Abstract
The objective of this study is to analyze the effectiveness of low power laser irradiation in the bone consolidation of tibial fractures in rats. An experimental, comparative, prospective study with control group was designed. Twenty Wistar rats were grouped into control (n = 10) and experimental groups (n = 10). A tibial fracture, with a mechanical drill, was inflicted in all rats. The experimental group received ten days of low power arsenide-gallium laser irradiation of 850 nm (KLD, Sao Paulo, Brasil)—100 mW, 8 J/cm2, 64 s. Before and after the laser treatment, a radiologic analysis was carried out in both groups, in which the rats were graded from 0 to IV according the Montoya scale of bone consolidation. Also, we histopathologically analyzed the bone to estimate the proliferation of fibroblasts, bone matrix, and angiogénesis with a microscopy, which were graded as I (thin layer of fibroblasts and osteoid matrix), II (thick layer of fibroblasts and osteoid matrix), or III (thick layer of fibroblasts and osteoid matrix and new blood vessels). Radiologic data showed that the experimental group had a higher bone consolidation of Montoya scale after ten days of laser irradiation compared to control group (P < 0.004). Histopathologic data showed more fibroblasts and angiogenesis presence in the group receiving laser irradiation, compared to control group (P < .002). The low power laser radiation therapy may expedite the bone repair after tibial fractures in rats, according to radiologic and histopathologic analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fractures involve an interruption to the continuity of the bone due to a traumatism, a stress overload, or a bone structure alteration due to a neoplasic, infectious, or genetic process [1]. Since the bone means the frame of the body, its functions involve the following: protection, movement, and storage and production of blood elements [2]. For this reason, the bone is continually being remodeled and renewed, a process which is influenced by mechanic and metabolic stimuli [3–5]. The irrigation and innervation of bones preserves its structure and functions, which may be jeopardized after a fracture [4, 3, 6].
The low power laser means an effective therapy showing analgesic, anti-inflammatory, and healing effects on the radiated region [7]. Despite its controversial biologic fundamentals, some authors recently reported increased microcirculation and tissue reparation, accelerating its metabolism [7, 8]. However, these effects may depend on the wavelength and the generated energy [9, 10].
Studies regarding low intensity laser irradiation reported an earlier initiation of reparation process in the bone and other tissues such as cartilage [11, 12] and earlier epithelization [13]. Also, studies showed that laser radiation may stimulate the growth of femoral condyles in rats, with these results being confirmed in soft and hard tissues [12, 14] (Barbosa).
Authors studying low power laser irradiation reported an increased fibrotic tissue production during the second week of the cartilage reparation process, with this therapy stimulating bone repair in vitro and in vivo studies [15].
However, very few studies indicated the wavelength, intensity, and power in the laser irradiation. Therefore, studies have employed different adjustments for laser irradiation, showing different beneficial results in tissue repair: diode laser irradiation with 650 nm and 39 mW in postsurgical phases [15]; infrared radiation with 850 nm in rats [16]; or low power laser with 660–808 nm in the femur of rats [17]. Barbosa et al. compared the laser therapy of 660 and 830 nm in the bone reparation of rats, reporting the best results from the 830-nm laser radiation [18]. Also, in humans, low power laser with 810 nm decreased pain perceptions when caused by orthodontic elastomeric separators [19], or low-power laser with 830 nm which improved the pain level and healing process in closed bone fractures of wrist and hand [20].
Due to the controversy about the most effective power, intensity, and wavelength applied in the repair process, we aimed to evaluate the effectiveness of continuous low power laser stimulation—100 mW, 850 nm, 64 s, and 8 J/cm2 as a low dose—in the consolidation of tibial fractures in rats.
Methods
Design
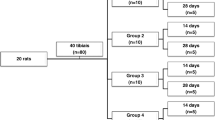
An experimental, comparative, prospective, unicentric study with a control group was designed. Twenty rats were chosen from the Bioterio Claude Bernard in Benemérita Autonomus University of Puebla and held in the Veterinary Hospital for Small Species—where the study was also conducted—in controlled conditions: light (12/12-h day/night), temperature (24 °C ± 2 °C), and free water and food. All rats should meet the inclusion criteria: male Wistar strain and weight of 160–200 g. This study was approved by the Universitary Biotherius and met with the Official Mexican Standard NOM-062-Z00 of, which refers to the technical specifications for production, care and use of laboratory animals.
The variables that we measured were the following: bone consolidation (graded according the Montoya Scale), and cell differentiation—proliferation of fibroblasts, bone matrix, and angiogenesis—(graded from the histhological evaluation).
Procedures
Preparation of sample
The sample was grouped into two groups: control group (n = 10 rats) (group A) and experimental group (n = 10 rats) (group B) which received the laser irradiation therapy. All rats were subjects of intraperitoneal anestesy, using ketamina-xilazina (first in control group). We shaved the medial region of the right shinbone of the rats and performed the traditional embrocate. When the asepsis was finished, we performed a 1-cm longitudinal incision in the proximal and medial third of the shinbone, involving only skin and subcutaneous tissue. The muscles were debrided, showing the bone to localize the medial region of tibial crest, where we made a hole with a Kirschner spike (0.062 mm) and a power drill. Then, we sutured the skin with a nylon suture (3–0 size) and simple stitches. All rats received an antibiotherapic and analgesy protocol according to their weight (Enrofloxacina—5 mg/kg, every 12 h for 5 days—and Meloxicam—2 mg/kg, every 24 h for 5 days—both orally). Thus, we performed the radiologic study with an equipment of Ray X Siemens Aktiengesellschaft Elema AB (Berlin, Germany) and a standardized dosimetry to avoid changes in bone density. The developing was performed with a Digital Equipment FCR Fujifilm Computed Radiography (Tokyo, Japan), which informed about the characteristics of the induced hole in the bone.
Laser irradiation
All rats of laser irradiation group were stimulated with a continuous low power irradiation Arsenide-Gallium laser (Ar-Ga) brand KLD®, model LASER IR (Sao Paulo, Brasil). All experimental rats received ten doses of 850 nm continuous laser radiation—100 mW, 8 J/cm2, 64 s—with punctal application on the medial side of the tibial crest where the hole were performed, during ten consecutive days (one dose/day). Before and after these ten days, all rats were radiologically and histopathologically studied to compare qualitatively both groups. We report the minimum eight most important parameters according to Jenkins and Carroll, 2011 as a summary [21]:
Wavelength = 830 nm; power = 100 mW; irradiation time = 64 s; beam area at the skin or culture surface (this is not necessarily the same as the aperture size) = 0.04 cm2, pulse parameters = continuous; anatomical location = punctal application on the medial side of the tibial crest, number of treatments = 10; and interval between treatments = every 24 h.
Radiologic and histopathological study
After the ten days of intervention, all rats were sacrificed in a chamber with an overdose of isoflurane gas (during the recommended time). In the end, we surgically disjoint the right lower limb, releasing the shinbone, which was allocated on a plastic recipient with 10 % of formol (to keep the tissue viable) and histopathologically study it.
All radiologic and histopathologic parameters of all rats were analyzed. The Montoya scale was used to measure bone consolidation grade [22, 23] (Table 1). The radiographs were performed by a Veterinarian Doctor and Zootechnician experienced in radiology (Fig. 1). A hematoxylin-eosin staining was used to the histhopatological evaluation (Fig. 2). The proximal epiphysis and distal diaphysis of all shinbones were analysed with a microscopy by a pathologist and were classified according the histologic evaluation criteria into the following: I (thin layer of fibroblasts and osteoid matrix), II (thick layer of fibroblasts and osteoid matrix), or III (thick layer of fibroblasts and osteoid matrix and new blood vessels).
Radiologic evaluation of control and experimental group. A1 the line of fracture is observable in the first day after performing the hole in the bone in the control group. A-1 the line of fracture is observable ten days after performing the hole in the bone in the control group. B2 the line of fracture is observable in the first day after performing the hole in the bone in the experimental group. B-2 the line of fracture is not observable ten days after performing the hole in the bone in the experimental group, which received the laser irradiation therapy during these ten days
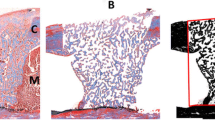
Histopathological study (hematoxylin-eosin staining). a Control group. Epiphysis with fibroblast proliferation (×10). b Control group. Epiphysis with fibroblast proliferation (×40). c Experimental group. Epiphysis with a thick layer of conjunctive tissue due to fibroblasts proliferation (×10); d Experimental group. Epiphysis with a thick layer of conjunctive tissue due to fibroblast proliferation and a neoformation of blood vessels (×40)
Statistical analysis
A Wilcoxon test for independent samples was used to compare both groups. Data were analyzed using SPSS for Windows (version 17; SPSS, Inc., Chicago, IL, USA), and significance was determined at P < 0.05.
Results
Table 2 showed the grade of Montoya scale after ten days with or without laser irradiation in the experimental and control group, respectively. Wilcoxon test for independent samples showed that rats from experimental group had higher radiologic grades (of the Montoya scale) compared to control group after the laser irradiation therapy (Z = −2.8; P = 0.004) (Fig. 3).
Table 3 showed the histopathological data about the proliferation of fibroblasts and neo-formation of blood vessels after ten days with or without laser irradiation in the experimental and control group, respectively. Wilcoxon test for independent samples showed statistical differences between both experimental and control groups (Z = −3.1; P = 0.002).
Discussion
The main findings of the present study showed that ten days of continuous low power laser irradiation—dose 8 J/cm2, wavelength 850 nm, power 100 mW, and time 64 s—expedited bone consolidation in tibial fractures in rats according to the Montoya scale, erasing the fracture line when compared with the control group rats. Supporting our results, Poppi et al., 2011, reported increased immature osteoblasts, osteocytes, and fibroblasts after applying laser irradiation of 660–808 nm as wavelength with 133 J/cm2 in femurs of rats [17] and Batista et al., showed positive local biostimulative effects of low-level laser therapy of 830 nm with 210 J/cm2, 210 s and 50 mW, in the normal bone (without previous radiotherapy). Similar results reported DeSouza et al., 2012 with laser irradiation of 100 mW and 660 nm [11]. However, the power remained unknown in both studies [11, 17]. In the present study, we showed the effect of a laser irradiation dose considerably lower than the studies up to date, which means a healthier radiologic treatment.
Our histopathologic study showed a higher fibroblast growth and proliferation, as well as higher bone matrix and neoformation of blood vessels compared to control rats. Very few studies designed a histopathologic study after laser irradiation in bone consolidation process. Pinheiro et al., 2012, showed histologic benefits after laser irradiation with 650 nm and 39 mW [16, 24]. Seifi et al., 2010, reported a growth of maxillary condyles in rats receiving laser irradiation compared to control rats, however none of both studies mention the intensity of the therapy [14]. Also, Barbosa et al., 2014 compared a laser radiation of 660 and 830 nm in the bone reparation of rats, reporting better results from the 830-nm laser radiation [18].
Some authors reported an increase in the microcirculation due to the release of nitric oxide in the irradiated tissues, providing the needed oxygen to repair the tissues. However, data about laser irradiation dose remain unknown [25]. Also, Wanq et al., 2014 reported improvements in the regeneration of the sciatic nerve after 20 days of low-level laser irradiation of 808 nm at 8 and 3 J/cm2, taken together.
The present study had some limitations: we only performed histologic and radiologic measurements 10 days after treatment, and an intermediate measurement may be interesting to estimate the bone consolidation process with more detail. However, our investigation informed about all characteristics of laser irradiation and showed the comparability of a control group.
Our study revealed an improvement in the bone consolidation process after the laser irradiation with a dose lower than usual, which increased the bone matrix and neoformation of blood vessels—cellular components that may lead the healing of bone injuries.
Conclusion
Ten days of diary continuous low power laser irradiation with 100 mW, 850 nm; 8 J/cm2 and 64 s with punctual application expedite the bone consolidation process and bone callus formation in shinbone of rats, as well as increase the fibroblast proliferation, bone matrix, and neoformation of blood vessels.
References
Zamudio L (2009) Ortopedia y traumatología de Zamudio 6ª Edición. Méndez Editores, Mexico
Guillén del Castillo M, Linares Girela D (2002) Bases biológicas y fisiológicas del movimiento humano. Panamericana, Madrid
Borysenko M, Ávila FAG (1985) Histología funcional. Limusa, Mexico
Latarjet M, Liard AR (2004) Anatomía humana, vol 2. Médica Panamericana, Madrid
Geneser F (2000) Histología 3a edición. Médica Panamericana, Buenos Aires
Beaty JH, Kasser JR (2003) Fracturas en el niño de Rockwood & Wilkims 5ª edición. Marbán, Madrid
Pérez JMG (2004) Física del láser. Alqua, California
Morillo MM, Portero FS, Vega JP (1998) Manual de medicina física. Elsevier España, Madrid
Martín JMR (2004) Electroterapia en fisioterapia. Médica Panamericana, Madrid
da Rosa AS, dos Santos AF, da Silva MM, Facco GG, Perreira DM, Alves ACA, Junior L, Pinto EC, de Carvalho PTC (2012) Effects of Low level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis. Photochem Photobiol 88(1):161–166
de Souza Merli LA, de Medeiros VP, Toma L, Reginato RD, Katchburian E, Nader HB, Faloppa F (2012) The low level laser therapy effect on the remodeling of bone extracellular matrix. Photochem Photobiol 88(5):1293–1301. doi:10.1111/j.1751-1097.2012.01172.x
Calatrava IR, Valenzuela JS, Gómez-Villamandos R, Redondo J, Gómez-Villamandos J, Jurado IA (1997) Histological and clinical responses of articular cartilage to low-level laser therapy: experimental study. Lasers Med Sci 12(2):117–121
Bisht D, Mehrotra R, Singh PA, Atri SC, Kumar A (1999) Effect of helium-neon laser on wound healing. Indian J Exp Biol 37(2):187–189
Seifi M, Maghzi A, Gutknecht N, Mir M, Asna-Ashari M (2010) The effect of 904 nm low level laser on condylar growth in rats. Lasers Med Sci 25(1):61–65. doi:10.1007/s10103-009-0651-x
Pacheco-Soares C, de Lurdes Moreira Gusmão C, Ferreira PD, Salgado MA (2012) The recover from the excision in head and neck of femoral after therapy with low level laser. J Biomed Sci Eng 5(2)
Pinheiro AL, Soares LG, Cangussu MC, Santos NR, Barbosa AF, Silveira Junior L (2012) Effects of LED phototherapy on bone defects grafted with MTA, bone morphogenetic proteins and guided bone regeneration: a Raman spectroscopic study. Lasers Med Sci 27(5):903–916. doi:10.1007/s10103-011-1010-2
Poppi RR, Da Silva AL, Nacer RS, Vieira RP, de Oliveira LVF, de Faria Júnior NS, Carvalho PTC (2011) Evaluation of the osteogenic effect of low-level laser therapy (808 nm and 660 nm) on bone defects induced in the femurs of female rats submitted to ovariectomy. Lasers Med Sci 26(4):515–522
Barbosa D, Villaverde AG, LoschiavoArisawa EA, de Souza RA (2014) Laser therapy in bone repair in rats: analysis of bone optical density. Acta Ortop Bras 22(2):71–74. doi:10.1590/1413-78522014220200438
Eslamian L, Borzabadi-Farahani A, Hassanzadeh-Azhiri A, Badiee MR, Fekrazad R (2014) The effect of 810-nm low-level laser therapy on pain caused by orthodontic elastomeric separators. Lasers Med Sci 29(2):559–564. doi:10.1007/s10103-012-1258-1
Chang WD, Wu JH, Wang HJ, Jiang JA (2014) Therapeutic outcomes of low-level laser therapy for closed bone fracture in the human wrist and hand. Photomed Laser Surg 32(4):212–218. doi:10.1089/pho.2012.3398
Jenkins PA, Carroll JD (2011) How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg 29(12):785–787. doi:10.1089/pho.2011.9895
Montoya A (1997) Tratamiento de las fracturas de diáfisis tibial. Tesis de recepción de grado. México DF IMSS–UNAM, 1977: 28–30
Antezana LS, Aceves HLV, Maldonado RM, Carlos J, Minero V, Rodríguez J, Cadena JLR, González AB (2005) Manejo quirúrgico de rodilla flotante en un hospital de urgencias. Acta Ortop Mex 19(5):200–206
Pinheiro AL, Soares LG, Barbosa AF, Ramalho LM, dos Santos JN (2012) Does LED phototherapy influence the repair of bone defects grafted with MTA, bone morphogenetic proteins, and guided bone regeneration? A description of the repair process on rodents. Lasers Med Sci 27(5):1013–1024. doi:10.1007/s10103-011-1033-8
Chen CH, Hung HS, Hsu SH (2008) Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibly via PI3K signal pathway. Lasers Surg Med 40(1):46–54. doi:10.1002/lsm.20589
Acknowledgments
Authors would like to thank all people who have collaborated in the present investigation and the Hospital and University for its supports.
Ethical standards
The Bioterio Claude Bernard in Benemérita Autonomus University of Puebla approved the present investigation. All procedures performed in this study were in accordance with the ethical standards laid down in the Declaration of Helsinki. The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest and any funding organization to mention.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Briteño-Vázquez, M., Santillán-Díaz, G., González-Pérez, M. et al. Low power laser stimulation of the bone consolidation in tibial fractures of rats: a radiologic and histopathological analysis. Lasers Med Sci 30, 333–338 (2015). https://doi.org/10.1007/s10103-014-1673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1673-6