Abstract
The present study aimed to evaluate the effects of LLLT (660- and 808-nm wavelengths) on the process of repairing bone defects induced in the femurs of female rats submitted to ovariectomy. Bilateral ovariectomies were performed on 18 female Wistar rats, which were divided into control and irradiated groups after the digital analysis of bone density showed decreased bone mass and after standardized drilling of the femurs. The irradiated groups received 133 J/cm2 of AsGaAl (660-nm) and InGaAlP (880-nm) laser radiation. The animals were euthanized on days 14 and 21 after the bone defects were established. Detailed descriptive histological evaluations were performed, followed by semi-quantitative histomorphometry. The results from days 14 and 21 showed that the irradiated groups presented increased density of osteoblasts, fibroblasts, and immature osteocytes on the tissue surface compared with the control (non-irradiated) groups (p < 0.05). Additionally, inflammatory infiltrate evaluations showed that LLLT decreased the accumulation of leukocytes when compared to the control treatment (p < 0.05). We concluded that, in our experimental model, both wavelengths (660-nm and 880-nm) inhibited the inflammatory process and induced the proliferation of cells responsible for bone remodeling and repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bone response to injury consists of ordered and differentiated events, which normally result in scarring of the injured tissue that is similar to the structure of healthy bone [1]. The bone remodeling process involves the integrated activity of osteoblasts to synthesize new tissue and osteoclasts to degrade damaged tissue [2, 3]. Both the synthesis and degradation of bone matrix proteins play essential roles in bone development and recovery from injuries [2, 3]. Bone fracture can have many different causes, but osteoporosis in particular leads to millions of fractures every year, constituting a major public health problem [4, 5]. Osteoporosis occurs in response to an imbalance between the activity of osteoclasts and osteoblasts, which results in an imbalance between bone formation and bone resorption, mostly due to postmenopausal hormonal changes [2, 3]. The disease is characterized by decreased bone mineral density (BMD), bone micro-architecture disruptions, and a variety of non-collagenous proteins in altered bone tissue [6, 7].
Low-level laser therapy (LLLT) has been shown to have beneficial effects on bone repair and development, such as improved vascularization and cellular proliferation, increased mechanical resistance of new bone tissue, and increased calcification of bone matrix [8–10]. Considering these beneficial effects, we hypothesized that LLLT could improve osteogenesis in osteopenic female rats submitted to ovariectomy and concomitant bone injury induced by femoral drilling. Therefore, the aim of the present study was to evaluate the effects of LLLT at 660 nm and 808 nm on the healing of bone injuries in ovariectomized rats.
Materials and methods
Animals
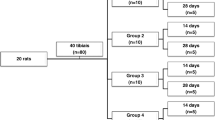
Eighteen adult female Wistar rats (Rattus norvegicus albinus) weighing 230–350 g were obtained from the biotery of the University for the Development of the State and Pantanal Region (UNIDERP), Campo Grande, MS. The animals were kept in 0.15-m2 cages with a controlled photoperiod of 12 h and controlled humidity and were given food and water ad libitum. The animals were rested under observation for 2 days prior to the experiment.
All experimental procedures were approved by the ethics committee for animal research of the Federal University of Mato Grosso do Sul (UFMS), Protocol 137/2007, and were performed in accordance with the Brazilian College for Animal Experimentation (COBEA).
Experimental groups
All animals were initially submitted to a surgical procedure (ovariectomy) and then randomized into the following groups: Group 1 (n = 6): ovariectomy + bone injury (OC); Group 2 (n = 6): ovariectomy + bone injury + 660-nm laser treatment (OR); or Group 3 (n = 6): ovariectomy + bone injury + 808-nm laser treatment (OI). These groups were further subdivided according to euthanasia date into the following sub-groups: Group 1: OC14 and OC28; Group 2: OR14 and OR28; and Group 3: OI14 and OI28 (Fig. 1).
Radiological examination
Radiology was performed using X-ray equipment from Dabi Atlante®, Model Spectro 70x, Class I, Type B. The equipment was programmed with 40 kVp with a diaphragm/chassis (focus/film) distance of 70 cm, and the exposition time was 0.25 mAs (milliampere-seconds). The animals were placed in the supine position, and radiographs were obtained with anteroposterior incidence. The radiological procedures were performed before the ovariectomy and repeated 90 days after ovariectomy, according to the parameters proposed by Lelovas et al. [11, 12].
Film processing (developing, fixation, washing, and storage) was performed in a dark chamber with the developer (Kodak Rp-X-Omat for 38 l) at ambient temperature. The film (Dental Film Speed E – 3.1 × 4.1 cm2) was developed as follows: 20-s immersion in developer, 30 s in running water, 10 min in fixing solution, 5 min in running water, and air-drying at room temperature. All radiographic procedures were performed in the X-ray room of the Federal University of Mato Grosso do Sul (UFMS). The X-rays were analyzed using the Digora digital system Version 1.51 for Windows (Orion Corporation SODEREX, Finland) by evaluating densities with the same sizes and localizations [11–13].
Surgical procedures
Ovariectomy
The bilateral ovariectomy was performed according to the procedure of Canettieri et al. [13]. After anesthesia with an intraperitoneal injection of ketamine 5% (Vetaset®, Fort Dodge, Campinas, SP, Brazil) plus xylazine 2%, (Kensol®, König–Avellaneda, Argentina) with a volume ratio of 1:2 at a dose of 0.1 ml/100 g of body weight, the animals were placed in the ventral decubitus position and prepared for abdominal trichotomy with iodide alcohol. A 6- to 8-cm incision was made, and the ovaries were exposed. Hemostasis was obtained by linking the upper part of the fallopian tube, and the ovaries were excised along with the adjacent fat tissue and a small portion of the uterus. The surgical planes were sutured with nylon polyamide thread number 4.
After the ovariectomy, all animals received analgesia with intramuscular buprenorphine 0.05 mg/kg (Temgesic®, Schering-Plough S/A, Cotia, SP, Brazil) and anti-inflammatory treatment with intramuscular sodium diclofenac 0.5 mg/kg (Medley Ltda, Campinas, SP, Brazil). This regimen was repeated every 12 h for 4 consecutive days.
Establishment of bone defects
After proof of osteoporosis was obtained via radiological examinations performed before and after the ovariectomy, the animals were anesthetized with an intraperitoneal injection of ketamine 5% plus xylazine 2% with a volume ratio of 1:2 at a dose of 0.1 ml/100 g of body weight. A trichotomy was then performed on the lateral face of each animal’s right thigh, sufficiently long to allow the region to be viewed. The animal was then positioned in the ventral decubitus position, and the front and hind paws were pinned in an abducted position. The incision location was then prepared with antiseptic (iodide alcohol), and a scalpel was used to incise the thigh to obtain direct access to the femur. Following incision of the fascia lata, the femoral diaphysis was located, and a bone defect was created on the craniolateral face approximately 50 mm from the proximal epiphysis. This procedure was performed using a 1016 spherical diamond-tipped drill bit (KG Sorensen, Cotia, SP, Brazil) coupled to a dental handpiece (Kawo, São Paulo, SP, Brazil) operated by an implant motor (Easy Implant, Easy Equipment) at 42,000 r.p.m. During the procedure, the site was constantly irrigated with physiological serum. The procedure consisted of drilling through the bone cortex until the medullary canal was reached [14].
Low-level laser therapy
Two days after the surgical procedure, the OR and OI groups underwent LLLT. An InGaAlP-type diode laser (continuous output power of 100 mW and a wavelength (λ) of 660 nm) was and a AsGaAl-type diode laser (output power of 100 mW and a wavelength (λ) of 808 nm), both from Photon Laser II DMC® (Sao Carlos, SP, Brazil) were used. The optical power was calibrated using a Newport multifunction optical meter, Model 1835 C. The spot size was 0.03 cm2, the power density was 3.3 W/cm2, and the energy density was 133.3 J/cm2 for 40 s. Irradiation (of Groups OR and OI) occurred 2 days after the surgical procedure that produced the bone defect. The animals were irradiated on four points of the femurs for 10 s at each point. Treatments were performed daily for either 14 (OR14 and OI14) or 28 (OR28 and OI28) days.
Histological procedures
The animals were identified, weighed, and euthanized using a lethal dose (200 mg/kg) of pentobarbital sodium. The femurs were then collected for histological analysis. The bone fragments were fixed in formalin 10% for 24 h, decalcified with ethylenediaminetetraacetic acid (EDTA), and then embedded in paraffin blocks, which were cut into 5-μm transverse sections and stained with hematoxylin & eosin (H&E) stain. All extensions of the bone defects were submitted to longitudinal sections to allow the evaluation of the surgical defect’s central region.
A descriptive histological analysis was performed using a semi-quantitative method based on knowledge of normal bone structure, according to the standards established by Leonel et al. [15] and Abo Elssad et al. [16]. To evaluate the osteogenic effects of LLLT on the bones of osteoporotic female rats, the following histological parameters were analyzed by light microscopy: the density of the inflammatory infiltrate, the density of osteoblasts, the density of immature osteocytes, the density of fibroblasts and the intensity of fibrosis. The histology results were used to conduct a semi-quantitative analysis in which the intensity was rated from zero to three plus signs: absence = (Ø); slight presence = (+); moderate presence = (++) and severe presence = (+++).
Statistical analysis
The Shapiro-Wilk test was used to evaluate the normal variance of the data. The level of statistical significance was set at p < 0.05. The Kruskal-Wallis test was used for comparisons, and Student’s t test was used as the post-test, also with the significance set at p < 0.05. BioEstat 5.0 software was used to perform the analysis.
Results
The data from days 14 and 21 for each group were compared, as were all parameters between the control and irradiated groups. The analysis of normal variance of the data revealed a predominantly non-normal variance distribution (p < 0.05).
Effects of LLLT on inflammatory parameters
The data presented in Table 1 show that the LLLT-treated groups exhibited reduced inflammatory processes at two different time points (14 and 21 days) when analyzed with the Kruskal-Wallis test. Inter-group comparisons revealed significant differences between the control group and both the 14-day group (p = 0.0017) and the 21-day group (p = 0.0018). However, Student’s t test showed no differences between the 14- and 21-day groups, demonstrating that both treatment periods were sufficient to decrease the inflammatory process.
LLLT effects on osteoblast numbers
The data presented in Table 2 demonstrate that at 14 days, both LLLT groups (660 nm and 808 nm) showed a proliferation of osteoblasts when compared with the controls group (p = 0.0026). The Kruskal-Wallis test was used for statistical analysis. After 21 days, no differences were found between the LLLT groups (660 nm and 808 nm) and controls.
LLLT effects on osteocyte numbers
The data presented in Table 3 demonstrate the average scores for the density of immature osteocytes on days 14 and 21, which were analyzed by the Kruskal-Wallis test. The results demonstrated that for both periods and both wavelengths (660 nm and 808 nm), LLLT induced a proliferation of immature osteocytes compared to controls (14 days: p = 0.0014, and 21 days: p = 0.0334). Student’s t test showed no differences between the 14- and 21-day LLLT groups.
LLLT effects on fibroblast numbers and fibrosis score
Table 4 compares the averages of scores for fibroblast density. The Kruskal-Wallis test did not show any difference between the treated groups (LLLT at 660 nm and 808 nm) and the control group (p = 0.053) at 14 days, although we observed a tendency toward the induction of fibroblast proliferation. At 21 days, the group treated with LLLT at 660 nm presented a modulated number of fibroblasts when compared to the control group (p = 0.0027). However, at 21 days, the group treated with LLLT at 808 nm was not different from the control group. Similarly, no differences were found when the treated groups were compared using Student’s t test.
Table 5 presents the scores obtained from the semi-quantitative analysis for fibrosis. No differences were found among the groups at 14 days (p = 0.5314) and 21 days (p = 0.304).
Specific LLLT effects on the trabecular bone
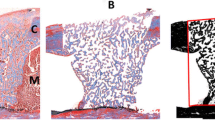
Hematoxylin and eosin-stained histological samples from the LLLT-treated groups (660 nm and 808 nm) showed that LLLT induced the filling of these bone defects by trabecular bone, with a substantial number of osteocytes and osteoblasts and the formation of the Haversian canal, which characterizes lamellar bone. We also observed a small inflammatory reaction and a substantial number of newly formed blood vessels. The control group also exhibited these changes but to a lesser degree (Fig. 2).
Representative photomicrographs of slides obtained from ovariectomized animals 14 days after bone defects were induced. a The group treated with a GaAlAs 808-nm infrared laser to observe the presence of Haversian canals (HC), the presence of primary bone (B1), and secondary bone (B2) and intense proliferation of osteocytes (arrow). b The group treated with an InGaAlP 660-nm visible red laser to observe the presence of Haversian canals (HC), proliferation of adipocytes, osteocytes (arrow), and the presence of secondary bone (B2). Hematoxylin and eosin
LLLT treatment also resulted in an increased number of osteocytes in the trabeculae lacunas of bone immersed in the bone matrix, suggesting the maturation of newly formed bone tissue. In addition, we detected secondary bone in the LLLT groups, along with a fine layer of conjunctive tissue surrounded by osteoprogenitor cells. In general, the treated groups exhibited more consistent bone formation and more compact bone with a larger number of the cells responsible for osteogenesis than in the untreated group (Fig. 3).
Representative photomicrographs of slides obtained from animals ovariectomized 21 days after bone defects were induced. a The group treated with a GaAlAs 808-nm infrared laser to observe the presence of newly formed bone (NB), primary bone (B1), blood vessels (Vb) and osteocytes (arrow). b The group treated with an InGaAlP 660-nm visible red laser to observe the presence of newly formed bone (NB), the proliferation of blood vessels (Vb), fibrous connective tissue (HR), osteocytes (arrow), and the presence of secondary bone (B2). Hematoxylin and eosin
Discussion
The osteogenesis process is a specialized response coordinated for different cell types, dependent on a physiological balance, which aims to form a new structure with the same constitution and function. Any imbalance in this phase will produce alterations in bone resistance, which can occur in pathological conditions such as osteoporosis, and has been the object of several investigations [1–3, 7, 17].
In general, the histological analysis showed that the animals treated with an InGaAlP-type diode laser (660 nm) and an AsGaAl-type diode laser (808 nm) for 14 or 21 days presented improvement of all aspects of bone repair when compared to the non-treated group. In the bone defect region, the irradiated groups presented increased numbers of osteoblasts, immature osteocytes, fibroblasts and new blood vessels, and decreased inflammatory infiltrate. Similar results for bone defects in rats were found by Abo Elssad et al. [16], who used an infrared laser (830 nm; 40 mV, density 16 J/cm2 and dose 4 J/cm2).
The histological results from the present study suggest that 14-day LLLT accelerated the repair of bone defects in osteoporotic female rats, specifically by increasing the proliferation of the cells involved in the osteogenesis process, such as fibroblasts, osteoblasts, and osteocytes. At 21 days, we observed a subtle decrease in the number of osteoblasts; however, the number of osteocytes remained elevated, which could indicate a continuing stimulatory effect on the mineralization of the osteoid matrix and an increased amount of newly formed bone. According to the literature, an increased number of osteocytes indicates the accumulation and integration of carbonates and calcium phosphate, which improves the synthesis of bone matrix and makes it more resistant [17, 18].
Lill et al. evaluated bone defects in ovariectomized animals and found that, in addition to decreasing bone density, osteoporosis negatively affected bone mineral mass gains post-fracture and impaired bone resistance at the end phase of remodeling [18]. Under these conditions, fractures still consolidate, but the process takes longer than in healthy bones. In addition to the final mineralization of the bone callus, osteoporosis also impairs the initial remodeling process of the bone [18].
Pinheiro et al. demonstrated that infrared laser treatment (830 nm) of bone injuries results in better repairing response than treatment with visible laser radiation (632.8 or 790 nm). According to the authors, infrared laser radiation has a photophysic effect, resulting in alterations in the membrane potential that produce an intracellular effect characterized by increased production of mitochondrial ATP and elevated cellular metabolism. Its photochemical biostimulatory effects stimulate the synthesis of enzymes, acting on lysosomes and mitochondria through a chemical reaction induced by the laser radiation [19].
In the present study, the chosen dose of 120 J/cm2 was efficient for both therapies. Other studies have found similar results, suggesting a probable dose-dependent effect in which higher doses could more efficiently stimulate bone repair [19–21]. Some studies have demonstrated that certain LLLT wavelengths induce cellular proliferation, releasing fibroblast growth factor and increasing fibroblast proliferation in vitro [21, 22]. Loevschall et al. evaluated the effects of different wavelengths on fibroblast proliferation, observing that LLLT significantly improved the mitosis rate and collagen production of these cells [22]. The histological data obtained in the present study demonstrated that the groups irradiated with LLLT (660 and 808 nm) exhibited increased fibroblast proliferation after 14 and 21 days. However, better results were achieved with 21 days of treatment at a 660-nm wavelength, demonstrating that irradiation with a red visible laser (660 nm) yielded better fibroblast proliferation, probably due to a proliferative effect or protein activation signaling.
The inflammatory reaction observed through the histological analysis showed that both therapies (660 and 808 nm at 14 and 21 days) effectively decreased the inflammatory process when compared with to the non-irradiated group. These results suggest that laser irradiation increased the resolution of inflammatory processes, most likely through lymphatic activation and neovascularization, which improved the influx of nutrients, such as calcium and phosphorus, to the bone tissue and thus increased bone resistance. Therefore, LLLT exerted anti-inflammatory effects on the proliferative process. Corroborating this idea, Coombe et al. [23] found an increased concentration of intracellular calcium in human osteoplastic cells treated with LLLT, indicating that these cells responded positively to LLLT.
In the present study, the histological findings after 14 days of LLLT revealed fibrosis only in the group treated with 808-nm laser radiation, demonstrating that LLLT at 660 nm was sufficient to reduce the fibrosis process. After 21 days with LLLT, all groups had fibrosis. However, it is important to highlight that the dense conjunctive tissue found in the irradiated groups was composed of collagen fibers and contained a large cell population that may have differentiated from osteoprogenitor cells.
In the present study, the increased numbers of osteoblasts in the LLLT-irradiated groups can be explained by the fact that the LLLT induces cellular proliferation, presenting biostimulatory effects on multipotent cells and leading them to differentiate into osteoblasts, which are the classic producers of bone matrix. These results are in agreement with the results of other studies [24–26].
Nicolau et al. [25] used LLLT (660 nm and 10 J/cm2) to repair bone defects in rat femurs irradiated for 2, 4, 6, or 8 days and killed at 5, 15, or 25 days. After the histomorphometric analysis, the authors concluded that LLLT stimulated the proliferation of all cell types, including osteoblasts and osteoclasts, in all periods but without changing bone microarchitecture.
Ozawa et al. [24] demonstrated LLLT’s positive effect on cellular proliferation in cell cultures of osteoblasts using an AsGaAl laser (830 nm) with an energy density of 3.82 J/cm2 and a potency of 500 mW. According to these authors, the therapy stimulated osteoblastic proliferation and differentiation as well as initiated bone formation. In osteoblast cell cultures originating from mesenchymal cells, Dortbudak et al. [26] applied GaAlAs laser irradiation (690 nm for 60 s) for 3, 5, or 7 days and observed that the irradiation produced significant biostimulatory effects.
Studies of strategies that improve bone deposition while minimizing bone loss are important, particularly within the context of osteoporosis. Osteoporotic fractures have high social and medical costs for city administrations around the world; therefore, solutions that could reduce these costs are extremely desirable. LLLT has been presented as an affordable and highly effective solution.
An absence of data in the literature prevents us from comparing the effects of different LLLT regimes on cellular proliferation, bone production, and the mechanical force of the bones in osteoporotic animals. Future research should address new techniques for LLLT standardization in scientific research.
Osteoporosis is known to be responsible for different negative alterations to bone structure and remodeling. The results of the present study suggest that LLLT had important beneficial effects on the early phase of bone repair, reducing the inflammatory process and stimulating the proliferation of cells responsible for synthesizing bone matrix. These effects resulted in a more resistant bone callus, demonstrating LLLT’s biomodulatory effect on the cellular metabolism.
We conclude that, in the our experimental model of bone defects in osteoporotic female Wistar rats, 660- and 808-nm LLLT induced osteogenesis and improved the repair of bone defects.
References
Dobbs MB, Buckwalter J, Saltzman C (1999) Osteoporosis: the increasing role of the orthopaedist. Iowa Orthop J 19:43–52
Kalfas IH (2001) Principles of bone healing. Neurosurg Focus 10:E1
Sha M, Guo Z, Fu J, Li J, Yuan CF, Shi L, Li SJ (2009) The effects of nail rigidity on fracture healing in rats with osteoporosis. Acta Orthop 80:135–138
Siris ES, Selby PL, Saag KG, Borgström F, Herings RM, Silverman SL (2009) Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med 122(2 Suppl):S3–13
Lorrain J, Paiement G, Chevrier N, Lalumière G, Laflamme GH, Caron P, Fillion A (2003) Population demographics and socioeconomic impact of osteoporotic fractures in Canada. Menopause 10:228–234
Kolios L, Sehmisch S, Daub F, Rack T, Tezval M, Stuermer KM, Stuermer EK (2009) Equol but not genistein improves early metaphyseal fracture healing in osteoporotic rats. Planta Med 76:459–465
Cooper C, Westlake S, Harvey N, Dennison E (2009) Developmental origins of osteoporotic fracture. Adv Exp Med Biol 639:217–236
Torres CS, dos Santos JN, Monteiro JS, Amorim PG, Pinheiro AL (2008) Does the use of laser photobiomodulation, bone morphogenetic proteins, and guided bone regeneration improve the outcome of autologous bone grafts? An in vivo study in a rodent model. Photomed Laser Surg 26:371–377
Pretel H, Lizarelli RF, Ramalho LT (2007) Effect of low-level laser therapy on bone repair: histological study in rats. Lasers Surg Med 39:788–796
Márquez Martínez ME, Pinheiro AL, Ramalho LM (2008) Effect of IR laser photobiomodulation on the repair of bone defects grafted with organic bovine bone. Lasers Med Sci 23:313–317
Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA (2008) The laboratory rat as an animal model for osteoporosis research. Comp Med 58:424–430
Sakakura CE, Giro G, Gonçalves D, Pereira RM, Orrico SR, Marcantonio E Jr (2006) Radiographic assessment of bone density around integrated titanium implants after ovariectomy in rats. Clin Oral Implants Res 17:134–138
Canettieri AC, Colombo CE, Chin CM, Faig-Leite H (2009) Femur bone repair in ovariectomized rats under the local action of alendronate, hydroxyapatite and the association of alendronate and hydroxyapatite. Int J Exp Pathol 90:520–526
Denadai AS, de Carvalho PT, dos Reis FA, Belchior AC, Pereira DM, Dourado DM, Silva IS, de Oliveira LV (2009) Morphometric and histological analysis of low-power laser influence on bone morphogenetic protein in bone defects repair. Lasers Med Sci 24:689–695
Leonel ECF, Porciúncula HF, Sobrinho JA, Ramalho LTO, Mangilli PD, Rapoport A (2004) The action of the castor bean polymer during the bone neoformation. Acta Cir Bras 19:342–350
AboElsaad NS, Soory M, Gadalla LM, Ragab LI, Dunne S, Zalata KR, Louca C (2009) Effect of soft laser and bioactive glass on bone regeneration in the treatment of bone defects (an experimental study). Lasers Med Sci 24:527–533
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephol 3:S131–S139
Lill CA, Hesseln J, Schlegel U, Eckhardt C, Goldhahn J, Schneider E (2003) Biomechanical evaluation of healing in a non-critical defect in a large animal model of osteoporosis. J Orthopaedic Res 21:836–842
Pinheiro ALB, Oliveira MG, Martins PPM, Ramalho LMP, Oliveira MAM, Junior AN, Nicolau RA (2001) Biomodulatory effects of LLLT on bone regeneration. Laser Therapy 13:73–79
Pinheiro ALB, Limeira Júnior FDA, Gerbi MLM, Ramalho LMP, Marzola C, Ponzi EAC (2003) Effect of low level laser therapy on the repair of bone defects grafted with inorganic bovine bone. Braz Dent J 14:177–181
Diniz JS, Nicolau RA, de Melo ON, Magalhães FC, Oliveira Pereira RD, Serakides R (2009) Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci 24:347–352
Loevschall H, Arenholt-Bindslev D (1994) Effect of low level diode laser irradiation of human oral mucosa fibroblasts in vitro. Lasers Surg Med 14:347–354
Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW (2001) The effects of low level laser irradiation on osteoblastic cells. Clin Orthod 4:3–14
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–54
Nicolau RA, Jorgetti V, Rigau J, Pacheco MT, dos Reis LM, Zangaro RA (2003) Effect of low-power GaAlAs laser (660 nm) on bone structure and cell activity an experimental animal study. Lasers Med Sci 18:89–94
Dörtbudak O, Hass R, Mailath-Pokorny G (2000) Biostimulation of bone marrow cells with a diode soft laser. Clin Oral Implants Res 11:540–545
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ré Poppi, R., Da Silva, A.L., Nacer, R.S. et al. Evaluation of the osteogenic effect of low-level laser therapy (808 nm and 660 nm) on bone defects induced in the femurs of female rats submitted to ovariectomy. Lasers Med Sci 26, 515–522 (2011). https://doi.org/10.1007/s10103-010-0867-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0867-9