Abstract
Goal of work
The aim of this study was to investigate the expression of pro-apoptotic protein p53 and anti-apoptotic proteins BCl-2 and MCl-1, as well as the expression of pro-inflammatory cytokines tumor necrosis factor (TNF) and interleukin-1beta (IL-1β) in patients developing mucositis during radiotherapy for head and neck cancer.
Materials and methods
Thirty-five patients receiving radiotherapy for head/neck cancer were included in this study. Patients were examined before radiotherapy. Oral mucositis was recorded weekly during radiotherapy. Cytologic smears from the oral cavity were taken with a brush. Immunocytochemical staining was performed by the use of p53, BCl-2, MCl-1 TNF and IL-1β monoclonal antibodies.
Main results
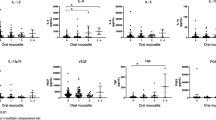
P53 was expressed in 1 of 15 smears before the initiation of radiotherapy (6.5%) compared to 3 of 7 smears from patients with grade III mucositis (43%) during radiotherapy. BCl-2 was expressed in 15 of 15 smears before radiotherapy (100%) and in three of seven patients with grade III mucositis (43%) during radiotherapy. MCl-1 was expressed in 10 of 14 samples before radiotherapy (71.5%) and in two of seven patients with grade III (28.5%) mucositis during radiotherapy. TNF was expressed in 9 of 14 patients before radiotherapy (64%) and in six of seven patients with grade III mucositis during radiotherapy (86%). IL-1β was detected in 7 of 14 patients before radiotherapy (50%) compared to 6 of 7 patients with grade III mucositis during radiotherapy (86%).
Conclusion
Our preliminary results indicate an induction of apoptosis and inflammation in the oral mucosa in patients developing mucositis during radiotherapy for head/neck cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucositis is a painful and debilitating side effect of many of the chemotherapy and radiation regimens used to treat cancer [47]. Oral mucositis occurs in approximately 40% of patients receiving conventional dose chemotherapy, 75% of patients receiving myeloablative chemotherapy for stem-cell transplantation and in nearly all patients receiving radiotherapy for head and neck cancer [28, 39, 50, 51].
Oral mucositis is associated with significant health and economic costs. It can result in pain and a decreased quality of life, and it is considered to be the most debilitating and troublesome side effect of therapy from the patient’s perspective [6, 43, 47]. Mucositis is also associated with higher risk of local and systemic infection (since the ulcerations provide a portal of entry for oral bacteria) and increased length of hospitalization [15]. In patients with severe mucositis, modification or even interruption of antineoplastic treatment is often required. Finally, the increased use of a variety of healthcare resources results in additional cost [49].
Current evidence indicates that the aetiology of mucositis is complex, involving change in gene expression, altered apoptosis and interaction between epithelial and subepithelial compartments [10]. It is now clear that mucosal injury results from a series of events in which cellular mediators play an important role [47]. Tumor necrosis factor (TNF), interleukin-1beta (IL-1β) and interleukin-6 (IL-6) are well-known pro-inflammatory cytokines involved in local and systemic inflammatory reactions [18]. Increased levels of TNF and IL-6 were found in the peripheral blood of patients receiving chemotherapy who demonstrated nonhematologic toxicities, compared with those who did not manifest such toxicities [22]. Similarly, patients during pre-transplant conditioning who developed post-transplant complications had significantly higher TNF levels than those without complications [42]. Subsequent animal studies demonstrated increased cytokine levels (IL-1β, TNF, IL-2 and TGFβ) not only in peripheral blood, but also within the oral mucosa following radiation [48].
Apoptotic cell death is an important component of regimen-related mucosal injury [4]. Both radiotherapy and chemotherapy damage the intestinal lining and cause apoptosis rather than necrosis of epithelial crypt cells and crypt hypoplasia [3, 9]. Keefe et al. [27] reported that in patients receiving chemotherapy, apoptosis increased sevenfold in intestinal crypts at 1 day and preceded hypoplastic villous atrophy and loss of enterocyte height. Potten et al. [40] found that apoptosis was markedly induced in intestinal crypts of mice after irradiation or combined radiotherapy and chemotherapy.
Several studies in animals and human cancer cell lines have described high pro-apoptotic (such as p53, Bax, Bak, Caspase-3) and low anti-apoptotic (such as BCl-2, MCl-1) protein expression in intestinal crypts of small intestine after the administration of chemotherapy and/or radiotherapy and correlated this to high levels of apoptosis which results in intestinal mucositis [8, 26, 29, 38].
Pro-apoptotic, anti-apoptotic and pro-inflammatory proteins expressed in the oral mucosa in head and neck cancer patients who receive radiotherapy and develop mucositis have not been investigated. Therefore, the aim of this study was to investigate the expression of pro-apoptotic protein p53 and anti-apoptotic proteins BCl-2 and MCl-1, as well as the expression of pro-inflammatory cytokines TNF and IL-1β, within the oral mucosa in patients developing mucositis after receiving radiotherapy for head and neck cancer. The hypothesis of our study was that pro-apoptotic proteins and pro-inflammatory cytokines would be increased, whereas anti-apoptotic markers would be decreased in patients developing oral mucositis after receiving radiotherapy for head/neck cancer.
Materials and methods
Patients
Thirty-five patients referred from the cancer centres of five Athens hospitals, with malignant head and neck tumor, eligible to receive radiotherapy, were included in the study. General blood tests and liver and renal functions were within normal limits. Karnofsky performance status ranged between 80 and 100%. All patients were thoroughly informed about their disease and the treatment they would receive. All patients signed an informed consent form. This study was approved by the Dental School of Athens Ethics Committee. Standard oral mucosal and dental care was introduced to all patients.
Radiotherapy
Patients were irradiated with a 6-MV linear accelerator. Approximately 16 patients received radical and 19 patients received postoperative radiotherapy (RT). The primary tumor and draining lymphatics were treated with parallel opposed fields. Supraclavicular and low neck nodes were treated with an anterior field. The daily and the total radiation dose are shown in Table 1. The lateral-field doses were reduced after 40–43 Gy to avoid overdosage to the spinal cord. The regional nodes were irradiated to a total dose of 45–61 Gy, depending on the nodal stage. Concomitant chemotherapy, including two cycles of cisplatinum (100 mg/m2 on days 1 and 28 of RT) and 5-fluorouracil (800 mg/m2 on days 1–5 and 28–32 of RT), was administered to 14 patients (40%). Table 1 shows patient characteristics, tumor diagnosis and type and dose of RT.
Oral clinical evaluation
Patients were examined before the initiation of radiotherapy and on weekly basis during the radiotherapy period. Oral mucosa evaluation was performed in every visit by the same two oral-medicine specialists. The scoring of oral mucositis was recorded using the EORTC/RTOG criteria (Table 2) [12].
In addition, oral mucositis was differentially diagnosed from local infection, which was treated accordingly. Oral mucositis grade I was differentially diagnosed from erythematous candidiasis. Oral ulcerative/pseudomembrane mucositis (grades II, III, IV) was differentiated from pseudomembranous candidiasis and herpes simplex virus-1 (HSV-1) infection.
The presumptive diagnosis of oral candidiasis was based on the criteria reported previously by Nicolatou-Galitis et al. [35, 37].
The presumptive diagnosis of HSV-1 infection was based on the criteria reported previously by Nicolatou-Galitis et al. [36].
Cytology
Cytologic smears were taken with a brush from the right and left buccal area of patients before the initiation of radiotherapy. During the radiotherapy period, smears were taken on a weekly basis. In patients who did not develop ulcerative mucositis, the samples were collected from the right and left buccal areas, whereas in patients who developed ulcerative mucositis (grades II, III and IV), the smears were taken from the ulcers and the surrounding area.
Eight glass slides with specimens were prepared from each collection site of the patients. Two of them were fixed with 96% alcohol and were stained with Papanicolaou stain for verification of the type of cells obtained (Fig. 1). The rest of the specimens were prepared for immunocytochemical study. These specimens were air dried, fixed for 10 min in cold acetone (−10°C) and stored at −70°C until use. Immunocytochemical staining was performed by the Avidin–Biotin Complex immunoperoxidase method [25]. Smears were incubated for 45 min with normal horse serum diluted 1:50 in phosphate-buffered saline (PBS; Dakopatts, Glostrup, Denmark) to reduce background staining. The primary antibodies p53 (1:50 dilution; Menarini, Italy), BCl-2 (1:50 dilution; Menarini), MCl-1 (1:50 dilution; Menarini), TNF (1:50 dilution; Menarini) and IL-1β (1:50 dilution; Menarini) were incubated for 40 min followed by incubation with the secondary antibody for 30 min and an avidin-biotin-peroxidase complex for 30 min. Peroxidase enzyme staining was achieved by incubation with 3-amino-9-ethyl carbazole and hydrogen peroxidase for 10 min. Between incubations, the smears were rinsed with PBS solution (0.05 μl/l, pH 7.6). Smears were counterstained with Mayer’s haematoxylin and covered with mounting medium (Glycergel, Dako, Glostrup, Denmark). Control slides were incubated with PBS solution. Immunocytochemical reactivities were evaluated by calculating the proportion of positively stained cells in at least ten visual fields. The intensity of staining was scored on a four-point scale: 0 = no staining, 1 = weak but unequivocal staining, 2 = definite staining of moderate intensity, 3 = strong staining.
Results
Seven of the smears were taken from patients with grade III mucositis during the study.
The pro-apoptotic marker p53 was weakly expressed in 1 of 15 smears from patients with grade 0 mucositis (6.5%) before the initiation of radiotherapy and in three of seven smears from patients with grade III mucositis (43%) during the radiotherapy period (Figs. 2 and 3).
The anti-apoptotic marker BCl-2 was weakly expressed in 15 of 15 smears from patients with grade 0 mucositis (100%) before the initiation of the treatment and in three of seven patients with grade III mucositis (43%) after the initiation of radiotherapy (Figs. 4 and 5).
In the pre-RT smears, the antiapoptotic marker MCl-1 was expressed in 10 of 14 samples with grade 0 mucositis (71.5%), in three of which the expression was moderate to strong (19%). In patients with grade III mucositis during RT, MCl-1 was weakly expressed in two of seven patients (28.5%; Figs. 6 and 7).
TNF was expressed in 9 of 14 patients with grade 0 mucositis (64%; strongly in 36% of them) before radiotherapy and in six of seven patients with grade III mucositis (86%; strongly in 57% of them) during radiotherapy (Figs. 8 and 9).
Before the initiation of radiotherapy, weak to moderate expression of IL-1β was detected in 7 of 14 patients with grade 0 mucositis (50%) compared to 6 of 7 patients with grade III mucositis (86%) during the radiotherapy period (Figs. 10 and 11).
In smears taken from patients with an increase of mucositis severity within two consecutive examinations, we observed up-regulation of p53 expression in 2 of 12 patients (17%), down-regulation of BCl-2 expression in 3 of 12 patients (25%), down-regulation of MCl-1 expression in 3 of 9 patients (33%), up-regulation of TNF expression in 4 of 9 patients (44%) and up-regulation of IL-1β expression in 7 of 9 patients (78%).
In smears taken from patients with a reduction of the grade of mucositis (healing), the expression of p53 appeared to be down-regulated in 50% of smears, whereas the expression of BCl-2 and MCl-1 appeared to be up-regulated in 75 and 100% of smears, respectively.
The same measurements were made for the patients with less severe mucositis (grade I and II), and the results showed a tendency towards the same direction but less clear. Thus, for the present preliminary report, only the data for patients with mucositis 0 and III are shown.
Discussion
This study investigated changes in pro-inflammatory, pro-apoptotic and anti-apoptotic markers in patients developing mucositis after receiving radiotherapy for head/neck malignant tumors. The hypothesis of our study was that pro-apoptotic proteins and pro-inflammatory cytokines would be increased, whereas anti-apoptotic proteins would be decreased in patients developing oral mucositis after receiving radiotherapy for head/neck cancer. An increase in the expression of pro-inflammatory cytokines TNF and IL-1β, as well as in the expression of pro-apoptotic protein p53 and a decrease in the expression of anti-apoptotic proteins BCl-2 and MCl-1 were recorded and were related to the radiation-induced oral mucositis. Our findings, therefore, are in agreement with our hypothesis.
Tumor necrosis factor is a pleiotropic protein that was initially isolated from mouse serum following exposure to bacterial endotoxin [11, 33]. It belongs to a family of proteins that have both beneficial, as well as potentially damaging, effects throughout the body [23, 33]. TNF plays an important role in inflammatory and immune responses, both protective and autoimmune such as rheumatoid arthritis and inflammatory bowel disease (IBD) [23, 33, 34].
Interleukin-1β is a cytokine with multiple biologic effects, including fever and increased gene expression of pro-inflammatory cytokines and mediators [13, 33]. Chemotherapy affects the release of both Interleukin-1β and TNF from the epithelium [30, 46]. Ionising radiation, at doses which in themselves are not directly damaging to tissue, also causes the release of these cytokines from the epithelium and connective tissue [46, 53].
Tumor necrosis factor is capable of causing tissue damage [2] and may be an accelerating and initiating event in the mucositis process [46]. Interleukin-1β incites an inflammatory response resulting in increased subepithelial vascularity [19] with a potential consequent increase in the local levels of cytotoxic agent [46]. Along with TNF, IL-1β is involved in the activation of NF-κB pathway. IL-1β and TNF have been reported to have a synergistic effect essential for the initial phases of the inflammatory response [14, 33].
Several researchers have demonstrated elevated TNF serum levels occurring in association with non-haematological toxicities [17, 22, 41, 42, 44]. Inhibition of TNF using agents such as pentoxyfylline reduced these non-haematological toxicities [7]. With respect to mucositis, various studies have shown a decrease in the occurrence or severity of mucositis following administration of TNF inhibitors [7, 16, 32]. Lima et al. [32] demonstrated, using a hamster model of 5-FU-induced mucositis, that administration of TNF inhibitors pentoxyfylline and thalidomide reduced the macroscopic and histological parameters of oral mucositis and concluded that these results indicated an important role for TNF in the pathophysiology of 5-FU induced oral mucositis. Local tissue levels of IL-1β and TNF have also been shown to increase markedly in animal models of radiation-induced oral mucositis concurrently with a development of mucositis [14]. Our results are in agreement with those of previous authors in serum and animal models, also demonstrating an increase in the expression of TNF and IL-1β in the oral mucosa in patients developing mucositis after receiving radiotherapy for head and neck cancer.
BCl-2 and MCl-1 are members of the BCl-2 gene family which controls apoptosis in response to a wide variety of stimuli, including chemotherapy and radiotherapy [1, 8, 21, 24]. The BCl-2 family consist of both pro- and anti- apoptotic members, and their main action appears to be the regulation of mitochondrial membrane permeability and caspase activation. Pro-apoptotic family members include Bax, Bak, Bcl-xS, Bad, BID, Bim, Noxa and Puma while anti-apoptotic family members include the Bcl-2, Mcl-1, Bcl-Xl, Bcl-w and A1 [1, 8, 21, 24].
P53 is a tumor-suppression gene due to its ability to suppress transformation by oncogenes and inhibit growth of transformed cells [5, 9]. The functions of p53 include cell cycle regulation, DNA repair and apoptosis [9, 45]. Apoptosis is a dominant mechanism by which p53 inhibits tumor development, and it is highly conserved through evolution [9]. The half life of the p53 protein is relatively short (about 20 min), and therefore, the cellular concentration remains normally very low. Levels of p53 protein are increased after various stimuli like oncogene activation, hypoxia, radiation, heat shock and cytotoxic drugs [9, 52].
Several studies in animals and human cancer cell lines have described high pro-apoptotic and low anti-apoptotic protein expression following chemotherapy and/or radiotherapy and correlated this to high level of apoptosis [20, 26, 29, 31, 38]. Gibson et al. [20] demonstrated an up-regulation of Bax protein and a down-regulation of Bcl-2 gene expression in breast cancer cell lines following chemotherapy (etoposide and cyclophosphamide combination). Leung and Wang [31] reported similar results in breast cancer cell lines following administration of adriamycin [31]. Inomata et al. [26] observed that when 5-fluorouracil was applied to murine intestinal crypts, Bax-positive cytoplasm was observed throughout the crypt epithelial cells, accompanied by the occurrence of apoptosis. In a 1996 report, the exposure of mice to gamma-radiation resulted in rapid elevations in the levels of the Bax protein. Increases in Bax were followed by massive apoptosis in lymphoid organs and in the small intestinal crypts [29]. Bowen et al. [8] have shown that cytotoxic chemotherapy up-regulates pro-apoptotic Bax, Bak and p53 proteins and down-regulates anti-apoptotic Mcl-1 protein in the small intestinal crypts of rats and humans. The increase of pro-apoptotic proteins coincided with elevated levels of apoptosis. Our results also show an increase in the expression of pro-apoptotic protein p53 and a decrease in anti-apoptotic proteins BCl-2 and MCl-1 in the oral mucosa of patients developing mucositis after receiving radiotherapy for head and neck cancer, being consistent with those of previous studies in animals, human cell lines and intestinal crypts.
In this study, we observed an up-regulation of pro-inflammatory and pro-apoptotic proteins, along with a down-regulation of anti-apoptotic proteins, not only in patients who developed mucositis after receiving radiotherapy for head/neck cancer, but also in patients with an increase in mucositis severity within two consecutive examinations. Conversely, we have recorded a down-regulation of the pro-apoptotic protein p53, along with an up-regulation of anti-apoptotic proteins BCl-2 and MCl-1 in patients with a reduction of the grade of mucositis (healing). Notably, these findings seem to correlate with the five-phase theoretical mucositis model proposed by Sonis [47].
While there is a sizable literature on the role of pro-inflammatory cytokines and apoptotic proteins in the development of mucositis, most researchers focus on animal or cancer cell line studies or measure the levels of the inflammatory markers in the serum. This study investigated the changes on the inflammatory cytokines and pro- and anti- apoptotic proteins in vivo, in the oral cavity of head and neck cancer patients, before the initiation of radiotherapy, after the development of radiotherapy-induced mucositis and after the healing of mucositis. The samples were taken from the oral cavity directly from the ulcerative lesions of mucositis. In addition, the present study investigates a combination of inflammatory and apoptotic markers in the same patients. An immunocytolochemical technique has been used, and cytologic smears were collected from the oral cavity. Cytology is a well-established method for the study of epithelial cell changes and this study is, as far as we know, the first time that this method is used for the study of radiotherapy-induced mucositis in the oral mucosa of head and neck cancer patients.
In conclusion, in this study, we have used an immunocytochemical technique to evaluate the changes in inflammatory and apoptotic markers expressed in the oral cavity of head and neck cancer patients before the initiation of radiotherapy and after the development of radiotherapy-induced mucositis. An increase in the expression of pro-inflammatory cytokines TNF and IL-1β as well as the expression of pro-apoptotic protein p53 and a decrease in the expression of anti-apoptotic proteins BCl-2 and MCl-1 were recorded and were related to the radiation-induced oral mucositis. To the best of our knowledge, our results represent the first attempt to identify significant mucositis-related markers using samples from the ulcerative lesions of oral cavity of head and neck cancer patients before and after the radiotherapy treatment. These results are in agreement with those of previous studies in animals, human cell lines, serum and intestinal crypts, further denoting the correlation of inflammatory and apoptotic proteins to the pathobiology of mucositis. The present preliminary report justifies the continuation of the study and the additional investigation in a larger number of patients to further explore the complex aetiopathological mechanisms which underlie the biology of oral mucositis. It would also be worthwhile to make blood measurements of the same markers, so that a comparison between blood and brushing measurements can be made. The study is continued.
References
Adams J, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Aggarwal B, Kohr W, Hass P (1985) Human tumor necrosis factor. Production, puri®cation and characterization. J Biol Chem 260:2345–2354
Anilkumar T, Sarraf C, Hunt T, Alison M (1992) The nature of cytotoxic drug-induced cell death in murine intestinal crypts. Br J Cancer 65:552–558
Anthony L, Bowen J, Garden A, Hewson I, Sonis S (2006) New thoughts on the pathobiology of regimen-related mucosal injury. Support Care Cancer 14:516–518
Baker S, Markowitz S, Fearon E, Willson J, Vogelstein B (1990) Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249:912–915
Bellm L, Epstein J, Rose-Ped A (2000) Patient reports of complications of bone marrow transplantation. Support Care Cancer 8:33–39
Bianco J, Appelbaum F, Nemunaitis J (1991) Phase I–II trial of pentoxifylline for the prevention of transplant-related toxicities following bone marrow transplantation. Blood 78:1205–1211
Bowen J, Gibson R, Keefe D, Cummins A (2005) Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37:56–62
Bowen J, Gibson R, Cummins A, Keefe D (2006) Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer 14:713–731
Bowen J, Gibson R, Cummins A, Tyskin A, Keefe D (2007) Irinotecan changes gene expression in the small intestine of the rat with breast cancer. Cancer Chemother Pharmacol 59:337–348
Carswell E, Old L, Kassel R (1975) An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 72:3666–3670
Cox J, Stetz J, Pajak T (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346
Dinarello C (1996) Biologic basis for interleukin-1 in disease. Blood 87:2095–2147
Dinarello C (2000) Proinflammatory cytokines. Chest 118:503–508
Elting L, Cooksley C, Chambers M (2003) The burdens of cancer therapy: clinical and economic outcomes of chemotherapy-induced mucositis. Cancer Nurs 98:153–159
Ferra C, de Sanjose S, Lastra C (1997) Pentoxifylline, ciprofloxacin and prednisone failed to prevent transplantrelated toxicities in bone marrow transplant recipients and were associated with an increased incidence of infectious complications. Bone Marrow Transplant 20:1075–1080
Ferra C, de Sanjose S, Gallardo D (1998) IL-6 and IL-8 levels in plasma during hematopoietic progenitor transplantation. Haematologica 83:1082–1087
Ferra C, de Sanjose S, Gallardo D, Berlanga JJ, Rueda F, Marin D, de la Banda E, Ancin I, Peris J, Garcia J, Granena A (1998) IL-6 and IL-8 levels in plasma during hematopoietic progenitor transplantation. Haematologica 83:1082–1087
Ferrara J, Abhyankar S, Gilliland D (1993) Cytokine stem of graft-versus-host disease: a critical e€ector role for interleukin-1. Transplant Proc 25:1216–1217
Gibson L, Fortney J, Magro G, Ericson S, Lynch J, Landreth K (1999) Regulation of BAX and BCL-2 expression in breast cancer cells by chemotherapy. Breast Cancer Res Treat 55:107–117
Gross A, McDonnell J, Korsmeyer S (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Hall P, Benko H, Hogan K (1995) The influence of serum tumor necrosis factor-alpha and interleukin-6 concentrations or nonhematologic toxicities and hematologic recovery in patients with acute myelogenous leukemia. Exp Hematol 23:1256–1260
Hehlgans T, Pfeffer K (2005) The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology 115:1–20
Herr I, Debatin K (2001) Cellular stress response and apoptosis in cancer therapy. Blood 98:2603–2614
Hsu S, Raine L, Fanger H (1981) Use of avidin–biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Inomata A, Horii I, Suzuki K (2002) 5-Fluorouracil-induced intestinal toxicity: what determines the severity of damage to murine intestinal crypt epithelia. Toxicol Lett 133:231–240
Keefe D, Brealey J, Goland G, Cummins A (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47:632–637
Khan S, Wingard J (2001) Infection and mucosal injury in cancer treatment. J Natl Cancer Inst Monogr:31–36
Kitada S, Krajewski S, Miyashita T, Krajewska M, Reed J (1996) Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene 12:187–192
Krenger W, Ferrara J (1996) Graft-vs-host disease and the Th1/Th2 paradigm. Immunol Res 15:50–73
Leung L, Wang T (1999) Differential effects of chemotherapeutic agents on the Bcl-2/Bax apoptosis pathway in human breast cancer cell line MCF-7. Breast Cancer Res Treat 55:73–83
Lima V, Brito G, Cunha F (2005) Effects of the tumour necrosis factor-a inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur J Oral Sci 113:210–217
Logan R, Stringer A, Bowen J, Yeoh A, Gibson R, Sonis S, Keefe D (2007) The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 33(5):448–460 (May 14)
Lorenz H-M, Kalden J (2002) Perspectives for TNF-a-targeting therapies. Arthritis Res 4:S17–S24
Nicolatou-Galitis O, Dardoufas K, Markoulatos P (2001) Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. J Oral Pathol Med 30:471–480
Nicolatou-Galitis O, Athanassiadou P, Kouloulias V, Sotiropoulou-Lontou A, Dardoufas K, Polychronopoulou A, Gonidi M, Kyprianou K, Kolitsi G, Skarleas C, Pissakas G, Papanikolaou I, Kouvaris J (2006) Herpes simplex virus-1 (HSV-1) infection in radiation-induced oral mucositis. Support Care Cancer 14:753–762
Nicolatou-Galitis O, Velegraki A, Sotiropoulou-Lontou A (2006) Effect of fluconazole antifungal prophylaxis on oral mucositis in head and neck cancer patients receiving radiotherapy. Support Care Cancer 14(1):44–51
Nita M, Nagawa H, Tominaga O, Tsuno N, Fujii S, Sasaki S, Fu C, Takenoue T, Tsuruo T, Muto T (1998) 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br J Cancer 78:986–992
Peterson DE, Schubert MM (2001) Oral toxicity. In: Perry MC (ed) The chemotherapy source book. 3rd edn. Lippincott, Williams and Wilkins, Philadelphia, pp 404–424
Potten C, Wilson J, Booth C (1997) Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells 15:82–93
Rabinowitz J, Petros W, Stuart A, Peters W (1993) Characterization of endogenous cytokine concentrations after high-dose chemotherapy with autologous bone marrow support. Blood 81:2452–2459
Remberger M, Ringdem O, Markling L (1995) TNF alpha levels are increased during bone marrow transplantation conditioning regimens in patients who develop acute GVHD. Bone Marrow Transplant 15:99–104
Rose-Ped A, Bellm L, Epstein J (2002) Complications of radiation therapy for head and neck cancers: the patient’s perspective. Cancer Nurs 25:461–467
Sleijfer S, Vujaskovic Z, Limburg P, Koops H, Mulder N (1998) Induction of tumor necrosis factor-a as a cause of bleomycinrelated toxicity. Cancer Chemother Pharmacol 82:970–974
Smith N, Rubenstein J, Eggener S, Kozlowski J (2003) The p53 tumor suppressor gene and nuclear protein: basic science review and relevance in the management of bladder cancer. J Urol 169:1219–1228
Sonis S (1998) Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 34:39–43
Sonis S (2004) A biological approach to mucositis. J Support Oncol 2:21–32
Sonis S, Peterson R, Edwards L (2000) Defining mechanisms of action of interleukin-11 on the progression of radiation-induced mucositis in hamsters. Oral Oncol 36:393–381
Sonis S, Oster G, Fuchs H (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem cell transplantation. J Clin Oncol 19:2201–2205
Stiff P (2001) Mucositis associated with stem cell transplantation: current status and innovative approaches to management. Bone Marrow Transplant 17:S3–S11
Sutherland S, Browman G (2001) Prophylaxis of oral mucositis in irradiated head-and-neck cancer patients: a proposed classification scheme of interventions and metaanalysis of randomized controlled trials. Int J Radiat Oncol Biol Phys 49:917–930
Vousden K, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604
Xun C, Thompson J, Jennings C, Brown S, Widmerru B (1994) E€ect of total body irradiation, busulfan-cyclophosphamide or cyclophasphamide conditioning on in¯ammatory cytokine release and development of acute and chronic graft-vs-host disease in H-2-incompatible transplanted ScID mice. Blood 83:2360–2367
Acknowledgements
We wish to thank Ms Antonia Sipsa and Ms Amalia Moumouri for their valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xanthinaki, A., Nicolatou-Galitis, O., Athanassiadou, P. et al. Apoptotic and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: preliminary report. Support Care Cancer 16, 1025–1033 (2008). https://doi.org/10.1007/s00520-007-0379-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0379-8