Abstract
This study evaluated the influence of photobiomodulation (PBM) using low-level laser therapy (PBM/LLLT) or light-emitting diode (PBM/LED) therapy on peri-implant tissue healing. A laboratory model was used to assess the adhesion and metabolism of osteoblasts (SaOs-2), human gingival fibroblasts (HGF), and normal oral keratinocytes (NOK) seeded on a titanium (Ti) surface. After seeding the cells on disks of Ti placed in wells of 24-well plates, three irradiations were performed every 24 h at energy density of 3 J/cm2. For PBM/LLLT, a LaserTABLE device was used with a wavelength of 780 nm and 25 mW, while for PBM/LED irradiation, a LEDTABLE device was used at 810 nm, 20 mW, at a density of 3 J/cm2. After irradiations, the number of cells (NC) attached and spread on the Ti surface, cell viability (CV), total protein (TP), and collagen (Col) synthesis were assessed. Alkaline phosphate activity (ALP) was evaluated only for SaOs-2. Data were submitted to ANOVA complemented by Turkey statistical tests at a 5% significance level. PBM significantly increased adherence of NOK to the Ti surface, while no significant effect was observed for SaOs-2 and HGF. PBM positively affected CV, as well as Col and TP synthesis, in distinct patterns according to the cell line. Increased ALP activity was observed only in those cells exposed to PBM/LLLT. Considering cell specificity, this investigation reports that photobiomodulation with low-power laser and LED at determined parameters enhances cellular functions related to peri-implant tissue healing in a laboratory model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photobiomodulation (PBM) using low-level laser therapy (LLLT) and light-emitting diodes (LED) has gained attention for treatment of several human conditions [1]. This therapeutic protocol is based on the absorption of light within a specific wavelength spectrum by organic molecules known as photoacceptors, which convert this luminous energy into biochemical effects such as increasing ATP synthesis, cell metabolism, and gene expression of proteins [2]. Mitochondrial enzymes, such as cytochrome C oxidase and other protein complexes involved in electron transport chain, resemble the major class of these components and are mainly stimulated by red and near-infrared light; therefore, this limited light spectrum has successfully been used to test the effectiveness of photobiomodulation in cells and tissues [1, 3,4,5].
Several studies demonstrate that PBM using low-level laser or LED devices applied to cells and tissues enhances cell migration and proliferation, as well as the expression of genes and proteins related to down-modulation of inflammatory response and tissue healing [6,7,8,9,10]. However, literature still lacks scientific data concerning the ideal PBM parameters for modulating oral tissues and cells.
For oral implantology, a long-term successful clinical outcome is achieved by two factors. The first is the osseointegration of the implant; this is where bone cells adhere to the implant’s titanium surface and synthesize a collagen-rich matrix, which is further mineralized by calcium deposition [11, 12]. The second is an effective peri-implant sealing, which is characterized by healing of the oral mucosa tissue surrounding the abutment [11, 12]. The processes governing tissue healing in peri-implant sealing are complex. Gingival epithelial tissue, resembled by oral keratinocytes and the subjacent connective tissue that is in contact with the abutment surface, is primarily responsible for achieving peri-implant sealing which is mediated by collagen fibrils and local fibroblasts [11,12,13]. Therefore, a number of studies have evaluated the efficacy of different therapies upon the metabolism and adhesion of bone [14,15,16] and oral mucosa cells to a titanium surface [17].
The epithelium and subjacent connective tissue, plus the maxillary bone, directly contribute to the functional and esthetic success of oral rehabilitation using intraosseous implants. It is therefore beneficial to evaluate specific therapies capable of up-regulating the metabolism and activities of cells within these soft and hard oral tissues. The effects of PBM on peri-implant tissue healing were already assessed by previous investigations, which demonstrated that this therapeutic protocol can improve the success of oral implants by increasing cell migration and proliferation and increase also local angiogenesis and down-modulating the inflammatory response [14]. In addition, PBM also increases osteoblastic differentiation, which accelerates bone deposition around implants [14, 15].
Here, a comparative evaluation of effects of PBM was demonstrated, using specific parameters of low-level laser therapy (PBM/LLLT) and light-emitting diodes (PBM/LED), to irradiate oral keratinocytes, gingival fibroblasts, and osteoblasts seeded onto a titanium surface.
Materials and methods
Cell lines and cell culture
This study was carried out using a human oral keratinocyte lineage (NOK-Si–CVCL # BW57), a primary cell culture of human gingival fibroblasts (HGF-CAAE #55629215.7.0000.5416) and a human osteoblastic lineage (SaOs-2–ATCC# HTB85). All cells were maintained in 75-cm2 flasks (Corning, New York, NY, USA) in Dulbecco’s Modified Eagle’s Medium (DMEM – # - Gibco, Carlsbad, CA, USA) supplemented with antibiotics (PenStrep–Gibco) and 10% of fetal bovine serum (FBS–Gibco). Oral keratinocytes and HGF were sub-cultured using .25% trypsin (Gibco) while osteoblasts were sub-cultured by .25% trypsin/EDTA (Gibco).
Experimental design
For this investigation, 13-mm-diameter machined titanium disks were polished in − 400, − 600, and − 1200 granulation bands and then cleaned with acetone, ethanol, and deionized water [18,19,20]. Surface roughness was analyzed by confocal microscope (OLYMPUS LEXT OLS4000, Japan) and then disks were sterilized in an autoclave. Prior to cell seeding, disks were individually placed in wells of sterilized 24-well plates (Techno Plastic Products-TPP, Trasadingen, CH, USA). Then, 1 mL of complete DMEM was added to each well, followed by cells for seeding (5 × 104 cells/well).
After 24 h of incubation, the complete DMEM was replaced by 1 mL of FBS-free DMEM and the cells were immediately subjected to PBM with LLLT or LED devices, at uniform parameters presented in Table 1 [22].
PBM was applied by means of two prototypes: LASERTable [8, 10, 19, 23] and LEDTable [24], both of which were specifically designed for in vitro studies. These devices provide full irradiation of each cell culture plate at a standardized distance and irradiation area, which allows for a uniform comparison of both therapies. The cells were irradiated three times at 24 h intervals, which corresponds to 9 J/cm2.
Twenty-four hours after the last irradiation, all cell types were assessed for adhesion, viability, and protein synthesis. Alkaline phosphatase activity (ALP) and mineral nodule deposition were detected only for osteoblasts. Cells seeded on Ti disks and not submitted to PBM were used as a control group.
Cell morphology
Morphological analysis by fluorescence microscopy was performed for the cells that remained attached to the Ti surface. Cells were fixed in 10% paraformaldehyde for 15 min and were permeabilized in .1% triton x-100 (Sigma-Aldrich, St Louis, MO, USA) for 10 min. Then, samples were incubated with Actin Red-probe (1:200-Molecular Probes, Carlsbad, CA, USA) for 30 min for visualization of cytoskeleton filaments while nuclei were stained with Hoescht DNA-intercalant (Molecular Probes) (1:5000) for 15 min. Samples (n = 4) were then assessed by inverted fluorescence microscope (EVOS Floid Cell Image Station, Thermo Fischer Scientific, Waltham, MA, USA) and photomicrographs were analyzed by ImageJ Software (US National Institutes of Health, Bethesda, MA USA). Five photomicrographs of each sample were analyzed to enable quantitative and qualitative data [19].
Cell viability
Viability of cells seeded onto a Ti surface and submitted to PBM by LLLT or LED was evaluated by an alamarBlue assay (Invitrogen, Carlsbad, CA, USA). For this protocol, cells were incubated at 37 °C with alamarblue solution at 10% in FBS-free DMEM for 4 h [19]. During this period, mitochondrial enzymes could cleave the resazurin salt in a fluorescent dye (resorufin), which was then detected in a fluorometer at 460/495 nm (Synergy H1 microplate reader, BioTek Instruments, Winooski, VT, USA).
Total protein synthesis
Total protein synthesis was assessed using the Lowry method and following the detailed protocol described by Basso et al. (2018) [19]. Briefly, after cell lysis with .1% sodium lauryl sulfate (Sigma-Aldrich), samples were incubated with Lowry reagent (.1%) for 40 min and protein conjugate was detected by Folin & Ciocalteu’s Phenol reagent (1:5, Sigma-Aldrich) for 20 min. Total protein amount was assessed by spectrophotometry (Synergy H1) at 655 nm. Bovine serum albumin (BSA) was used to obtain a standard curve.
Collagen synthesis
Collagen synthesis was determined by the Sirius Red method. This assay recognizes collagen types I, II, III, and IV, which resemble the collagen tissue of oral mucosa and bone [25]. Therefore, all cells were subjected to this protocol.
Supernatant of each sample was incubated (1:1) with Direct Red reagent at .1% (Sigma-Aldrich) for 1 h under agitation (400 rpm) at room temperature. Then, samples were centrifuged at 104 rpm following washing with hydrochloric acid (HCl-5M). After washing, new centrifugation pellets were dissolved in sodium hydroxide solution (NaOH-.5 M). A 200-μL aliquot of each sample was analyzed using spectrophotometry (Synergy H1) at 555 nm.
ALP activity
ALP is an ectoenzyme involved in the initial phases of bone mineralization. It acts by binding to collagen fibrils and enhancing calcium adherence; it also contributes to the conformational structure of this protein [20, 24]. Therefore, higher ALP activity is an indicator of increased mineralization activity and it can be used as a proxy. The in vitro ALP activity was detected by an end-point assay (Labtest Diagnóstico S.A., Lagoa Santa, MG, BR), as previously described [20].
Statistical data analysis
After normality and homoscedasticity evaluation (Shapiro-Wilk, p < 0.05), data were subjected to ANOVA and Tukey tests at a 5% significance level. The adhesion of cells to titanium surfaces was also qualitatively presented.
Results
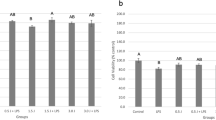
Similar quantitative and qualitative adhesion of osteoblasts and fibroblasts was observed for all groups (Fig. 1a and b; Tables 2 and 3) (p > 0.05). Increased cell population on Ti disks was observed after PBM only for keratinocytes, regardless of the light source used (Fig. 1c; Table 4) (p < 0.05).
Cell viability was increased for osteoblasts irradiated with PBM/LLLT and PBM/LED (p < 0.05; Table 2) as well for fibroblasts, which showed higher viability rates for PBM/LLLT (p < 0.05; Table 3). Viability of keratinocytes was up-regulated by PBM, especially when these cells were irradiated with PBM/LED (p < 0.05; Table 4).
Despite that the protein synthesis was enhanced for osteoblasts submitted to PBM/LLLT or PBM/LED irradiation (p < 0.05; Table 2), this cell activity was observed only in PBM/LLLT-treated fibroblasts (p < 0.05; Table 3). Keratinocytes subjected to PBM exhibited protein synthesis similar to the control group (p > 0.05; Table 4).
Collagen synthesis was also increased for PBM-treated osteoblasts and fibroblasts (p < 0.05). However, when these cells were irradiated with PBM/LED, they underwent a higher synthesis of collagen than those submitted to PBM/LLLT (Tables 2 and 3). Collagen synthesis by keratinocytes was unaffected by both the PBM protocols used in this study (p > 0.05; Table 4).
While the ALP activity was up-regulated in PBM/LLLT-treated osteoblasts, the irradiation of these cells with PBM/LED decreased their ALP activity, even in comparison with the control group (p < 0.05; Table 2).
Discussion
In general, both PBM treatment modalities (PBM/LLLT and PBM/LED) evaluated in this study improved all cell parameters related to healing of peri-implant tissues. The migration and adhesion of osteoblasts to the Ti surface, which is the first step that drives the osseointegration process, trigger a cascade of cellular and molecular events related to the synthesis and mineralization of collagen-rich matrix [26,27,28,29]. This investigation demonstrated that the adhesion of osteoblasts to a Ti surface was not influenced by PBM. However, both PBM treatment modalities increased cell metabolism, characterized by cell viability, total protein production, collagen synthesis, and ALP activity. Moreover, cell functions were distinctly affected by each PBM source; PBM/LLLT in particular demonstrated better results than PBM/LED, at selected parameters.
In a previous in vitro study, Khadra et al. (2005) [30] reported the positive response of osteoblasts submitted to PBM/LLLT at parameters similar to those evaluated by this investigation. Other studies have also evaluated the effects of PBM using LLLT or LED on osteoblasts [23, 31]. However, comparison of both therapy modalities using similar parameters is scarce [29]. In addition, the poor standardization of PBM protocols applied to the oral implantology field and the lack of detailed information regarding the irradiation parameters inhibit adequate comparison of current scientific data available in the literature [16, 32].
There are a limited number of studies that have used Ti as a substrate to evaluate the effects of PBM on peri-implant healing using human cells [21, 30, 33]. PBM investigations on bone and mucosa healing were performed using polystyrene substrate [34, 35], which is suitable for cell response but does not mimic clinical peri-implant conditions. Ross et al. (2012) [36] reported that cells display distinct behavior according to the substrate on which they are seeded. Therefore, it follows that the selection of adequate substrate for cell culture should be carefully considered for in vitro studies related to implant repair. This investigation used a Ti surface, oral mucosa cells, and osteoblasts to simulate in vivo conditions. The results of this in vitro investigation might predict the in vivo interaction of PBM-treated cells in a clinical setting to amplify osseointegration and soft tissue sealing to titanium—a material that is widely used to fabricate screws and abutments for oral implants.
Pagin et al. (2014) [31] demonstrated that PBM/LLLT enhanced the metabolism of osteoblasts and caused greater cell growth and differentiation in comparison to PBM/LED. The authors emphasized that this result may be related to the stimulation of specific chromophores that accelerate osteoblast differentiation. The stimulation of specific chromophores by distinct light sources may also be a relevant factor on PBM effects for each cell type [2]. Additionally, Hamblin et al. (2018) [5] reported that the sensitivity, phenotype, responsiveness, and homeostasis status of cells may influence differing responses when these cells are submitted PBM. However, while the primary effects of PBM may be to activate a similar pathway for different cell types—characterized by increased ATP synthesis and phosphorylation of proteins related to cell cycle—lasers and LEDs may present peculiarities regarding cell-light interaction [1, 5, 36].
It has been shown that the success of oral osseointegrated implants also depends on the formation of a peri-implant biological sealing by soft oral tissues [13, 37, 38]. The fast attachment of keratinocytes and HGF to prosthetic surfaces is mandatory for the establishment of such biological sealing, which acts as a physical and biological barrier against periodontal pathogens [13]. In this vein, the adhesion of fibroblasts and epithelial cells to a Ti surface is decisive for the successful outcome of oral osseointegrated implants over time [13]. Therefore, in the present study, the adhesion of PBM-treated human gingival fibroblasts (HGF) and oral keratinocytes (NOK) to the surface of Ti discs was assessed. In general, both modalities of irradiation enhanced keratinocyte population, which was demonstrated by qualitative and quantitative data (Table 4). Besides attaching to the Ti surface, epithelial cells also play a fundamental role in biological sealing, since they release collagenous proteins to strengthen the physical barrier, and immunoglobulins, which also protect the oral mucosa tissue against peri-implant pathogens [13]. Overall, the application of PBM at the selected parameters improved the adhesion and proliferation of keratinocytes to the Ti disks and also their viability when a LED treatment was used. On the other hand, total protein production and collagen synthesis were not influenced by either of the two PBM modalities.
In evaluating the response of fibroblasts to FBM, the application of PBM/LED enhanced the viability of HGF, while PBM/LLLT increased the total protein production by these cells, mainly via the synthesis of collagen. The latter observation was significant because collage is a major extracellular protein of periodontal tissue and plays a role in connective tissue healing [22]. Based on these data, these results provide evidence that the PBM/LLLT parameters assessed in this study may induce the fast deposition of collagenous tissue around a Ti abutment surface and improve the biological sealing at the implantation site. Previous studies also demonstrated the biostimulation of PBM/LLLT-treated fibroblasts [8, 19, 39, 40]. For instance, the data of this in vitro study highlight the distinct sensitivity and responsiveness of oral keratinocytes and fibroblasts, which were directly related to the light source used. Therefore, careful standardization of the PBM protocols is recommended and selection of specific light therapies for the target tissue characteristics and its cell responsiveness, such as those previously reported by Arany et al. (2016) [7] and Engel et al. (2016) [40, 41]. In general, PBM/LED therapy promoted higher positive biological effects on cultured oral keratinocytes and fibroblasts than PBM/LLLT for the PBM parameters assessed in this investigation.
In this study, the authors showed the photobiomodulation of osteoblasts as well as oral fibroblasts and keratinocytes seeded on a Ti surface and then submitted to specific parameters of LED and low-level laser therapies. The methodology used in this investigation was established to mimic in vivo conditions in which specific cells play key roles in the osseointegration and biological soft tissue sealing around dental implants. While recognizing that the results from laboratorial studies cannot be extrapolated directly to clinical situations [41], such as extremely controlled conditions, single-cell source for each cell line, and the absence of interaction among different tissues, the original scientific data reported from our investigation is promising and should drive further in vitro and in vivo studies to improve the research field and clinical outcomes for patients with dental implants.
Conclusion
At selected parameters, and considering cellular and tissue specificities, PBM may be a suitable therapy to promote peri-implant healing.
References
De Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):7000417
Al-Ghamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27:237–249
Passarella S, Karu T (2014) Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B Biol 140:344–358
Tsai S, Hamblin MR (2017) Biological effects and medical applications of infrared radiation. J Photochem Photobiol B Biol 170:197–207
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94(2):199–212
Saquib S, Jadhav V, Priyanka N, Perla N (2014) Low-level laser therapy in dentistry: a review. Int J Contemp Dent Med Rev:111214-111217
Arany PR (2016) Craniofacial wound healing with photobiomodulation therapy: new insights and current challenges. J Dent Res 95(9):977–984
Basso FG, Soares DG, Pansani TN, Cardoso LM, Scheffel DL, de Souza Costa CA, Hebling J (2016) Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Lasers Surg Med 48(10):1006–1014
Basso FG, Pansani TN, Soares DG, Hebling J, de Souza Costa CA (2018) LLLT Effects on oral keratinocytes in an organotypic 3D model. Photochem Photobiol 94(1):190–194
Cardoso LM, Pansani TN, Hebling J, de Souza Costa CA, Basso FG (2020) Photobiomodulation of inflammatory-cytokine-related effects in a 3-D culture model with gingival fibroblasts. Lasers Med Sci 35(5):1205–1212
Jayesh RS, Dhinakarsamy V (2015) Osseointegration. J Pharm Bioallied Sci 7(Suppl 1):S226–S229
Trindade R, Albrektsoon T, Wennerberg A (2015) Current concepts for the biological basis of dental implants. Oral Maxillofac Surg Clin North Am 27(2):175–183
An N, Rausch-fan X, Wieland M, Matejka M, Andrukhov O, Schedle A (2012) Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent Mater 28(12):1207–1214
Tang E, Arany P (2013) Photobiomodulation and implants: implications for dentistry. J Periodontal Implant Sci 43(6):262–268
Zayed SA, Hakim AAA (2020) Clinical efficacy of photobiomodulation on dental implant osseointegration: a systematic review. Saudi J Med Med Sci 8(2):80–86
Aoki A, Mizutani K, Schwarz F, Scullean A, Yukna RA, Takasaki AA, Romanos GE, Taniguchi Y, Sasaki KM, Zeredo JL, Koshy G, Coluzzi DJ, White JM, Abiko Y, Ishikawa I, Izumi Y (2015) Periodontal and peri-implant wound healing following laser therapy. Periodontol 68(1):217–269
Hosseinpour S, Fekrazad R, Arany PR, Ye Q (2019) Molecular impacts of photobiomodulation on bone regeneration: a systematic review. Prog Biophys Mol Biol 149:147–159
Pansani TN, Basso FG, Souza IDR, Hebling J, de Souza Costa CA (2019) Characterization of titanium surface coated with epidermal growth factor and its effect on human gingival fibroblasts. Arch Oral Biol 102:48–54
Basso FG, Pansani TN, Soares DG, Cardoso LM, Hebling J, de Souza Costa CA (2018) Influence of bisphosphonates on the adherence and metabolism of epithelial cells and gingival fibroblasts to titanium surfaces. Clin Oral Investig 22(2):893–900
Basso FG, Pansani TN, Cardoso LM, Hebling J, Vila Real RP, de Souza Costa CA (2020) Influence of bisphosphonates on the behaviour of osteoblasts seeded onto titanium discs. Braz Dent J 31(3):304–309
Ates GB, Can AA, Gülsoy M (2017) Investigation of photobiomodulation potentiality by 634 and 809 nm lasers on human osteoblasts. Lasers Med Sci 32(3):591–599
Jenkins PA, Carroll JD (2011) How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg 29(12):785–787
Lins EC, Oliveira CF, Guimarães OC, Costa CA, Kurachi C, Bagnato VS (2013) A novel 785-nm laser diode-based system for standardization of cell culture irradiation. Photomed Laser Surg 31(10):466–473
Turrioni AP, Basso FG, Montoro LA (2014) Almeida LdeF, de Souza Costa CA, Hebling J. Phototherapy up-regulates dentin matrix proteins expression and synthesis by stem cells from human-exfoliated deciduous teeth. J Dent 42(10):1292–1299
Bartold PM, Walsh LJ, Narayanan S (2000) Molecular and cell biology of the gingiva. Periodontol 24:28–55
Joos U, Wiessmann HP, Szuwart T, Meyer U (2006) Mineralization at the interface of implants. Int J Oral Maxillofac Surg 35(9):783–790
Chang P, Lang NP, Gianobile WV (2010) Evaluation of functional dynamics during osseointegration and regeneration associated with oral implants: a review. Clin Oral Implants Res 21(1):1–12
Esfahanizadeh N, Motalebi S, Daneshparvar N, Akhoundi N, Bonakdar S (2016) Morphology, proliferation, and gene expression of gingival fibroblasts on Laser-Lok, titanium, and zirconia surfaces. Lasers Med Sci 31(5):863–873
Khadra M, Lyngstadaas SP, Haanæs HR, Mustafa K (2005) Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 26(17):3503–3509
Pagin MT, Oliveira FA, Oliveira RC, Sant’Ana ACP, Rezende MLR, Greghi SLA, Damante CA (2014) Laser and light-emitting diode effects on pre-osteoblast growth and differentiation. Lasers Med Sci 29(1):55–59
Deana AM, Souza AM, Teixeira VP, Mesquita-Ferrari RA, Bussadori SK, Fernandes KPS (2018) The impact of photobiomodulation on osteoblast-like cell: a review. Lasers Med Sci 33(5):1147–1158
Cancakya AB, Erdem MA, Erdem AP, Erguven M, Aybar B, Kasapoglu C, Bilir A (2011) Evaluation of light-emmiting diode (LED-660 nm) application over primary osteoblast-like cells on titanium surfaces: an in vitro study. Int J Med Sci 8(7):584–593
Saracino S, Mozzati M, Martinasso G, Pol R, Canuto RA, Muzio G (2009) Superpulsed laser irradiation increases osteoblast activity via modulation of bone morphogenetic factors. Lasers Surg Med 41(4):298–304
Ross AM, Jiang Z, Bastmeyer M, Lahann J (2012) Physical aspects of cell culture substrates: topography, roughness, and elasticity. Small 8(3):336–355
Salvi GE, Bosshardt DD, Lang NP, Abrahamsoon I, Berglundh T, Lindhe J, Ivanovski S, Donos N (2015) Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontol 68(1):135–152
Heiskanen V, Hamblin MR (2018) Photobiomodulation: lasers vs. light emitting diodes? Photochem Photobiol Sci 17(8):1003–1017
Al-Wattar WMA, Al-Wattar WM, Al-Radha ASD (2017) Microbiological and cytological response to dental implant healing abutment. J Int Dental Med Res 10(3):891–898
Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K (2005) Determining optimal dose of laser therapy for attachment and proliferation of human oral fibroblasts cultured on titanium implant material. J Biomed Mater Res A 73(1):55–62
Roncati M, Lauritano D, Cura F, Carinci F (2016) Evaluation of light-emitting diode (LED-835 nm) application over human gingival fibroblast: an in vitro study. J Biol Regul Homeost Agents 30(2 Suppl 1):161–167
Engel KW, Khan I, Arany PR (2016) Cell lineage responses to photobiomodulation therapy. J Biophotonics 9(11-12):1148–1156
De Souza Costa CA, Hebling J, Scheffel DLS, Soares DG, Basso FG, Ribeiro APD (2014) Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 30(7):769–784
Funding
The National Council for Scientific and Technological Development, CNPq (Grant # 302108/2019-0) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) provided financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rech, C.A., Pansani, T.N., Cardoso, L.M. et al. Photobiomodulation using LLLT and LED of cells involved in osseointegration and peri-implant soft tissue healing. Lasers Med Sci 37, 573–580 (2022). https://doi.org/10.1007/s10103-021-03299-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03299-w