Mann-Whitney, p < 0.05)
VEGF synthesis decreased by 25% by both interleukins compared with the control group (p < 0.05), and this result was also observed when the interleukin-treated cells were subjected to PBM (p < 0.05). PBM alone increased 10% VEGF synthesis compared with the control group, but this result was not statistically significant (Fig. 2).
VEGF synthesis by human gingival fibroblasts (HGF) seeded onto a 3-D cell culture model and exposed or not to IL-6/IL-8 and L (low-level laser therapy – PBM, 0.5 J/cm2). Boxplots indicate median values as 25% and the 95th percentile. Groups identified by different letters show a statistically significant difference (Kruskal-Wallis and Mann-Whitney, p < 0.05)
IL-6 enhanced 10% TNF-α synthesis compared with the control group, but this increase was not statistically significant. PBM alone decreased 30% TNF-α synthesis (p < 0.05) and when cells were submitted to PBM associated with IL-6 and IL-8 treatment, it was observed respectively, TNF-α synthesis similar to the control group and a decreased of 18% compared with the control group (p < 0.05). IL-1β synthesis was similar for all experimental groups (Fig. 3).
Synthesis of TNF-α and IL-1β by human gingival fibroblasts (HGF) seeded onto a 3-D cell culture model and exposed or not to IL-6/IL-8 and L (low-level laser therapy – PBM, 0.5 J/cm2). Boxplots indicate median values as 25% and the 95th percentile. Groups identified by different letters show a statistically significant difference (Kruskal-Wallis and Mann-Whitney, p < 0.05)
Gene expression of collagen type I was 20% diminished in those cells treated with IL-8 compared with the control group, PBM promoted 15% increase on collagen type I expression compared with the control group, but these results were statistically similar. VEGF gene expression was significantly stimulated in PBM groups exposed or not to IL-6 compared with the control group: PBM alone enhanced 10% VEGF expression (p < 0.05) and, in the presence of IL-6, an increase of 40% was observed (p < 0.05) (Fig. 4).
Gene expression of hCOL-I and hVEGF by human gingival fibroblasts (HGF) seeded onto a 3-D cell culture model and exposed or not to IL-6/IL-8 and L (low-level laser therapy – PBM, 0.5 J/cm2). Boxplots indicate median values as 25% and the 95th percentile. Groups identified by different letters show a statistically significant difference (Kruskal-Wallis and Mann-Whitney, p < 0.05)
The microscopic assessment of the 3-D cell-culture morphology showed no significant differences among the distinct groups (Fig. 5). For all groups, HGF with elongated morphology were homogeneously distributed within the matrices. However, numerous rounded cells were observed in those groups in which HGF were exposed to the cytokines. Apparently, a high cell density occurred when the 3-D culture model was subjected solely to PBM.
Discussion
During the initial phase of tissue healing, an acute inflammatory reaction takes place. This local inflammation is responsible for mesenchymal cell and leukocyte migration as well as for the expression of inflammatory cytokines and other chemo-attractive and mitogenic molecules that mediate the progression of tissue healing [8, 9]. However, the persistent presence of high concentrations of these cytokines interferes with the healing process, caused by decreased mitogenic capacity and increased local oxidative stress that enhance the local synthesis and activity of matrix metalloproteinases (MMPs), capable of disorganizing collagenous extracellular matrix [6, 7, 23,24,25,26]. TNF-α, IL-6, IL-8, and IL-1β are proinflammatory cytokines related to the development of OM, characterized as ulcerative lesions, in patients receiving cancer treatment [24, 25]. The synthesis of these mediators is moderated by the NF-κB pathway, which is also associated with cell viability, proliferation, migration, and survival [27, 28]. Despite the relevant knowledge currently available in this specific research field, the direct influence of inflammatory cytokines on oral mucosal cells and the effects of PBM by low-level laser therapy on such cells have not yet been fully elucidated.
Nevertheless, the positive effects of PBM have been shown by several in vitro studies in which monolayer cell cultures were used [11,12,13,14,15, 22] and the data obtained in those investigations may not fully represent the light interaction with cells and proteins [30, 31]. The feasibility of using a type I collagen matrix to establish a 3-D culture model has been shown [17]. Thus, this research model may help investigators elucidate, in in vitro conditions, some mechanisms involving interactions of laser-irradiated cells under inflammatory stimuli. 3-D cell culture models provide more representative data and may show diverse cell responses when compared with two-dimensional cell-culture models [16, 17]. Based on this fact, in the present study, we used a 3-D model for HGF culture in type I collagen, which is the main component of the oral mucosa extracellular matrix [29].
In general, the 3-D culture model developed for this investigation and assessed under light microscopy exhibited homogeneous distribution of spindle-shaped HGF within a well-organized collagen matrix. This model provided a more suitable environment for cell elongation and phenotypic expression. However, HGF exposed to cytokines presented rounded morphology, demonstrating that our experimental model allowed cytokines to stimulate cells, modifying their morphology (shape) and functions (phenotype), like promoting enhanced TNF-α synthesis and decreasing collagen type I gene expression and cells viability, as observed in other studies [13, 15, 32]. The differences on cell morphology among the experimental groups were related to cytokines treatment or not, once the groups not submitted to cytokine exposure showed elongated morphology, similar to HGF morphology observed in previously investigation [17], which used the same 3-D experimental model, but not submitted to inflammatory challenge. Furthermore, it was possible to observe the light interaction through the 3-D model and its possible PBM effects on HGF submitted to proinflammatory cytokine challenge, providing an interesting in vitro simulation of connective tissue exposure, mimicking an in vivo condition of ulcerative lesions [30, 31].
For PBM, 0.5 J/cm2 energy density was selected based on previous studies which showed that this dose promoted increasing cell metabolism, number, migration, and cytokines’ negative effect modulation, as well as enhancing gene expression of hCOL-I and hEGF [13, 14, 17]. Distinct cell responses can be observed at different energy density levels: very low energy densities may not provide considerable observable effects, while higher energy densities may result in cellular function inhibition [35]. However, there is no documentation of the precise limit values of light intensity thresholds, but there is evidence that the threshold parameters energy density and intensity are biologically independent of each other. This independence is very important for understanding the photobiological effects achieved at low energy density levels [35]. Our study demonstrated that 0.5 J/cm2 promoted positive effects on HGF cultured on 3-D model, which were described below.
Here, HGF showed decreased viability when exposed to IL-8, as demonstrated in previous investigations [15, 32], which reported that this cytokine may negatively affect local wound healing. IL-6 did not interfere significantly with cell viability, such as described in a prior in vitro study, in which this cytokine affected cell migration with no impairment of cell proliferation [13]. PBM enhanced HGF viability when the cells were exposed to IL-8, confirming the biostimulation capacity of this therapy in 3-D or monolayer in vitro models [15,16,17].
Proinflammatory cytokines may decrease the mitogenic activity of oral mucosal cells, which can be exacerbated by the positive feedback of the local inflammatory response, resulting in delayed oral healing [10, 12, 13, 32]. This event was also observed in the present investigation: HGF exposed to IL-6 had a positive feedback TNF-α, but this increase was statistically similar to the control group TNF-α synthesis. PBM application was capable of modulating this cell response, diminishing TNF-α synthesis levels in the groups submitted or not to cytokines stimuli, as previously demonstrated [12].
Besides the similar functions and responses of HGF to IL-1β and TNF-α, it is important to focus on the fact that these mediators are not chemically similar and have specific receptors and pathways. These cytokines respond as positive feedback during the inflammatory phase of the wound healing process and contribute to local oxidative stress [26]. In the present study, the cells’ exposure to IL-6 or IL-8, followed by the application or not of specific PBM, did not affect IL-1β synthesis—scientific data that corroborate with previous laboratory studies [12, 26].
VEGF is a growth factor that is directly related to neovascularization and plays a main role in the tissue healing process by stimulating the proliferation and migration of several cell types [17]. We demonstrated that HGF exposed to IL-6 and IL-8 with or without PBM presented reduced synthesis of VEGF and when cells were exposed solely to PBM, VEGF synthesis was similar to the control group. The presence of cytokines did not influence VEGF expression, as reported in other investigation [13]. PBM alone or in the presence of IL-6 enhanced VEGF gene expression, findings observed in other studies [16, 22] that highlight the potential of this therapy for improving tissue healing.
Collagen synthesis is another crucial cell function that plays a fundamental role in wound closure [13]. In the present study, gene expression of collagen type I was negatively affected by IL-8, as previously demonstrated [13]. Additionally, HGF exposed to PBM exhibit enhanced gene expression of collagen type I, but this result was statistically similar to the control group collagen expression.
Analysis of these data demonstrates the relevance of using a 3-D cell culture model to assess the PBM effects on cell phenotypes deeper into collagen-rich matrix, promoting more suitable and reliable results. Therefore, PBM on the selected parameters (0.5 J/cm2, 0.025 W, 780 nm) was capable of adequately penetrating the collagen matrix and positively stimulating HGF wound healing–related functions and decreasing TNF-α synthesis, even in the presence of inflammatory challenge.
References
Keefe DM, Schubert MM, Elting LS et al (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109(5):820–831. https://doi.org/10.1002/cncr.22484
Treister N, Sonis S (2007) Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg 15(2):123–129. https://doi.org/10.1097/MOO.0b013e3280523ad6
Logan RM, Stringer AM, Bowen JM et al (2007) The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 33(5):448–460. https://doi.org/10.1016/j.ctrv.2007.03.001
Sonis ST, Costa JW Jr, Evitts SM et al (1992) Effect of epidermal growth factor on ulcerative mucositis in hamsters that receive cancer chemotherapy. Oral Surg Oral Med Oral Pathol 74(6):749–755
Sonis ST, Elting LS, Keefe D et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025. https://doi.org/10.1002/cncr.20162
Meirovitz A, Kuten M, Billan S et al (2010) Cytokines levels, severity of acute mucositis and the need of PEG tube installation during chemo-radiation for head and neck cancer—a prospective pilot study. Radiat Oncol 5:16. https://doi.org/10.1186/1748-717X-5-16
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229. https://doi.org/10.1177/0022034509359125
Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M (2014) Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 22(5):569–578. https://doi.org/10.1111/wrr.12205
Barrientos S, Stojadinovic O, Golinko MS et al (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16(5):585–601. https://doi.org/10.1111/j.1524-475X.2008.00410.x
Basso FG, Pansani TN, Turrioni AP et al (2016) Tumor necrosis factor-alpha and interleukin (IL)-1beta, IL-6, and IL-8 impair in vitro migration and induce apoptosis of gingival fibroblasts and epithelial cells, delaying wound healing. J Periodontol 87(8):990–996. https://doi.org/10.1902/jop.2016.150713
Arany PR, Nayak RS, Hallikerimath S et al (2007) Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen 15(6):866–874. https://doi.org/10.1111/j.1524-475X.2007.00306.x
Basso FG, Pansani TN, Soares DG et al (2015) Biomodulation of inflammatory cytokines related to oral mucositis by low-level laser therapy. Photochem Photobiol 91(4):952–956. https://doi.org/10.1111/php.12445
Basso FG, Soares DG, Pansani TN et al (2016) Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Lasers Surg Med 48(10):1006–1014. https://doi.org/10.1002/lsm.22553
Basso FG, Pansani TN, Turrioni AP et al (2012) In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int J Dent 2012:719452. https://doi.org/10.1155/2012/719452
Pansani TN, Basso FG, Soares DG et al (2018) Photobiomodulation in the metabolism of lipopolysaccharides-exposed epithelial cells and gingival fibroblasts. Photochem Photobiol 94(3):598–603. https://doi.org/10.1111/php.12877
Basso FG, Pansani TN, Soares DG et al (2018) LLLT effects on oral keratinocytes in an organotypic 3-D model. Photochem Photobiol 94(1):190–194. https://doi.org/10.1111/php.12845
Basso FG, Soares DG, de Souza Costa CA, Hebling J (2016) Low-level laser therapy in 3D cell culture model using gingival fibroblasts. Lasers Med Sci 31(5):973–978. https://doi.org/10.1007/s10103-016-1945-4
Moharamzadeh K, Colley H, Murdoch C et al (2012) Tissue-engineered oral mucosa. J Dent Res 91(7):642–650. https://doi.org/10.1177/0022034511435702
Tra WM, van Neck JW, Hovius SE (2012) Characterization of a three-dimensional mucosal equivalent: similarities and differences with native oral mucosa. Cells Tissues Organs 195(3):185–196. https://doi.org/10.1159/000324918
Basso FG, Hebling J, Marcelo CL et al (2017) Development of an oral mucosa equivalent using a porcine dermal matrix. Br J Oral Maxillofac Surg 55(3):308–311. https://doi.org/10.1016/j.bjoms.2016.09.019
Bucchieri F, Fucarino A, Marino Gammazza A et al (2012) Medium-term culture of normal human oral mucosa: a novel three-dimensional model to study the effectiveness of drugs administration. Curr Pharm Des 18(34):5421–5430
Basso FG, Oliveira CF, Kurachi C et al (2013) Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med Sci 28(2):367–374. https://doi.org/10.1007/s10103-012-1057-8
Menke NB, Ward KR, Witten TM et al (2007) Impaired wound healing. Clin Dermatol 25(1):19–25. https://doi.org/10.1016/j.clindermatol.2006.12.005
Morales-Rojas T, Viera N, Moron-Medina A et al (2012) Proinflammatory cytokines during the initial phase of oral mucositis in patients with acute lymphoblastic leukaemia. Int J Paediatr Dent 22(3):191–196. https://doi.org/10.1111/j.1365-263X.2011.01175.x
Xanthinaki A, Nicolatou-Galitis O, Athanassiadou P et al (2008) Apoptotic and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: preliminary report. Support Care Cancer 16(9):1025–1033. https://doi.org/10.1007/s00520-007-0379-8
Houreld NN, Sekhejane PR, Abrahamse H (2010) Irradiation at 830 nm stimulates nitric oxide production and inhibits pro-inflammatory cytokines in diabetic wounded fibroblast cells. Lasers Surg Med 42(6):494–502. https://doi.org/10.1002/lsm.20812
Gasparini C, Feldmann M (2012) NF-kappaB as a target for modulating inflammatory responses. Curr Pharm Des 18(35):5735–5745
Palmqvist P, Lundberg P, Lundgren I et al (2008) IL-1beta and TNF-alpha regulate IL-6-type cytokines in gingival fibroblasts. J Dent Res 87(6):558–563. https://doi.org/10.1177/154405910808700614
Bartold PM, Walsh LJ, Narayanan AS (2000) Molecular and cell biology of the gingiva. Periodontol 24:28–55
Ottaviani G, Gobbo M, Sturnega M et al (2013) Effect of class IV laser therapy on chemotherapy-induced oral mucositis: a clinical and experimental study. Am J Pathol 183(6):1747–1757. https://doi.org/10.1016/j.ajpath.2013.09.003
Schwarz F, Aoki A, Sculean A, Becker J (2009) The impact of laser application on periodontal and peri-implant wound healing. Periodontol 51:79–108. https://doi.org/10.1111/j.1600-0757.2009.00301.x
Basso FG, Soares DG, Pansani TN et al (2015) Effect of LPS treatment on the viability and chemokine synthesis by epithelial cells and gingival fibroblasts. Arch Oral Biol 60(8):1117–1121. https://doi.org/10.1016/j.archoralbio.2015.04.010
Lins EC, Oliveira CF, Guimarães OC et al (2013) A novel 785-nm laser diode-based system for standardization of cell culture irradiation. Photomed Laser Surg 31(10):466–473. https://doi.org/10.1089/pho.2012.3310
Jenkins PA, Carroll JD (2011) How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg 29(12):785–787. https://doi.org/10.1089/pho.2011.9895
Sommer AP, Pinheiro AL, Mester AR et al (2001) Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg 19(1):29–33. https://doi.org/10.1089/104454701750066910
Funding
This study received financial support from the Coordination of Superior Level Staff Improvement (CAPES) and the Brazilian National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was performed in accordance with the local Ethics Committee (CAAE: 74823317.0.0000.5416).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardoso, L.M., Pansani, T.N., Hebling, J. et al. Photobiomodulation of inflammatory-cytokine-related effects in a 3-D culture model with gingival fibroblasts. Lasers Med Sci 35, 1205–1212 (2020). https://doi.org/10.1007/s10103-020-02974-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-02974-8