Abstract
Banana peduncle waste (BPW) is a major fibrous waste generated from the wholesale vegetable markets in urban areas that is less degradable due to the presence of cellulose, hemicellulose and lignin contents. In order to recover energy and produce biochar as a by-product, pyrolysis is a promising alternate method for the treatment and disposal of BPW. The novel aspect of the study is to use response surface methodology (RSM) with central composite design by Minitab Software to identify the optimum process parameters, such as pyrolysis temperature (PT), holding time (HT), and heating rate (HR) for the responses biochar yield (%), carbon content (g), and pH of the biochar. For RSM the minimum and maximum values are considered in the range of 400–600 °C, 80–240 min and 10–20 °C min−1 for PT, HT and HR, respectively. Experiments of 20 sets at different parameters as per RSM were carried out in lab scale pyrolysis unit and results showed that PT and HT have a significant effect on biochar yield, carbon content and pH, while HR did not show a considerable impact. The proximate and ultimate analyses of BPW and biochar obtained (BPW–BC) were also investigated including SEM and FTIR. From the results, biochar yield of 50%, carbon content of 3.18 g and pH of 11 were obtained at optimum conditions of PT: 462 °C, HT: 80 min and HR-10 °C min−1 and found that the experimental responses are very close to the predicted values. Further, the characteristics of BPW–BC obtained vary with process conditions and could be potentially used for various applications such as soil conditioners, adsorbent pyrogenic carbon capture and storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Banana is one of the main fruits ranked second next to citrus fruits and has international trade market potential. Banana fruit is cultivated in tropical and subtropical regions and consumed globally (Workneh and Belew 2013). The banana peduncle contributes to about 12% of the weight of the fruit bunch and is conveyed along with the fruits to the cities for local distribution and consumption. This part of the peduncle is discarded as waste (Pazmiño-Hernandez et al. 2019). The discarded banana peduncle is fibrous in nature due to the presence of lignocellulose and hemicellulose content. The lignin content forms the structure that acts as a shield for both cellulose and hemicellulose content, which inhibits bacterial activity, and hence conventional biological treatment methods like anaerobic digestion and composting are not suitable for BPW (Beis et al. 2002).
There are few studies reported for anaerobic digestion of BPW with pretreatment for biogas production (Benish et al. 2022). But, BPW can be easily treated and disposed using thermochemical conversion method which is a novel approach in this study. The thermochemical conversions of these waste biomasses are carried out either by incineration, gasification, liquefaction or pyrolysis processes (Dhyani and Bhaskar 2018). Among thermochemical biomass conversion technologies, pyrolysis is one of the simple methods by which biomass is burnt in the absence of oxygen resulting in carbon capture and storage. During pyrolysis, the naturally present lignin, cellulose and hemicellulose contents are converted into biochar, bio-oil and synthetic gas (Mohan et al. 2006). The different types of pyrolysis adopted for biomass conversion are fast pyrolysis, flash pyrolysis, slow pyrolysis, catalytic pyrolysis and plasma pyrolysis (Uddin et al. 2018). During fast pyrolysis, the biomass is burnt to high temperatures in a short time. During fast pyrolysis, 60–70% of bio-oil, 12–15% biochar and 15–28% of gas were obtained (Isahak et al. 2012). Similarly, during flash pyrolysis, bio-oil generation is predominant in comparison with biochar and syngas. During slow pyrolysis, the major products are biochar and non-condensable gases. The quality of biochar such as porosity, surface area and H/Corg molar ratio vary depending on the substrate, their organic and inorganic composition, genotype and other parameters during the pyrolysis process, i.e., particle size, pyrolytic temperature, heating rate and holding time (Angın 2013; Wati 2019; Zhao et al. 2021). Higher temperatures affect biochar characteristics and decrease biochar yield due to the escape of organic components (Zhao et al. 2018). The pyrolytic temperature is a significant factor that determines the composition and stability of the biochar. On the other hand, higher holding time results in carbonization of biochar with less organic content (Zornoza et al. 2016).
Most of the researchers focused their research on treatment of banana pseudo-stems only, but it is pertinent to mention that the banana peduncle transported along with banana fruit to the urban areas needs to be studied (Manimaran et al. 2020) for treatment and safe disposal as currently most of BPW is disposed in open dumping or landfill. In addition, the greenhouse gases emission from fossil fuels and recent increase in the cost of energy have encouraged researchers to find new treatment technology to obtain energy and value-added products, especially bioenergy from waste biomass, and potential options for carbon sequestration and storage to mitigate climate change including thermochemical conversion processes (Ronsse et al. 2013).
Further on the other hand, the agricultural soil in many regions is under serious threat due to changes in farming methods, the use of artificial fertilizers and changes in climatic conditions. Under these circumstances, there is an urgent requirement to improve the agricultural land by remediating the soil and regaining its fertility by natural methods (Johns 2017). Potassium (K) is a macronutrient for plant growth and it is deficient in the soil due its availability in insoluble forms and sometimes the available potassium is leached out during irrigation runoff. Hence, in the agriculture field, the potash fertilizer is used to increase potassium content in the soil for plant growth (Römheld and Kirkby 2010). In addition to K content, the organic carbon (C) and water retention in soil is also important factors, as tropical countries experience only periodical rainfall. Hence, the addition of carbon (C) in the form of biochar can increase water retention due to its porosity and structure and also meet the soil’s carbon requirement (Suravi et al. 2021). The BPW is rich in the minerals such as potassium (K) and phosphorus (P) which can be retained during pyrolysis after the removal of the volatile substances and could use to improve the K content in soil. The elements present in the biomass such as sodium, magnesium, calcium, potassium and phosphorus are retained in biochar, which increases the soil nutrient value when used as a soil conditioner. The biochar addition also varies the soil density and thus has a strong effect on the growth and morphology of the root (Suravi et al. 2021). The porosity in biochar also increases the microbial activity in the soil. The microbial count in the soil would increase considerably and the soil turning would be higher. Hence, the root and formation of root hairs become easier (Jacoby et al. 2017). In addition, the availability of minerals in biomass is also concentrated in the biochar (Karim et al. 2017).
The agriculture land is falling short of macro- and micro-nutrients while on the other hand BPW has macro- and micro-nutrients that is disposed as waste in open dumps or landfills. The novelty of this study is to bridge the nutrient demand by using BPW as substrate to recover nutrients and carbon-rich biochar using pyrolysis process and to identify the optimized process parameter. This study can give a complete treatment method for disposal of BPW in urban cities and reduce the emission of greenhouse gases due to disposal of BPW in large quantities in landfill or open dumpsites in addition to carbon capture by biochar.
The current objective of the study is to use an effective response surface methodology using central composite design (CCD) to determine the impact of pyrolytic process parameters (PT, HT and HR) on responses such as biochar yield, carbon content and pH of biochar. RSM enables statistical analysis of these responses resulting from multiple process parameters and their main and interaction effects (Brown and Brown 2012). Second-order quadratic regression model equations for responses is also obtained from central composite design (CCD) (Izadiyan and Hemmateenejad 2016). The detailed characterization of biochar obtained from the study was investigated for various potential applications.
Materials and methods
BPW (raw biomass) was collected from the wholesale vegetable market in Koyambedu, Chennai, and was then reduced in size to less than 20 mm using a shredder (Netzsch N-Mac). The shredded BPW is oven-dried and stored in airtight closed container at room temperature. Proximate analysis, fiber content and ultimate analysis were carried out to characterize the BPW sample.
Pyrolysis of BPW is carried out using a custom-made lab-scale pyrolysis unit to get biochar. The flue gas from the pyrolysis unit is passed through a chiller line, and condensable gases are collected as bio-oil. The schematic drawing of the lab-scale pyrolysis unit is shown in Fig. 1.
Pyrolysis of BPW at different process conditions using RSM
The response surface methodology using CCD has been carried out using Minitab Software (version 14). Biochar samples were produced as a result of experiments carried out in a lab-scale pyrolysis unit at various pyrolysis temperatures (PT), holding times (HT) and heating rates (HR) conditions according to CCD. Biochar yield (%), carbon content (g) and pH of the biochar are the responses taken into account. Table 1 displays the experimental ranges of the independent process parameters (PT, HT and HR) investigated in accordance with the design.
The biochar yield (%) is calculated by Eq. 1.
The carbon content (g) of the biochar samples was calculated by multiplying the % of C from the CHNS analyzer and amount of biochar during the pyrolysis. The pH of the biochar samples was measured as per the ASTM (D4972-19) standards using pH meter (Eutech).
Based on the experimental investigations, the responses obtained at various process parameters were statistically analyzed in Minitab Software to obtain a regression equation linking the responses to process parameters.
The following polynomial regression expression relates the responses and the process conditions to estimate the predicted values. The typical regression equation for responses analysis is given in Eq. 2.
where Y s the response, β0 is the intercept, β1 to β9 is the coefficient of the factors, PT is the pyrolysis temperature, HT is the holding time and HR is the heating rate.
Two-dimensional graphs displaying the reactions at various levels of independent process parameters for the responses were created. Furthermore, analysis of variance (ANOVA) was performed to compare the experimental and predicted responses (Behera et al. 2018) to validate the regression equation prediction.
The actual experimental results of biochar yield, carbon content and pH were obtained by carrying out the pyrolysis experiments with BPW at the optimum condition obtained from CCD, and the results are compared.
Characterization of BPW and biochar obtained from BPW (BPW–BC)
The characteristics of BPW and BPW–BC were analyzed by proximate analysis (moisture, dry matter and ash content, volatile matter, fixed carbon), ultimate analysis (CHNS) and fiber analysis (hemi-cellulose, cellulose, lignin). In addition, the BPW–BC obtained from BPW was analyzed for specific parameters such as pH, electrical conductivity, cation exchange capacity, determination of surface functional groups using FTIR and scanning electron microscopy (SEM).
Proximate and ultimate analyses
The moisture and dry matter content were determined in accordance with the ASTM standard (D 3173). Volatile matter (VM) was determined by measuring the weight loss after combustion of the samples in a furnace at 550 °C for 2 h as per APHA standards (Shafiq 2017). CHNS analysis is performed using a CHNS analyzer (Eurovector-EA3000 model).
Fiber analysis
The Van-Soest fiber analysis method (Wolfrum et al. 2009) was used to determine the neutral detergent fiber (NDF) and acid detergent fiber (ADF). The following formula was used to compute the hemicellulose, lignin and cellulose contents.
Determination of functional groups and surface morphology
Functional groups in the raw BPW samples and BPW–BC were analyzed using JASCO FTIR-4700 equipment. Fourier transform infrared was carried out with wavelength range from 400 to 4000 cm−1 with a resolution of 4 cm−1. Similarly, scanning electron microscopy (SEM) was used to determine the surface morphology of raw BPW and BPW–BC (TESCAN-CLARA model).
Determination of pH, EC, CEC and organic carbon
The pH, electrical conductivity (EC), organic carbon and cation exchange capacity (CEC) were also analyzed. The pH of the BPW–BC was measured as per the ASTM standard (D4972-19) using pH meter (Eutech), and EC of biochar was analyzed as per ASTM standard (E1004-17) using conductivity meter (Hach) (Han et al. 2022). The organic carbon was analyzed using the Walkley and Black technique (Enang et al. 2018). The barium acetate method was used to determine the cation exchange capacity (CEC) (Batista et al. 2018).
Results and discussion
Characterization of banana peduncle waste (BPW)
The results of proximate, ultimate and fiber content analysis of banana peduncle waste (BPW) are given in Table 2. It is also observed that the VS content is about 6.1% of wet weight (approx. 75% of the total solids). It was found that lignocellulosic substances (cellulose, hemicellulose and lignin contents) in BPW contribute to about 70% of total solids. It is observed that the presence of higher lignocellulosic content in the BPW requires pretreatment methods either mechanically or enzymatically for biological treatment (Kumar and Sharma 2017). Higher lignocellulosic content indicates that BPW is better suited for thermal treatment methods compared to conventional biological process (Zhang et al. 2018). The analysis results have also confirmed that the biochar obtained from the BPW is rich in potash and phosphates than other biomasses which acts as macronutrients to the plants when used as fertilizer in the soil.
It is observed that there were wide variations in the cellulose, hemicellulose and lignin contents of triplicate samples indicated by higher standard deviation and it is mainly due to the presence of different varieties of BPW samples available and collected from market.
Optimization study of pyrolysis process parameters
Based on lab scale pyrolysis experiments with BPW, the responses obtained in terms of biochar yield (%), carbon content (g) and pH of the biochar from 20 experimental conditions. RSM was used to develop second-order quadratic regression models that relate responses (biochar yield, carbon content and pH) to experimental conditions (PT, HT and HR). The experimental and the predicted values of the responses-based models equations are given in Table 3.
The different process parameters for 20 combinations of experimental runs were obtained from the CCD and the pyrolysis of BPW was carried out at the experimental conditions. The impact of pyrolysis process parameters on responses such biochar yield, carbon content and pH of the collected biochar is clearly confirmed by the main effect plot in Figs. 2, 3 and 4.
The highest biochar yield of 54.6% was obtained at pyrolysis condition PT of 500 °C, HT of 25.45 min and HR of 15 °C min−1 and the lowest biochar yield of 32.3% was obtained with pyrolysis condition PT of 600 °C, HT of 240 min and HR of 20 °C min−1. Similarly, the highest carbon content of 3.6 g was obtained with pyrolysis conditions PT of 500 °C, HT of 25.45 min and HR of 15 °C min−1 and the lowest carbon content of 2.2 g was obtained with pyrolysis conditions PT of 600 °C, HT of 240 min and HR of 10 °C min−1.
From the results, it is observed that there will be a decrease in the biochar yield and carbon content with increase in PT from 331 to 668 °C. This is due to volatilization of organic matters and free carbon molecules present in substrates and hence the reduction in the biochar yield and carbon content is observed. On the other hand, the highest pH of the biochar is observed with PT of 668 °C, HT of 160 min and HR of 20 °C min−1 and the results are quite evident that the pH of biochar increases with an increase in the PT and HT. Higher pH is observed due to increase in ash content with increase in PT and HT (Mohamed Noor et al. 2019; Zheng et al. 2013).
Main effect plot
The main effect plots for the effect of three process parameters (PT, HT & HR) on the responses biochar yield, carbon content and pH are shown in Figs. 2, 3 and 4, respectively. According to Fig. 2, the drop in biochar yield from 47.7 to 34.6% was obtained when the PT is raised from 331 to 668 °C which could be due to the greater volatility and breakdown of the cellulose and hemicellulose components present in the BPW (Uzun et al. 2010). Further, the biochar yield is also reduced from 54.6 to 33.5% as the HT increased from 25 to 294 min and similar trends were reported by other researchers also (Sevgi 2015; Siddiqui 2017). It was hypothesized that as the pyrolysis temperature is raised, the amount of volatile matter drops and the amount of fixed carbon rises and causes a drop in the O/C ratio of the biochar generated (Te et al. 2021). It can be seen that the HR in the pyrolysis does not show any significant effect on biochar yield as shown in Fig. 2.
From Fig. 3, it is observed that the carbon content of biochar also got reduced with an increase in PT and HT while the HR did not show much impact similar to biochar yield response. The main effect plot showed that there is a considerable drop in carbon content till 500 °C and thereafter showed that there is less reduction (Söderqvist 2019; Weber and Quicker 2018). At higher PT and HT, the free carbon present in the BPW gets volatized which was reflected in decrease in carbon content as observed in Fig. 3 (Cao and Harris 2010; Safdari et al. 2019). However, O/C ratio will be decreased due to increase in carbon content from the biochar which interprets the carbonization and aromaticity reveals how to interact with the soil. Interaction of biochar with the soil helps the microorganisms present in the soil to break down organic matter and indirectly influences nitrogen mineralization (Haryati et al. 2018).
From Fig. 4, it is observed that an increase in PT also increases the pH of the biochar due to a decrease in acidic functional groups. The biochar obtained at higher pyrolysis temperatures becomes more alkaline due to an increase in ash content (Rajkovich et al. 2012; Zea et al. 2016). Similar to PT, HT also showed a similar trend, i.e., an increase in HT increases pH of the biochar. The biochar is more alkaline at a higher holding time which could be due to the separation of minerals present in the biochar from the organic content (Wang et al. 2018). The main effect plot of the pH of biochar showed slight reduction in pH from 11.6 to 10.75 with an increase in HR.
The central composite design also gives the individual and interaction effects of the process conditions and their impact on responses analyzed by t test and the results are given in Table 4.
Based on the results of the student’s t test, the significance of process parameters on the responses is determined. From the results, it is evident that the process parameters PT and HT showed significant effects on biochar yield and carbon content (p < 0.05), whereas the factor HR did not show any significant effect on these responses (p > 0.05). In respect of pH response, PT, HT and HR showed significant effects (p < 0.05). The negative t-values indicate their negative effect of PT and HT on the responses biochar yield and carbon content. The positive t-value indicates the positive effect of HR on the pH of the biochar response. It could be observed that the interaction effects of PT and HT also showed a significant impact on the response biochar yield and pH (p < 0.05). It is also observed that the other interaction effects of process parameters are not significant for any of the responses with higher p values (p > 0.05).
The regression model equations for biochar yield, carbon content and pH of the biochar with three independent process parameters are given in Eqs. 3, 4 and 5, respectively.
The predicted values of the responses were determined using regression analysis derived equations. R2 and adjusted R2 values (also known as the coefficient of determination) are used to assess the effectiveness of regression models.
The R2 values of the polynomial regression equations are 0.99 for biochar yield, 0.95 for carbon content and 0.96 for pH of the biochar implying that only 1%, 5% and 3% of variations in the responses are not explained by the regression analysis model. In a similar manner, the difference between the adjusted R2 and projected R2 is determined to be 0.0117 for biochar yield, 0.1908 for carbon content and 0.135 for pH all of which are less than 0.2, showing that the evaluated model fit the experimental data according to the output of the Minitab Software.
Analysis of variance analysis of biochar yield, carbon content and pH
The predicted values of the responses based on quadratic regression models and experimental values were analyzed using analysis of variance (ANOVA) and statistical models were tested with F-values and lack of fit. The results obtained from the analysis of variance for three responses are given in Table 5.
F-values calculated on the regression models are 416.12, 19.32 and 31.30 for biochar yield, carbon content and pH of biochar, respectively, as shown in Table 5, which is found to be higher than the F tabular value of 2.82. The p values were also less than 0.05, confirming that the confidence levels are more than 99%, indicating that the analyzed regression models are exceptionally well fitted to the experimental data. The models’ lower p values and higher F-values also imply that the independent factors and responses are in a good relationship. In this regression models, p values of the lack of fit are greater than 0.05 indicating that the experimental data obtained fit in the regression model and that there is significantly less chance that the F-values could be more significant due to noise (Wong et al. 2015).
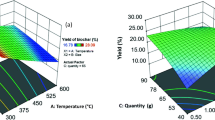
The typical two-dimensional plots for biochar yield, carbon content and pH for significant process conditions are shown in Fig. 5. From Fig. 5a, it was observed that the biochar yield reduces with an increase in PT and HT confirming the results obtained from the main effect plot and student t tests. Similarly, the carbon content of biochar also showed a similar trend with PT and HT shown in Fig. 5b. In case of pH response, a slight increase in pH is observed in Fig. 5c.
Optimal process parameters prediction and experimentation
Based on the biochar yield, carbon content and pH of the biochar responses, it was observed that higher PT and HT reduced the biochar yield and carbon content. In respect of pH, higher PT and HT resulted in alkaline biochar, whereas HR did not show any significant effect on all three responses. Further, the optimum conditions obtained from output of central composite design (CCD) using Minitab Software are 462 °C, 82 min and 10 °C min−1 for the production of biochar. The theoretical responses for the optimum conditions for biochar production result in 50% of biochar yield, 3.198 g of carbon content and pH of 11 from CCD. Under these conditions, high biochar yield and carbon content can be maintained without damaging the structure of biochar due to PT and HT (Yu et al. 2022). Biochar production values of 49.9%, carbon content of 3.014 g and pH values of 10.78 were observed from the laboratory experimental, which are extremely close to the results for the optimum conditions from CCD. This confirms that the generated regression model was acceptable and significant (Fig. 6).
Characterization of BPW and BPW–BC
FTIR analysis of BPW and BPW–BC
The existing functional groups of the BPW and BPW–BC under optimum conditions were analyzed using the FTIR spectrum with wave numbers ranging from 4000 to 400 cm−1. A broad peak at wave number 3297 cm−1 in BPW is caused by O–H stretching in intermolecular bonding water molecules or indicates the presence of carboxylic, phenolic or alcoholic groups (Qian et al. 2013). The peak at wave number 2922 cm−1 was due to C–H stretching bands along with aliphatic functional groups or denotes the presence of elements with the series CHn (Siddiqui 2017). The peaks at wave numbers 1637 and 1387 cm−1 represent the aromatic ring and carboxylate groups. These peaks are recorded due to stretching of C=C, aromatic ring components, and stretching of C=O, conjugated ketones, chinones, quinones, amide and amine (Soja and Bielsk 2021; Street 2010). The extensive peak at 1022 cm−1 confirms the cellulose, hemicellulose and lignin content present in BPW (Pang et al. 2014). However, in BPW–BC, only two considerable peaks were observed. The spectrum peak at wave number 3437 cm−1 of less intensive biochar confirms the presence of dehydrated water molecules (Siddiqui 2017), which could be due to the absorption property of BPW–BC toward water molecules. The peaks at wave number 1608 cm−1 are due to the presence of aromatic compounds associated with ketones and quinones. The aromatic compounds are retained in the biochar during cellulosic compounds degradation (Barrera-zapata 2017).
SEM analysis of BPW and BPW–BC
The SEM images of BPW and BPW–BC obtained to understand the visual and surface morphology are shown in Fig. 7. From the SEM image of the BPW Fig. 7a, the continuous structure morphology was observed, while many pores were observed in the BPW–BC Fig. 7b, because of thermal degradation of the lignocellulosic content and repeated to increase the surface area (Azmier et al. 2014). Many researchers have reported similar observations that thermal degradation occurs in two stages, i.e., degradation of hydrocarbons and increase in pores sizes due to the removal of organic contents (Ma et al. 2016).
pH, electrical conductivity, CEC and organic carbon BPW–BC and its potential application
The pH of BPW–BC obtained from laboratory experiments with optimum conditions is 10.78 which is alkaline in nature. This is due to the disappearance of acidic functional groups and alkalic groups from carbonates and inorganic salts (Tomczyk et al. 2020). The presence of the inorganic salts is also confirmed by higher electrical conductivity (EC) of 5.040 ± 0.15 mS/cm. The BPW–BC has high amount of cation exchange capacity with CEC value of 82 ± 0.24 cmolc/kg. The organic carbon of the BPW–BC is found to be 6.78 ± 0.54% of the dry weight. These characteristics of biochar obtained from the study are useful for soil reclamation applications. The soil found in the earth are mostly acidic in nature which is due to anthropogenic activities. Hence, the addition of this alkaline BPW–BC can be used to reduce the acidity of soil (Geng et al. 2022). In addition, BPW–BC can also increase the water holding capacity of the soil in the tropical regions (El-Naggar et al. 2019). The use of biochar in the soil can also act as carbon sink which reduces the greenhouse gases emission which is found to be sustainable method for disposing of BPW. The inorganic salts present in the BPW–BC act as macro- and micronutrient to the crops and the BPW–BC also reduces the leaching of nutrient in water during surface runoff (Allohverdi et al. 2021).
Conclusion
From the present investigation on the pyrolysis of BPW at various pyrolysis parameters, it can be concluded that PT and HT have a significant effect on biochar yield, carbon content and pH of the biochar, but the HR does not have any significant effect on all three responses. Higher PT and HT have decreased the biochar yield and improved the presence of carbon content in the biochar. All these process parameters (PT, HT and HR) have a significant effect on the biochar and alkaline biochar was obtained. The alkaline biochar produced from BPW from this study has potential application for reclamation of acidic soil, water retention capacity in soil reclamation and in agriculture as fertilizer.
From the results of lab scale batch reactor studies, it is concluded that the biochar could be produced at optimum condition for using it as valuable product. Further continuous studies in pilot scale need to be carried out for scale up and large-scale production of biochar from BPW. Currently, pilot scale implementation is under progress as conversion of BPW to valuable biochar could efficiently decrease greenhouse gas emissions and store the carbon in biochar meeting the objectives of sustainable development goals—climate action (SDG13).
Data availability
Data sharing is not applicable to this article.
References
Allohverdi T, Mohanty AK, Roy P, Misra M (2021) A review on current status of biochar uses in agriculture. Molecules. https://doi.org/10.3390/molecules26185584
Angın D (2013) Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour Technol 128:593–597. https://doi.org/10.1016/j.biortech.2012.10.150
Azmier M, Azreen N, Puad A, Solomon O (2014) Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour Ind. https://doi.org/10.1016/j.wri.2014.06.002
Barrera-zapata R (2017) Morphological and physicochemical characterization of biochar produced by gasification of selected forestry species. Caracterización Morfológica y Fisico-Química de Biocarbones Producidos 26(46):123–130
Beis SH, Onay Ö, Koçkar ÖM (2002) Fixed-bed pyrolysis of safflower seed: Influence of pyrolysis parameters on product yields and compositions. Renew Energy 26(1):21–32. https://doi.org/10.1016/S0960-1481(01)00109-4
Benish PMR, Mozhiarasi V, Nagabalaji V, Weichgrebe D, Srinivasan SV (2022) Optimization of process parameters for enhanced methane production from banana peduncle by thermal pretreatment. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02917-z
Brown JN, Brown RC (2012) Process optimization of an auger pyrolyzer with heat carrier using response surface methodology. Bioresour Technol 103(1):405–414. https://doi.org/10.1016/j.biortech.2011.09.117
Cao X, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101(14):5222–5228. https://doi.org/10.1016/j.biortech.2010.02.052
Dhyani V, Bhaskar T (2018) A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy 129:695–716. https://doi.org/10.1016/j.renene.2017.04.035
El-Naggar A, Lee SS, Rinklebe J, Farooq M, Song H, Sarmah AK, Zimmerman AR, Ahmad M, Shaheen SM, Ok YS (2019) Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma 337(September 2018):536–554. https://doi.org/10.1016/j.geoderma.2018.09.034
Geng N, Kang X, Yan X, Yin N, Wang H, Pan H, Yang Q, Lou Y, Zhuge Y (2022) Biochar mitigation of soil acidification and carbon sequestration is influenced by materials and temperature. Ecotoxicol Environ Saf 232:113241. https://doi.org/10.1016/j.ecoenv.2022.113241
Han X, Jiang N, Jin F, Reddy KR, Wang Y, Liu K, Du Y (2022) Effects of biochar-amended alkali-activated slag on the stabilization of coral sand in coastal areas. J Rock Mech Geotech Eng. https://doi.org/10.1016/j.jrmge.2022.04.010
Haryati Z, Loh SK, Kong SH, Bachmann RT (2018) Pilot scale biochar production from palm kernel shell (PKS) in a fixed bed allothermal reactor. J Oil Palm Res 30(3):485–494. https://doi.org/10.21894/jopr.2018.0043
Isahak WNRW, Hisham MWM, Yarmo MA, Yun Hin TY (2012) A review on bio-oil production from biomass by using pyrolysis method. Renew Sustain Energy Rev 16(8):5910–5923. https://doi.org/10.1016/j.rser.2012.05.039
Izadiyan P, Hemmateenejad B (2016) Multi-response optimization of factors affecting ultrasonic assisted extraction from Iranian basil using central composite design. Food Chem 190:864–870. https://doi.org/10.1016/j.foodchem.2015.06.036
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01617
Johns C (2017) Living soils: the role of microorganisms in soil health. Future Directions International, June, 7. https://www.futuredirections.org.au/publication/living-soils-role-microorganisms-soil-health/
Karim AA, Kumar M, Singh SK, Panda CR, Mishra BK (2017) Potassium enriched biochar production by thermal plasma processing of banana peduncle for soil application. J Anal Appl Pyrol. https://doi.org/10.1016/j.jaap.2016.12.009
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess. https://doi.org/10.1186/s40643-017-0137-9
Ma X, Zhou B, Budai A, Jeng A, Hao X, Wei D, Zhang Y, Rasse D (2016) Study of biochar properties by scanning electron microscope—energy dispersive X-ray spectroscopy (SEM-EDX). Commun Soil Sci Plant Anal 47(5):593–601. https://doi.org/10.1080/00103624.2016.1146742
Manimaran P, Pillai GP, Vignesh V, Prithiviraj M (2020) Characterization of natural cellulosic fibers from Nendran Banana Peduncle plants. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.08.111
Mohamed Noor N, Shariff A, Abdullah N, Mohamad Aziz NS (2019) Temperature effect on biochar properties from slow pyrolysis of coconut flesh waste. Malays J Fundam Appl Sci 15(2):153–158. https://doi.org/10.11113/mjfas.v15n2.1015
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 4:848–889
Pang J, Zheng M, Wang A, Sun R, Wang H, Jiang Y, Zhang T (2014) Catalytic conversion of concentrated miscanthus in water for ethylene glycol production. AIChE J 60(6):2254–2262. https://doi.org/10.1002/aic
Pazmiño-Hernandez M, Moreira CM, Pullammanappallil P (2019) Feasibility assessment of waste banana peduncle as feedstock for biofuel production. Biofuels 10(4):473–484. https://doi.org/10.1080/17597269.2017.1323321
Qian K, Kumar A, Patil K, Bellmer D, Wang D, Yuan W, Huhnke RL (2013) Effects of biomass feedstocks and gasification conditions on the physiochemical properties of char. Energies. https://doi.org/10.3390/en6083972
Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR (2012) Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils. https://doi.org/10.1007/s00374-011-0624-7
Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant Soil 335(1):155–180. https://doi.org/10.1007/s11104-010-0520-1
Ronsse F, van Hecke S, Dickinson D, Prins W (2013) Production and characterization of slow pyrolysis biochar: influence of feedstock type and pyrolysis conditions. GCB Bioenergy 5(2):104–115. https://doi.org/10.1111/gcbb.12018
Safdari M, Amini E, Weise DR, Fletcher TH (2019) Heating rate and temperature effects on pyrolysis products from live wildland fuels. Fuel 242:295–304. https://doi.org/10.1016/j.fuel.2019.01.040
Sevgi S (2015) Pyrolysis of hornbeam shell (Carpinus betulus L.) in a fixed bed reactor: characterization of bio-oil and bio-char. Fuel 150:672–678. https://doi.org/10.1016/j.fuel.2015.02.095
Shafiq U (2017) Proximate analysis of low and high quality pure coal and their blends from Pakistan. Austin Chem Eng 4(1):2–5. https://doi.org/10.26420/austinchemeng.2017.1048
Siddiqui MTH (2017) Characterization and process optimization of biochar produced using novel biomass, waste pomegranate peel: a response surface methodology approach. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-017-0091-y
Soderqvist H (2019) Carbon stability of biochar. Degree Proj Energy Environ Second Cycle, 30 Credit Stock SWEDEN2019
Soja G, Bielsk L (2021) Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour Convers 4(November 2020):36–46. https://doi.org/10.1016/j.crcon.2021.01.003
Street EM (2010) Supercritical ethanol liquefaction of swine manure for bio-oils production. Am J Eng Appl Sci 3(2):494–500
Suravi KN, Attenborough K, Taherzadeh S, Macdonald AJ, Powlson DS, Ashton RW, Whalley WR (2021) The effect of organic carbon content on soil compression characteristics. Soil Tillage Res 209(February):104975. https://doi.org/10.1016/j.still.2021.104975
Te WZ, Muhanin KNM, Chu YM, Selvarajoo A, Singh A, Ahmed SF, Vo DVN, Show PL (2021) Optimization of pyrolysis parameters for production of biochar from banana peels: evaluation of biochar application on the growth of Ipomoea aquatica. Front Energy Res. https://doi.org/10.3389/fenrg.2020.637846
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19(1):191–215. https://doi.org/10.1007/s11157-020-09523-3
Uddin MN, Techato K, Taweekun J, Rahman MM, Rasul MG, Mahlia TMI, Ashrafur SM (2018) An overview of recent developments in biomass pyrolysis technologies. Energies. https://doi.org/10.3390/en11113115
Uzun B, Apaydin-varol E, Ates F, Pütün E (2010) Synthetic Fuel Production from Tea Waste : Characterisation of Bio-Oil and Bio-Char 89:176–184. https://doi.org/10.1016/j.fuel.2009.08.040
Wang K, Peng N, Lu G, Dang Z (2018) Effects of pyrolysis temperature and holding time on physicochemical properties of swine-manure-derived biochar. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0435-2
Wati (2019) Pyrolysis of banana pseudo-stem and leaf through fast and slow processes in producing bio-char and bio-oil. Αγαη 8(5):55
Weber K, Quicker P (2018) Properties of biochar. Fuel 217(December 2017):240–261. https://doi.org/10.1016/j.fuel.2017.12.054
Wong JE, Muñiz DB, Martínez GCG, Belmares RE, Aguilar CN (2015) Ultrasonics sonochemistry ultrasound-assisted extraction of polyphenols from native plants in the Mexican desert. Ultrason Sonochem 22:474–481. https://doi.org/10.1016/j.ultsonch.2014.06.001
Workneh TS, Belew D (2013) Review on postharvest technology of banana fruit. Afr J Biotech 12(7):658–664. https://doi.org/10.5897/AJB12.2015
Yu J, Song M, Li Z (2022) Optimization of biochar preparation process and carbon sequestration effect of pruned wolfberry branches. Green Process Synth 11(1):423–434. https://doi.org/10.1515/gps-2022-0044
Zea S, Biochar L, Capture C, Bachmann RT, Rafiq MK, Bachmann RT, Rafiq MT (2016) Influence of pyrolysis temperature on physico-chemical properties of corn influence of pyrolysis temperature on physico-chemical properties of corn stover (Zea mays L.) biochar and feasibility for carbon capture and energy balance. Bioresour Technol. https://doi.org/10.1371/journal.pone.0156894
Zhang J, Kan X, Shen Y, Loh KC, Wang CH, Dai Y, Tong YW (2018) A hybrid biological and thermal waste-to-energy system with heat energy recovery and utilization for solid organic waste treatment. Energy 152:214–222. https://doi.org/10.1016/j.energy.2018.03.143
Zhao B, O’Connor D, Zhang J, Peng T, Shen Z, Tsang DCW, Hou D (2018) Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J Clean Prod 174:977–987. https://doi.org/10.1016/j.jclepro.2017.11.013
Zhao J, Wang D, Pidlisnyuk V, Erickson LE (2021) Miscanthus biomass for alternative energy production. Phytotechnol Biomass Prod. https://doi.org/10.1201/9781003082613-10
Zheng H, Wang Z, Deng X, Xing B (2013) Impact of pyrolysis temperature on nutrient properties of biochar. Funct Nat Org Matter Chang Environ 9789400756:975–978. https://doi.org/10.1007/978-94-007-5634-2_179
Zornoza R, Moreno-Barriga F, Acosta JA, Muñoz MA, Faz A (2016) Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 144:122–130. https://doi.org/10.1016/j.chemosphere.2015.08.046
Acknowledgements
The authors are grateful to Director, CSIR-Central Leather Research Institute (CLRI), for providing the laboratory facilities and permitting this research work. The authors also thank Indian and German Industrial partners (M/s. Ramky Enviro Engineers Ltd., India, and M/s. Biomacon Gmbh, Germany) for their support in this 2+2 PYRaSOL project. The authors also acknowledge ISAH team, Leibniz University, Hannover, Germany, for their technical support for this work. The CSIR-CLRI Communication number is 1673. This research work is carried as a part of the PhD programme of the candidate M/s. R. Nishanthi, registered under Anna University, Chennai, India.
Funding
This work was funded by Indo-German Science and Technology Centre (IGSTC), New Delhi, a joint initiative of the Department of Science and Technology (DST), Government of India, and the Federal Ministry of Education and Research (BMBF), Germany, under 2 + 2 Project “Smart Cities integrated energy supply, carbon sequestration and urban organic waste treatment through combined solar sludge drying and pyrolysis (PYRaSOL)” (Funding code: 01DQ18001A).
Author information
Authors and Affiliations
Contributions
NR was involved in methodology, data curation, validation, original draft preparation; SG was involved in data curation, investigation; SSV and DW were involved in conceptualization, supervision, validation and review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper entitled “Optimization of Pyrolysis process parameters for the production of biochar from Banana peduncle fibrous waste and its Characterization.”
Ethics approval
The above study was carried out using the waste collected from the market. Hence, ethical approval is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajendiran, N., Ganesan, S., Weichgrebe, D. et al. Optimization of pyrolysis process parameters for the production of biochar from banana peduncle fibrous waste and its characterization. Clean Techn Environ Policy 25, 3189–3201 (2023). https://doi.org/10.1007/s10098-023-02592-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-023-02592-2