Abstract
The aim of this study was to investigate the effects of low-level red laser on tissue repair in rats submitted to second-degree burn, evaluating if the timing of laser treatment influences the healing process. The animals had their backs shaved and divided as follows: control group (n = 12)—rats burned and not irradiated, early laser group (n = 12)—rats burned and irradiated from day 1 after injury for five consecutive days, and late laser group (n = 14)—rats burned and irradiated from day 4 after injury for five consecutive days. Laser irradiation was according to a clinical protocol (20 J/cm2, 100 mW, continuous wave emission mode, 660 nm) as recommended by the laser device manufacturer. Half of the animals were sacrificed 10 days after burn, and the other animals were sacrificed 21 days after burn. The late laser group accelerated wound contraction 10 and 21 days after burn. The late laser group accelerated reepithelialization 18 days after burn. The late laser group increases the granulation tissue 10 and 21 days after burn. Both irradiated groups increased type III collagen expression and TGF-β 21 days after burn. Both irradiated groups increased macrophage and myofibroblast numbers 10 days after burn and decreased 21 days after. Low-level red laser exposure contributes to the process of tissue repair of second-degree burns, but the intervention during proliferative phase is crucial in the final outcome of the repair process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the International Society of Burns, the burn is defined as an injury to the skin or other body tissue caused mainly by acute thermal trauma such as hot liquid, fire, radiation, electricity, and friction [1]. In the USA, every year, 450,000 patients receive medical treatment related to burns, with over 40,000 hospital admissions and 3500 deaths due to burns [2].
After skin burn injury, the repair process occurs in three overlapping but distinct phases: inflammation, granulation tissue formation, and remodeling [3]. Inflammation is characterized mainly by the recruitment of inflammatory cells, platelet activation, and secretion of pro-inflammatory cytokines; granulation tissue formation follows with the formation of new vessels, myofibroblastic differentiation, and deposition of extracellular matrix, and during remodeling, reduction in cellularity occurs, and extracellular matrix reorganization toward normal dermis with formation of larger collagen bundles with higher content of intermolecular cross-links between collagen molecules is a main feature [4]. Nowadays, therapeutic strategies to restore the burned tissue are mostly unsatisfactory in the aesthetic and functional standpoint. Thus, new therapeutic strategies with the aim to reduce the damage caused by the burn are necessary [5, 6].
Therapy with low-level laser has been scientifically proven as an important therapeutic modality for various clinical indications [7]. This therapy is based on the excitation of endogenous chromophores which occurs when the biological tissue absorbs the radiation at a wavelength corresponding to red or near infrared [8]. As a consequence of light energy absorption, cell metabolism and synthesis of biomolecules (nucleic acids, proteins, and ATP) are increased [9], due to biomodulation effect [8]. Some studies have demonstrated the beneficial effects of laser in burn models. Meireles and coworkers [10] reported that diabetic rats subjected to third-degree burn and exposed to red (660 nm) and infrared (780 nm) low-level lasers presented accelerated tissue repair. Also, pulsed infrared laser improved tissue repair in rats subjected to third-degree burn [10]. However, there is no consensus about benefits of low-level lasers for treating burn skin, and other authors reported no improvement on burn skin in mice exposed to low-level red lasers [11].
Moreover, different conditions of laser exposure, such as wavelength, fluence, and emission mode (continuous wave or pulsed emission mode), are used in experimental and clinical protocols [12–14] making difficult the comparison between the studies. Also, the phase of tissue repair when laser exposure is more effective is not determined to second-degree burn.
Thus, the aim of this study was to investigate the effects of low-level red laser on tissue repair in rats submitted to second-degree burn, evaluating if the timing of laser treatment influences the healing process. Fluence, power, emission mode, and schedule laser irradiation were used according to clinical protocols proposed by the device’s manufacturer.
Methods
Animals
Male Wistar rats weighing between 250 and 350 g were maintained with free access to food and water on a 12-h light/dark cycle. All experimental animal work was carried out in accordance with the Brazilian Legislation (no. 11.794, from October 8, 2008) and approved by the Ethical Committee for Animal Use of Universidade do Estado do Rio de Janeiro (CEUA/023/2014).
Low-level red laser
Experimental procedures utilized a low-level laser (Photon Lase III, AlGaInP, 100 mW), with emission in 660 nm in a continuous wave mode. Laser device was purchased from D.M.C. Equipamentos Ltda (São Paulo, Brazil).
Experimental design
The animals were sedated with a intramuscular injection of a combination of ketamine (150 mg/kg) and xylazine (15 mg/mg); then, the animals had their backs shaved and were divided as follows: control group (n = 12)—rats burned and not irradiated, early laser group (n = 12)—rats burned and irradiated from day 1 after injury for five consecutive days, and late laser group (n = 14)—rats burned and irradiated from day 4 after injury for five consecutive days. Laser irradiation was performed with a grid system in which the lesion area was covered twice with an average of 22 irradiations per lesion according to a clinical protocol (660 nm, 20 J/cm2, 100 mW, continuous wave emission mode) in each quadrant as recommended by the laser device manufacturer. Half of the animals were sacrificed 10 days after burn, and the other animals were sacrificed 21 days after burn. All animals were sacrificed by anesthetic overdose.
Burn model
A device and a positioning described by our group were used to burn the animals. Briefly, a 23-mm diameter aluminum cylinder was attached to a soldering iron and was heated to 80 °C; once the temperature reached the desired level, the metal rod was placed in contact with the back skin of the animal for 15 s [15].
Macroscopic analysis
To measure the lesion area, a transparent plastic sheet was placed over the burn, and burn margins were traced. When present, the reepithelized area was also traced. After digitalization, burn areas were evaluated using ImageJ software image-processing system (National Institutes of Health, Bethesda, MD). Reepithelization was estimated by the difference between the total burn area and the burn area still uncovered by neoepidermis at each time point. Data are expressed as percentage of early burn area and percentage of reepithelized burn area at each time point.
Histological examination
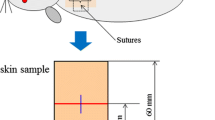
The skin samples were carefully collected to include the adjacent healthy tissue and the healed tissue. Tissue fragments were formalin fixed (pH 7.2) and paraffin embedded. Sections (5 μm) were stained with hematoxylin and eosin to analyze microscopically the burn area, epidermis thickness (μm), and granulation tissue area (μm2) using ImageJ software. Sections were also stained with Picro-Sirius and observed under polarization, to evaluate collagen fiber organization.
Western blotting analysis
Five frozen lesions per group were macerated in a lysis buffer, and total protein concentration was determined using the bicinchoninic acid protein assay (Thermo Fisher Scientific, Rockwood, TN). Proteins (30 μg) were resolved by 8 or 10 % sodium dodecylsulfate-polyacrylamide gels and were transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5 % nonfat milk in powder form (Molico, São Paulo, Brazil) and probed with a rabbit anti-precursor transforming growth factor (TGF)-β 1/2/3 antibody (Santa Cruz Biotechnology, Buffalo, NY/1:200), rabbit anti-type 1 collagen precursor (Col-1) (Millipore, Temecula, CA/1:500), mouse anti-type 3 collagen (Col-3) (Millipore, Temecula, CA/1:600), and mouse anti-β-actin antibody (Sigma-Aldrich, Saint Louis MO/1:1000) overnight at 4 °C. Membranes were then washed and incubated with anti-rabbit (1:100) or anti-mouse (1:100) secondary antibody conjugated with peroxidase (DAKO, Carpinteria, CA). Bound antibodies were detected by enhanced chemiluminescence (Santa Cruz Biotechnology), densitometry analysis was performed using Adobe Photoshop version 7.01 (Adobe Systems, San Jose, CA), and results were expressed as arbitrary units.
Immunohistochemistry and quantification
For quantification of CD68-positive macrophages, cellular proliferation, myofibroblasts, and blood vessels presenting α-smooth muscle actin-positive cells, sections were incubated with mouse anti-CD68 (1:300; Serotec, Kidlington, UK), mouse anti-smooth muscle actin (1:1000; DAKO), and mouse anti-proliferating cell nuclear antigen (DAKO; 1:1000), respectively. For antigen retrieval, sections were digested with 0.1 % trypsin (Difco Laboratories, Detroit, MI) or incubated with citrate buffer (pH 6.0) before labeling. Subsequently, sections were incubated with 3 % hydrogen peroxide in methanol to inhibit endogenous peroxidase. After washing, all primary antibodies were detected using the EnVision system (DAKO), and diaminobenzidine was used as a chromogen. Sections were counterstained with hematoxylin. No labeling was observed on sections where primary antibody was omitted.
Slides were digitalized using a Pannoramic MIDI digital slide scanner (3Dhistech Kft; Budapeste, Hungria). To quantify the number of cells or vessels, ten random fields per animal were captured (70,000 μm2) through an image analysis software (Pannoramic Viewer; 3Dhistech Kft). Data are presented as cells per square millimeter.
Statistical analysis
Data are reported as means ± standard deviation. The one-way analysis of variance (ANOVA) test was performed to verify possible statistical differences followed by Bonferroni posttest with p < 0.05 as less significant level. InStat GraphPad software was used to perform statistical analysis (GraphPad InStat version 6.00 for Windows XP, GraphPad Software, San Diego, CA).
Results
Macroscopic analysis
The late laser group presented accelerated wound contraction by 36 % after 10 days (p < 0.001) and 69 % after 21 days (p < 0.01) compared to their respective control group. Also, data in Fig. 1a show a significant (p < 0.001) difference between the early and late irradiated groups after 10 days of laser exposure (p < 0.001). The late laser group presented accelerated reepithelialization by 18 % after 18 days of laser exposure compared to the control group (p < 0.01) and 13 % compared to the early laser group (p < 0.05) according to Fig. 1b.
Macroscopic analysis. a Percentage of initial burn area 3, 10, 18, and 21 days after burn and b percentage of reepithelialized burn area in 18 days after burn. Data are expressed as the mean ± standard deviation. **p < 0.01 irradiated vs. control; ***p < 0.001 irradiated vs. control; #p < 0.05 early laser vs. late laser; ###p < 0.001 early laser vs. late laser
Microscopic analyzes
Neoepidermis thickness in Wistar rats burned and exposed to low-level red laser is shown in Fig. 2a, b. The early laser group presented a significant (p < 0.05) increase (40 %) of neoepidermis thickness compared to the control group. Also, a significant (p < 0.01) increase was observed when the early group was compared to the late one 10 days after burn. It was observed, 21 days after burn, a significant (p < 0.001) decrease in neoepidermis thickness by 39 % between irradiated groups compared to the control group.
Microscopic analysis. a Epidermal thickness 10 and 21 days after burn. b Microscopic aspect of granulation tissue. Bar = 200 μm, hematoxylin and eosin. c Quantification of granulation tissue area 10 and 21 days after burn. Data are expressed as the mean ± standard deviation. *p < 0.05 irradiated vs. control; ***p < 0.001 irradiated vs. control; ##p < 0.01 early laser vs. late laser; ###p < 0.001 early laser vs. late laser. d Collagen fibers in granulation tissue 10 and 21 days after burn. Observe an increase in the amount of collagen fibers in irradiated groups 21 days after burn. Bar = 100 μm, Picro-Sirius stained observed under polarization
Granulation tissue area in the skin of Wistar rats burned and exposed to low-level red laser is shown in Fig. 2c. The late laser group presented a significant increase of 1515 % and 1232 % in the area of granulation tissue 10 days after burn compared to the control group and early laser group (p < 0.001), respectively. Twenty-one days after burn, the early and late laser groups presented a similar significant increase (p < 0.05) in granulation tissue area (102 % and 100 %, respectively) compared to the control group. Collagen fibers in rat skin exposed to low-level red laser are shown in Fig. 2d. By Picro-Sirius staining, observed under polarized light, an increase in the amount of collagen fibers in granulation tissue of the irradiated groups 10 and, especially, 21 days after burn was observed.
Western blotting analysis
Regarding the expression of collagen I, no difference was observed between the early laser group and the control group, only a reduction of 15 % in the late laser group compared to the control group, but not significant according to Fig. 3a. The early laser group presented a significant increase of 15 %, and the late laser group of 5 %, in the expression of collagen III when compared to the control group (p < 0.05) according to Fig. 3b. The early laser group presented a significant increase of 21 %, and the late laser group of 13 %, in the expression of TGF-β, both compared to the control group (p < 0.05) according to Fig. 3c.
Protein expression. a Type I collagen expression 21 days after burn. b Type III collagen expression 21 days after burn. c TGF-β expression 21 days after burn. β-Actin was used as a constitutive protein for normalization. Densitometry expressed as arbitrary units (a.u.). Data are expressed as the mean ± standard deviation. *p < 0.05 irradiated vs. control
Immunohistochemistry
Data in Fig. 4a shows an increase in macrophage number by 135 % in the early laser group and 103 % in the late laser group, when compared to the control group (p < 0.001) 10 days after burn. But after 21 days, a decrease of 44 % in the early laser group and 63 % in late laser group was observed, both compared to the control group (p < 0.001).
Immunohistochemistry analysis. a Macrophages CD68-positive 10 and 21 days after burn. b PCNA-positive cells 10 and 21 days after burn. c Myofibroblast 10 and 21 days after burn. d Vessels. Bar = 50 μm. Data are expressed as the mean ± standard deviation. *p < 0.05 irradiated vs. control; **p < 0.01 irradiated vs. control; ***p < 0.001 irradiated vs. control; #p < 0.05 early laser vs. late laser; ##p < 0.01 early laser vs. late laser; ###p < 0.001 early laser vs. late laser
Data in Fig. 4b show a decrease of proliferating cell nuclear antigen (PCNA)-positive cells of 29 % in the early laser group compared to the control group (p < 0.05) and 37 % compared to the late laser group (p < 0.01) 10 days after burn. But after 21 days of laser exposure, a decrease of 52 % in the early laser group and 40 % in the late laser group, when compared to the control group (p < 0.001), was observed.
Data in Fig. 4c show an increased amount of myofibroblasts (91 %) (p < 0.01) in the early laser group and in the late laser group (237 %) (p < 0.001). However, after 21 days of laser exposure, the early laser group shows a decrease of 60 % (p < 0.01) in myofibroblast number, while a reduction of 45 % (p < 0.001) was observed in the late laser group when compared to the control group.
Data in Fig. 4d show an increase of vessel number of 91 % in the early laser group compared to the control group (p < 0.01) and 55 % compared to the late laser group (p < 0.001) 10 days after burn. Also, after 21 days of laser exposure, vessel number increased 129 % (p < 0.01) in the early laser group and 118 % (p < 0.001) in the late laser group, when compared to the control group.
Discussion
Although the comprehension about the effects of low-level laser therapy is increasing, there are conflicting results due to the use of different parameters, such as wavelength, fluence, emission mode, and power, as well as absence of consensus in the clinical protocols [16]. In laser device guides, clinical protocols are suggested for the treatment of a number of diseases, but sometimes they do not present a scientific basis [17]; there is no suggestion about physiologic tissue condition and/or indication whether exposure is more effective in a specific phase of the disease. Data obtained from experimental models can validate these protocols and confirm the positive effects of laser radiation. This study employed parameters suggested by the device’s manufacturer, and laser exposure was carried out at different phases of second-degree burn.
Low-level red laser accelerated the closing time of the injury at 10 days, especially in the late laser group. Wound contraction occurs mainly by the action of myofibroblasts approaching the edges of the lesion [18]. During the second phase of wound healing, proliferation, fibroblasts differentiate into myofibroblasts [19]. The late laser group was applied during the second phase and potentiated this phase.
Epidermis thickness can be used as an indicator of repair process. Migration and proliferation of keratinocytes occur parallel to lesion contraction; when neoepidermis is completely formed, its thickness reduces [19]. This study showed an increase in epidermis thickness 10 days after burn and a decrease 21 days after burn in treated animals, showing that the whole process was improved. An increase in the area of granulation tissue in animals from the late laser group 10 days after burn was observed, and after 21 days, both the irradiated groups presented increased granulation tissue. Final stage of the proliferative phase is characterized by the formation of granulation tissue in which fibroblasts migrate to the site of injury with intent to synthesize collagen [20]. Our results agree with Meireles and coworkers [10] that observed a moderate increase of tissue granulation in diabetic rats subjected to third-degree burn and irradiated with 660 nm after 21 days. Irradiation of the late group began just in the proliferative phase, suggesting that the laser stimulated fibroblasts to migrate to the burn site and synthesize extracellular matrix components, such as collagen.
In the qualitative analysis of collagen staining by Picro-Sirius, an increase of collagen fibers, not well organized, in the irradiated groups was observed. The type of collagen deposited in the wound area was evaluated by Western blotting analysis. Expression of type III collagen was increased compared to the control group. These results agree with other studies that observed in rats subjected to third-degree burn treated with red laser a predominance of type III collagen after 16 days [21]. Usually, in a normal process of skin repair, there is deposition of type III collagen in the early days after the injury and that is replaced by type III collagen in the final phase of repair [22]. Furthermore, the production of collagen in irradiated groups is probably stimulated by the increased expression of TGF-β. This growth factor is responsible for stimulating the fibroblasts to synthesize collagen [23]. Laser irradiation stimulated the closure and also the synthesis of matrix components.
Reddy [16] suggested that the time of intervention of the laser is crucial in the tissue repair process and highlighted the inflammatory and proliferative phases as critical moments. Studies suggest that the laser is capable of modulating anti-inflammatory and pro-inflammatory mediators during the process of tissue repair [24, 25]. Also, it has been suggested that macrophages play an important role in the early stages of skin repair and that their depletion could adversely affect reepithelialization, angiogenesis, and contraction of the lesion [26–28]. In our study, laser irradiation modulated inflammation, and we observed a higher amount of inflammatory cells 10 days after burn and a reduced amount 21 days after burn.
We observed a reduction of some important parameters in skin repair process as the number of PCNA-positive cells and myofibroblasts, mainly 21 days after burn. A progressive increase in the number of vessels, in both the irradiated groups, was observed mainly 21 days after burn. Similar results were reported by Núñez and coworkers [5] who observed an increase in blood vessels in rats subjected to burn and exposed to low-level red laser 21 days after injury. In addition, a study by Chiarotto and coworkers [29] in rats subjected to second-degree burn and irradiated with 670 and 830 nm showed that the laser increased the expression of vascular endothelial growth factor (VEGF) that can explain the increase in the amount of vessels.
A recent study reported an improvement of third-degree burn healing after low-level red laser exposure [30]. However, third-degree burns are very different from second-degree burns. In third-degree burns, the whole dermis is destructed and the healing starts from the remaining tissue at borders of the lesion. In laser therapy for the treatment of third-degree burns, the angle of lesions is exposed to laser, but in second-degree burns, the whole lesion area is exposed to laser because in these burns, part of the dermis remains and is sufficient to begin the healing process. Our research demonstrated that wound closure is improved after exposure to low-level red laser due to the increase of reepithelization, granulation tissue volume, type III collagen deposition, neoepidermis maturation (thinner neoepidermis), as well as increase of TGF-β levels and macrophage amount parallel to the increase of cell proliferation and myofibroblast amount during the initial phase, and decrease in the advanced phase.
Thus, our study advances in the comprehension of the effects of low-level red laser on second-degree burns demonstrating that this radiation accelerates the wound healing process modulating parameters involved in tissue repair mainly when laser exposure is carried out in the proliferative phase.
Conclusion
In conclusion, this study showed that low-level red laser exposure contributes to the process of tissue repair of second-degree burns, but that the intervention during proliferative phase is crucial in the final outcome of the repair process. In this study, clinical parameters were used to set the laser device, what makes easily to extrapolate the results, although all the limitations in comparing animal and human studies must be considered.
References
Peck MD (2011) Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 37:1087–1100
American burn association Online. http://www.ameriburn.org/resources_factsheet.php. 2012
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–746
Nunez SC, Franca CM, Silva DF, Nogueira GE, Prates RA, Ribeiro MS (2013) The influence of red laser irradiation timeline on burn healing in rats. Lasers Med Sci 28:633–641
Zhang X, Liu L, Wei X, Tan YS, Tong L, Chang R, Ghanamah MS, Reinblatt M, Marti GP, Harmon JW, Semenza GP (2010) Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1alpha heterozygous-null mice after burn wounding. Wound Repair Regen 18:193–201
Abrahamse H (2012) Regenerative medicine, stem cells, and low-level laser therapy: future directives. Photomed Laser Surg 30:681–682
Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62:607–610
da Silva JP, da Silva MA, Almeida AP, Lombardi Junior I, Matos AP (2010) Laser therapy in the tissue repair process: a literature review. Photomed Laser Surg 28:17–21
Meireles GC, Santos JN, Chagas PO, Moura AP, Pinheiro AL (2008) Effectiveness of laser photobiomodulation at 660 or 780 nanometers on the repair of third-degree burns in diabetic rats. Photomed Laser Surg 26:47–54
Vasheghani MM, Bayat M, Rezaei F, Bayat A, Karimipour M (2008) Effect of low-level laser therapy on mast cells in second degree burns in rats. Photomed Laser Surg 26:1–5
Gupta A, Daí T, Hamblin MR (2014) Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med Sci 29:257–265
Goncalves RV, Novaes RD, Cupertino Mdo C, Moraes B, Leite JP, Peluzio Mdo C, Pinto MV, da Matta SL (2013) Time-dependent effects of low-level laser therapy on the morphology and oxidative response in the skin wound healing in rats. Lasers Med Sci 28:383–390
de Moraes JM, de Oliveira E, Mendonca D, Moura VB, Oliveira MA, Afonso CL, Vinaud MC, Bachion MM, de Souza Lino R Jr (2013) Anti-inflammatory effect of low-intensity laser on the healing of third-degree burn wounds in rats. Lasers Med Sci 28:1169–1176
Venter NG, Monte-Alto-Costa A, Marques RG (2014) A new model for the standardization of experimental burn wounds. Burns. doi:10.1016/j.burns.2014.08.002
Reddy GK (2004) Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg 22:141–150
Fonseca AS, Geller M, Bernardo Filho M, Valenca SS, de Paoli F (2012) Low-level infrared laser effect on plasmid DNA. Lasers Med Sci 27:121–130
Farahani RM, Kloth LC (2008) The hypothesis of ‘biophysical matrix contraction’: wound contraction revisited. Int Wound J 5:477–482
Li J, Chen J, Kirsner R (2007) Pathophysiology of acute wound healing. Clin Dermatol 25:9–18
Broughton G 2nd, Janis JE, Attinger CE (2006) Wound healing: an overview. Plast Reconstr Surg 117:1e-S–32e-S
Fiorio FB, Albertini R, Leal-Junior EC, de Carvalho PT (2014) Effect of low-level laser therapy on types I and III collagen and inflammatory cells in rats with induced third-degree burns. Lasers Med Sci 29:313–319
Wong VW, Gurtner GC, Longaker MT (2013) Wound healing: a paradigm for regeneration. Mayo Clin Proc 88:1022–1031
Boo S, Dagnino L (2013) Integrins as modulators of transforming growth factor beta signaling in dermal fibroblasts during skin regeneration after injury. Adv Wound Care (New Rochelle) 2:238–246
dos Santos SA, Alves AC, Leal-Junior EC, Albertini R, Vieira Rde P, Ligeiro AP, Junior JA, de Carvalho PT (2014) Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med Sci 29:1051–1058
Alves AC, Vieira R, Leal-Junior E, dos Santos S, Ligeiro AP, Albertini R (2013) Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther 15:R116
Mirza R, DiPietro LA, Koh TJ (2009) Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 175:2454–2462
Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W (2010) Differential roles of macrophages in diverse phases of skin repair. J Immunol 184:3964–3977
Jetten N, Roumans N, Gijbels MJ, Romano A, Post MJ, de Winther MP (2014) Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PLoS One 9:e102994
Chiarotto GB, Neves LM, Esquisatto MA, do Amaral ME, Dos Santos GM, Mendonça FA (2014) Effects of laser irradiation (670-nm InGaP and 830-nm GaAlAs) on burn of second degree in rats. Lasers Med Sci 29:1685–1693
de Vasconcelos Catao MH, Nonaka CF, de Albuquerque RL Jr, Bento PM, de Oliveira Costa R. (2014) Effects of red laser, infrared, photodynamic therapy, and green LED on the healing process of third-degree burns: clinical and histological study in rats. Lasers Med Sci 30(1):421-428
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trajano, E.T.L., da Trajano, L.A., dos Santos Silva, M.A. et al. Low-level red laser improves healing of second-degree burn when applied during proliferative phase. Lasers Med Sci 30, 1297–1304 (2015). https://doi.org/10.1007/s10103-015-1729-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1729-2