Abstract
Algal biofuels serve as a promising alternative energy source for liquid fuels. However, one of the bottlenecks in the conversion of microalgae to biofuels is the drying process. A moisture content of at most 10 % is desired for algal biomass prior to oil extraction to maximise biofuel yield. Conventional means of drying results to longer drying time and uneven drying of algal biomass. This study investigated the drying characteristics of microwave for microalgae (Chlorella vulgaris). Three microwave intensity levels (300, 600, and 900 W) were considered to dry 10, 20, and 30 of algal mass. Page model gave a better fit on the moisture ratio with time of microwave drying than the exponential model. Furthermore, the specific energy requirement was computed, and a relationship was found between moisture ratio with power and mass. Fourier transform infrared spectroscopy results showed significant reduction of infrared signal intensities of the functional groups present in the algae after drying at higher microwave power level. It was concluded that the 20 W/g microwave drying setting gave a lower specific energy requirement with good quality of remaining high lipid content qualitatively. Furthermore, it was recommended to use gas chromatography mass spectroscopy to further quantify the algal lipids and other functional groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global oil demand from the transportation sector has been continuously increasing while this sector contributes one fifth of the overall carbon dioxide (CO2) emissions (Balat and Balat 2010). Moreover, light vehicles are projected to grow to 2 billion by the end of 2050 which lead to an additional demand of fossil fuels. The eminent depletion of fossil fuels together with the projected increasing demand is forecasted to trigger an elevated fuel price (Rawat et al. 2013). Hence, biofuels are introduced to reduce the dependence on imported fossil-based fuels and to reduce CO2 emissions (Zhu 2015). Experimentally, biofuels have less ecotoxicity than of petroleum fuels (Bulatov and Klemeš 2011). Experimental results from the study of Lapinskiene et al. (2006) showed that toxicity to soil organisms was greater by 3 % at petroleum diesel concentrations than of biodiesel. Biodiesel are categorised as first generation biofuels, which are harvested from edible plant material, and second generation biofuels, which are produced from lignocellulosic or biomass material (Singh and Olsen 2011). There are numerous potential feedstocks that can be utilised for biofuel production: coconut, soybean, corn, canola, jatropha, and palm oil (Du et al. 2008). However, to meet 1 % of global fuel demand, 1 % of available land globally is used for first generation biofuels (Brennan and Owende 2010). To address the issue of food versus fuel and production per land area, third generation biofuels are proposed. Third generation biofuels are derived from microorganisms such as bacteria, yeast, and microalgae that produce oleaginous material. These microorganisms can grow in heterotrophic and photoautotrophic conditions (Singh and Olsen 2011).
Microalgae is one of the promising alternative energy sources due to its high oil yield per land area (Chisti 2007) while CO2 is reduced for its cultivation (Glaser 2009). Microalgae are photosynthetic micro-organism that converts sunlight into different kinds of nutrients in various applications: nutraceutical, pharmaceutical, agricultural feed, and energy. There are benefits that microalgae can offer (Pokoo-Aikins et al. 2010). First, energy sources such as methane, bio-ethanol, bio-hydrogen (Brau et al. 2013), bio-oil (Grima 1994), and biodiesel (Gouveia and Oliveira 2009) can be produced from microalgae using various conversion methods (Koller et al. 2014). Second, the 60 % of dewatered microalgae’s weight are pure biomass (Avagyan 2008), which prove that they are rich in oil prior to extraction process (Yanfen et al. 2012). Specifically, the production of biodiesel from microalgae involves stages from cultivation to harvesting, drying, oil extraction, and finally, transesterification (O’Connell et al. 2013). However, almost 60 % of the total energy consumed in the production of biofuels from microalgae is the drying process (Yanfen et al. 2012). It increases the effectiveness of the solvent-based extraction of oil (Iqbal 2012) and to prevent the formation of water and oil emulsion, resulting to higher quality of biofuels (Viswanathan et al. 2012). Moreover, to avoid spoilage due to hot climate environment, harvested biomass must be dried rapidly up to 5–10 % (dry solid) (Grima 2003).
Previous studies showed the various alternative drying methods used in drying microalgae for biofuels such as convective dryer, solar drying, rotary drying, spray drying, cross-flow drying, vacuum shelf drying, and flashing drying. Viswanathan et al. (2012) employed convective drying with a constant parallel air flow velocity of 0.25 m/s subjected at different drying temperatures to determine the lipid yield in the solvent extraction method. Becker and Venkataraman (1982) utilised solar heat to assess its feasibility to dry Spirulina. Prakash et al. (1997) also used solar drying for Spirulina and Scenedesmus algal species. However, the disadvantages of solar dryer are dehydration and disintegration of algal chlorophyll which alter the quality of the final algal product, and it is weather-dependent technology (Show et al. 2015). Similarly, Culaba et al. (2013) conducted an experiment on the drying characteristics of Tetraselmis sp using solar dryer. Issues such as uneven drying and charring of product occurred during the study. Rotary drying showed impressive dried algal product when drying Scenedesmus algae (Soeder and Pabst 1975). However, using this method consumed high energy for different kinds of species such as Botryococcus braunii (Singh and Olsen 2011). Spray drying showed high efficiency in drying the algae for human consumption. However, it is costly to operate, and it degrades the quality of the microalgae biomass (Chen et al. 2011). To acknowledge such concern of drying method issues, the microwave drying characteristics of microalgae was investigated.
Microwave drying is known for its rapid and effective heat distribution in the sample, and its high yield output such as for orange slices (Diaz et al. 2003) and for apple slices (Feng and Tang 1998). Microwave is part of the electromagnetic spectra whose frequency ranges from 300 MHz to 300 GHz. The usual microwave frequencies that are used in domestic microwave application and for drying agricultural products are 915 MHz and 2.45 GHz (Datta and Davidson 2000). Based on the theory of electromagnetic waves, the microwave is absorbed by ions. In the case of drying, the microwave energy is absorbed by the water molecules in the sample (Nikolić et al. 2011). The dipolar water molecules align themselves constantly with the oscillating electric field generating heat caused by friction between molecules, hence, drying the sample (Chanrasekaran et al. 2013). The difference of microwave mechanism to other conventional heating mechanism is the non-surface heating that penetrates the overall target (Teo et al. 2014). On the contrary, conventional heating applies heat from the outside surface although mass transfer occurs from inside to the outside.
Other studies used microwave irradiation for different purposes (Maskan 2000, 2001; Özbek and Dadali 2007; Soysal et al. 2006). Lee et al. (2010) used microwave assisted extraction to Chlorella vulgaris, Botryococcus sp., and Scenedesmus sp. in solvent extraction using Bligh and dyer method. To extract lipids from microalgae, Cheng et al. (2013) used microwave treatment in Chlorella PY-ZU1 investigating its effect on the dynamic microalgal cell wall microstructures. Similarly, Iqbal (2012) used microwave to extract oil from Nannochloropsis sp. On the other hand, several computational studies were performed determining the effects of temperature and pressure on the water transport through lipid membrane (Manrique et al. 2014) and aquaporin (Ducut et al. 2014) of microalgae.
In this study, the microwave drying characteristics of Chlorella vulgaris were investigated, and its chemical composition were analysed by Fourier transform infrared spectroscopy before and after drying. This algal strain was chosen because of its abundance (Shih-Hsin Ho et al. 2014) and high oil content of about 14–40 % of dried biomass (Koller et al. 2014).
Methodology
This study comprises of microalgae cultivation, harvesting, microwave drying, mathematical modelling, and characterisation by Fourier transform infrared spectroscopy. Details for each procedure are shown in the succeeding subsections.
Microalgae cultivation
The initial strain of Chlorella vulgaris was obtained from the culture collection of the Plant Biology division, Institute of Biological Sciences, University of the Philippines Los Baños, Los Baños, Philippines.
The algal strain was cultured in a sterilised 6 L bottle container embellished with BG 11 medium at a pH level between 7.0 to 7.5 (Stanier et al. 1971). It contained NaNO3, K2HPO4·H20, MgSO4·7H2O, CaCl2·2H2O, Citric Acid, Ferric Citrate, Na2 EDTA, Na2CO3, and trace metals specifically H3BO3, MnCl2·4H2O, ZnSO4·7H2O, Na2MoO4·2H2O, CuSO4·5H2O, and Co(NO3)2·6H2O. The medium was sterilised in a batch bag using autoclave system at 15 psi for 15 min. During inoculation, the conditions were maintained at 23 ± 1 °C, under a fluorescent light of 100 μmol m−2 s−1 with 24 h of aeration.
Harvesting
The mature 6 L freshwater microalgae were dewatered by centrifugation method at 2000 rpm using Megafuge 1.0 R manufactured by Heraeus-Christ GMBH mode. A concentration of 3.33 g/L of microalgae were collected from the 6 L of cultured microalgae.
Microwave drying
The drying of biomass was carried out in a 2M265-M12WJ magnetron IBF Electronic microwave generator with the maximum incident power of 3,000 W at a frequency of 2,450 MHz.
The schematic diagram of the experimental setup is shown in Fig. 1a. The microwave equipment is composed of the magnetron, a directional coupler with a dummy load to prevent the microwave energy to reflect back to the source. The power level of the microwave was controlled using a programmable Logic controller (PLc) to ensure consistency of the microwave intensity. Based on the standard R26 waveguide set by the International Electrotechnical Commission (IEC), the dimensions used in designing the drying chamber are 86.36 mm × 43.18 mm x 87.14 mm as shown in Fig. 1b. The third dimension is about half the waveguide wavelength (λ g = 174.28) of the microwave at 2.45 GHz.
The experiments were conducted for 10 g, 20 g, and 30 g of microalgae exposed to microwave at three (3) different power settings 300, 600, and 900 W. The instantaneous weight of the microalgae was monitored using (Denver Instrument XE-4100 analytical balance) every 2 min. The microalgae drying was performed until the microalgae reached its bone-dried mass.
Mathematical modelling of microwave drying curves
Several studies have mathematically modelled the drying curves of different drying methods as shown in Table 1. Two moisture ratio models namely, Newton’ Model (exponential model) and the Page Model were used in this study. These models are derived from the Fick’s second law by simplifying the general series solution. The exponential model was initially used to describe the drying of mint leaves (Park et al. 2002) and mulberry (Doymaz 2004a, b). This model is written as
where m i (g) is the initial mass, m e (g) is the equilibrium mass or bone-dried mass, and m t (g) is the mass at time t, a, and k are constants in the model.
The exponential model was modified resulting to the Page Model to accommodate nonlinear exponent of time, and given as
where k and y are constants in this model. Page model was used in microwave drying such as for parsley by Soysal (2004), sardine fish by Darvishi et al. (2013), coriander leaves by Sarimeseli (2011), and sorbus fruits by Lule and Koyuncu (2015).
To evaluate the goodness of fit of these mathematical models, a regression analysis was performed calculating the coefficient of determination (R 2) and reduced Chi-square (χ2) between the predicted and experimental values. An R 2 close to 1 (Özdemir and Devres 1999) and lower χ2 values indicate the goodness of fit (Ertekin and Yaldiz 2004). The reduced Chi-square is given by
where MR pre,i is the predicted moisture ratio, MR exp,i is the experimental moisture ratio, N is the number of observations, and z is the number of constants.
Fourier transform infrared (FTIR) spectroscopy
The microalgae were characterised with FTIR spectroscopy using a NICOLET 6700 FTIR spectrophotometer in order to identify the chemical compounds present before and after microwave drying. The 20 g samples were prepared using potassium bromide (KBr) pellet method, in a 1:10 ratio of the dried microalgae and potassium bromide. It was ground and mixed using mortar and pestle, and pressed to form a thin pellet sample. The samples were scanned 32 times between the 4000 and 400 cm−1 region. This was conducted on the 20 g samples before and after exposure to microwave at three different specific power settings: 300, 600, and 900 W.
Results and discussions
In this section, the results on microwave drying, mathematical modelling, and FTIR spectroscopy are presented.
Microwave drying
Influence of power level
The effect of three power levels on the drying curve with different initial mass of microalgae is shown in Fig. 2. Generally, it shows that the drying curves are all in the falling period. There is no constant rate of drying period. The decay of the microwave drying curve is faster than that of the other drying methods performed by Gogus and Maskan (1999) and Gupta et al. (2002).
Figure 2 also shows that the drying power level caused an important increase in the drying rate leading to a shorter drying time. It means that the time required to reduce the moisture ratio is dependent on the power level of the microwave.
Mathematical model of the microwave drying characteristics
The drying curve shown in Fig. 2 can be generally characterised as an exponential function with time as modelled by the Newton model and Page model. It shows that the Page Model is a better fit than the Newton for the moisture ratio as a function of time at different power settings and initial mass. This can be validated by a higher coefficient of determination and lower Chi-square as shown in Fig. 3. The previous models are further evaluated by considering the power level and initial mass of the sample being dried.
Figure 4 shows the plot of the linear fit of the natural logarithm of moisture ratio as a function of time. Generally, it shows a good fit as indicated by the coefficient of determination (R2). It can be seen that as the power level is increased, the linear fit becomes more accurate and the slope becomes steeper. This clearly shows the dependence of the slope on the power level. On the other hand, the initial mass of the sample appears to be insignificant to the drying curve since there is no variation in the slopes of the drying curve except on the moisture ratio curve of the 20 g at 300 W power level data.
As seen in Fig. 4, the microwave drying curve of moisture ratio is best modelled as
where k is dependent on the power level and initial mass of the sample, which resembles the Page model. This model takes consideration of the curvature at lower power levels.
Using nonlinear regression calculations, the best fit parameters for all data corresponding to the highest coefficient of determination of 0.9744 and reduced Chi-square (χ 2) of 0.002 is given by
Table 2 shows the coefficient of determination for each power level using Eqs. (5) and (6). It shows a good fit for all dataset. Based on the model, the power level needed for drying the microalgae to the desired 10 % moisture ratio with a time requirement t r (in min) is given by
Energy of drying
The data were further analysed to determine the energy consumption in drying the algae. The specific energy requirements during the drying time were calculated. The specific energy requirement based on the study of Varith et al. (2007) and Soysal (2004), defined as the amount of energy required to remove a unit mass of moisture, were computed using Eq. (8):
where P is the power setting of the microwave oven in W, and t is the elapsed time in seconds.
The specific power settings were determined based on the power settings and microalgae sample size. The following specific power settings are 10, 15, 20, 30, 45, 60, and 90 W/g. Note that for 30 W/g, the combined results of the following power settings/sample size were used: 300 W/10 g, 600 W/20 g, and 900 W/30 g.
Figure 5 summarises the specific energy requirements for the various specific power settings as the samples are being dried. It shows that the 15 W/g setting requires the least energy to dry the sample until it reached its 42.5 % moisture content. Lower than its 42.5 % moisture content, the 20 W/g has the lowest specific energy requirement.
Derived from Eqs. (5) and (6), the specific energy requirement (joules) to dry the microalgae to 10 % moisture ratio is given by
Equation (9) indicates that the specific energy requirement decreases when large amount of algae is used in drying the algae at a given constant power level. This result agrees well with Fig. 4 which shows that the specific energy requirement is lower for higher initial mass given a constant power level.
Comparing the energy consumption with the other studies, microwave drying has shown a big potential for efficient and low cost drying method as shown in Table 3.
Fourier transform infrared spectroscopy results
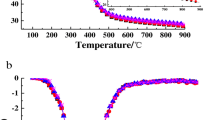
The FTIR spectroscopy result for the undried sample is provided in Fig. 6a. The strong peaks at point C (1536 and 1422 cm−1) corresponds to the bending modes of protein methyl groups. The intensity of the peak at point B (2924.56 cm−1) suggests high content of lipid and carbohydrate in the sample indicating that Chlorella vulgaris is a good biofuel feedstock. It is interesting to note that these spectral peaks are consistent with the findings of a similar investigation (Ponnuswamy 2013). Moreover, nitriles and alkynes intensity peaks appeared in the post-drying of microalgae.
FTIR spectroscopy of Chlorella vulgaris of a pre-drying and b post-drying at different microwave power. (a Water v(O–H) stretching, Protein v(N–H) stretching (amide A), b Lipid–hydrocarbons mainly due to C–H stretching, c Protein amide I band Mainly (C=O) stretching d Nitriles and Alkynes due to (C≡N) and (C≡C) adsorption.)
To investigate the effects of microwave irradiation on the quality of the algal samples, IR spectra were also recorded after irradiation as shown in Fig. 6b. The quality of the algae was checked by monitoring the changes in the IR peak intensities, specifically on the lipid–hydrocarbons (2809–3639 cm−1) and the protein amide (1583–3012 cm−1) signals, at different power levels. Results clearly demonstrate a significant change in the IR spectra obtained after irradiation. Note that a lower percentage of transmittance is more desirable as this indicates higher lipid content, thus a better biofuel feedstock which corresponds to the power settings 20 and 15 W/g. Moreover, it shows that there is a higher percentage transmittance at higher specific power settings indicating a decrease in lipid content, most probably due to thermal degradation. This is particularly true for the 45 W/g setting where the percentage transmittance is higher than that of the undried sample as shown in Fig. 6a.
Conclusions
The effects of microwave drying on the cultivated microalgae Chlorella vulgaris were analysed for its suitability in biofuel production. Based from the FTIR spectroscopy results, the cultivated algal species has a high content of lipid and carbohydrate indicating that Chlorella vulgaris is a good source of biomass for biofuel production. Microwave drying results suggested the usage of power settings 20 W/g and 15 W/g in drying Chlorella vulgaris for biofuel production. Page model shows a good fit better than Newton’s model for the drying curve. A mathematical model of the microwave drying curve was formulated as a function of microwave power and mass of the algae. Specific energy requirement and power level requirement were also modelled to obtain a 10 % moisture ratio. FTIR spectroscopy results revealed a significant reduction of infrared signal intensities as microwave power is increased. Further investigation will be pursued to quantify the algal oil, and the effects of microwave using Gas Chromatography Spectroscopy (GC–MS) method and microwave design for large-scale application.
References
Avagyan AB (2008) A contribution to global sustainable development: inclusion of microalgae and their biomass in production and bio cycles. Clean Technol Environ Policy 10(4):313–317
Balat M, Balat H (2010) Progress in biodiesel processing. Appl Energy 87:1815–1835
Becker EW, Venkataraman LV (1982) Biotechnology and exploitation of algae: the Indian approach. Deutsche Gesellschaft fur Technische Zusammenarbeit
Bennion EP, Ginosar DM, Moses J, Agblevor F, Quinn JC (2015) Lifecycle assessment of microalgae to biofuel: comparison of thermochemical processing pathways. Appl Energy 154:1062–1071
Brau JF, Morandin M, Berntsson T (2013) Hydrogen for oil refining via biomass indirect steam gasification: energy and environmental targets. Clean Technol Environ Policy 15(3):501–512
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies forproduction, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Bulatov I, Klemeš JJ (2011) Clean fuel technologies and clean and reliable energy: a summary. Clean Technol Environ Policy 13(4):543–546
Chanrasekaran S, Ramanathan S, Basak T (2013) Microwave food processing—a review. Food Res Int 52:243–261
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102(1):71–81
Cheng J, Yu T, Li T, Zhou J, Cen K (2013) Using wet microalgae for direct biodiesel production via microwave irradiation. Bioresour Technol 131:531–535
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Culaba AB, Tan RR, Biona JBM, Ubando AT, Lopez NSA, Tanchuco JQ, Garibay SS, Toledo NA, Jimenez CN, Pahila IG, Ami LS (2013) A mathematical model for the drying characteristics of microalgae (Tetraselmis sp.). Philippine Sci Lett 6(2)
Darvishi H, Azadbakht M, Rezaeiasl A, Farhang A (2013) Drying characteristics of sardine fish dried with microwave heating. J Saudi Soc Agric Sci 12(2):121–127
Datta AK, Davidson PM (2000) Microwave and radio frequency processing. J Food Sci 65:32–41
Dıaz GR, Martínez-Monzó J, Fito P, Chiralt A (2003) Modelling of dehydration–rehydration of orange slices in combined microwave/air drying. Innovat Food Sci Emerging Technol 4(2):203–209
Dissa AO, Bathiebo DJ, Desmorieux H, Coulibaly O, Koulidiati J (2011) Experimental characterization and modellling of thin layer direct solar drying of Amelie and Brooks mangoes. Energy 2011(36):2517–2527
Doymaz I (2004a) Convective air drying characteristics of thin layer carrots. J Food Eng 61:359–364
Doymaz I (2004b) Pretreatment effect on sun drying of mulberry fruits (Morus alba L.). J Food Eng 65:205–209
Doymaz İ (2005) Drying characteristics and kinetics of okra. J Food Eng 69(3):275–279
Du W, Li W, Sun T, Chen X, Liu D (2008) Perspectives for biotechnological production of biodiesel and impacts. Appl Microbiol Biotechnol 79(3):331–337
Ducut MRD, Villagracia ARC, Corpuz J, Arboleda NB Jr., David MY, Manrique RB, Ubando AT, Culaba AB (2014) Molecular dynamics study on the effects of varying temperature and pressure on phosphatidycholine lipids for microalgae drying. In Humanoid Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM), 2014 International Conference pp 1–4. IEEE, 2014
El-Sebaii AA, Aboul-Enein S, Ramadan MRI, El-Gohary HG (2002) Empirical correlations for drying kinetics of some fruits and vegetables. Energy 27(9):845–859
Ertekin C, Yaldiz O (2004) Drying of eggplant and selection of a suitable thin layer drying model. J Food Eng 63:349–359
Feng H, Tang J (1998) Microwave finish drying of diced apple slices in a spouted bed. J Food Sci 63(4):679–683
Glaser JA (2009) Carbon dioxide recycling. Clean Technol Environ Policy 11(3):253–257
Gogus F, Maskan M (1999) Water adsorption and drying characteristics of okra (Hibiscus esculentus L.). Drying Technol 17:883–894
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36(2):269–274
Grima EM, Medina AR, Giménez AG, Pérez JS, Camacho FG, Sánchez JG (1994) Comparison between extraction of lipids and fatty acids from microalgal biomass. J Am Oil Chem Soc 71(9):955–959
Grima EM, Belarbi EH, Fernández FA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20(7):491–515
Gupta O, Ahmed K, Shivhare US, Raghavan GSV (2002) Drying characteristics of red chilli. Drying Technol 20:1975–1987
Henderson SM, Pabis S (1961) Grain drying theory I. Temperature effect on drying coefficient. J Agric Eng Res 6(3):169–174
Ho Shih-Hsin et al (2014) Perspective on engineering strategies for improving biofuel production from micoalgae. Biotechnol Adv 32:1448–1459
Iqbal J (2012) Development of cost-effective and benign lipid extraction system for microalgae (Doctoral dissertation, Louisiana State University)
Koller M et al (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6(2014):52–63
Koua KB, Fassinou WF, Gbaha P, Toure S (2009) Mathematical modelling of the thin layer solar drying of banana, mango and cassava. Energy 34(10):1594–1602
Lahsasni S, Kouhila M, Mahrouz M, Idlimam A, Jamali A (2004) Thin layer convective solar drying and mathematical modeling of prickly pear peel (Opuntia ficus indica). Energy 29(2):211–224
Lapinskiene A, Martinkus P, Rebzdaite V (2006) Eco-toxicological studies of diesel and biodiesel fuels in aerated soil. Environ Pollut 142:432–437
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:S75–S77
Lule F, Koyuncu T (2015) Convective and microwave drying characteristics of sorbus fruits (Sorbus domestica L.). Proc Soc Behav Sci 195:2634–2643
Manrique R, Villagracia ARC, Ubando AT, Corpuz J, Padama AA, David MY, Arboleda NB Jr, Culaba AB, Kasai H (2014) A molecular dynamics investigation of water migration in a lipid bilayer for microalgae drying. Philipp Sci Let 7(01):138–145
Maskan M (2000) Microwave/air and microwave finish drying of banana. J Food Eng 44(2):71–78
Maskan M (2001) Kinetics of colour change of kiwifruits during hot air and microwave drying. J Food Eng 48(2):169–175
Midilli A, Kucuk H (2003) Energy and exergy analyses of solar drying process of pistachio. Energy 28(6):539–556
Minowa T, Sawayama S (1999) A novel microalgal system for energy production with nitrogen cycling. Fuel 78(10):1213–1215
Nikolić S, Mojović L, Rakin M, Pejin D, Pejin J (2011) Utilization of microwave and ultrasound pretreatments in the production of bioethanol from corn. Clean Technol Environ Policy 13(4):587–594
O’Connell D, Savelski M, Slater CS (2013) Life cycle assessment of dewatering routes for algae derived biodiesel processes. Clean Technol Environ Policy 15(4):567–577
Özbek B, Dadali G (2007) Thin-layer drying characteristics and modelling of mint leaves undergoing microwave treatment. J Food Eng 83(4):541–549
Özdemir M, Devres YO (1999) The thin layer drying characteristics of hazelnuts during roasting. J Food Eng 42(4):225–233
Park KJ, Vohnikova Z, Brod FPR (2002) Evaluation of drying parameters and desorption isotherms of garden mint leaves (Mentha crispa L.). J Food Eng 51:193–199
Pokoo-Aikins G, Nadim A, El-Halwagi MM, Mahalec V (2010) Design and analysis of biodiesel production from algae grown through carbon sequestration. Clean Technol Environ Policy 12(3):239–254
Ponnuswamy I (2013) Isolation and characterization of green microalgae for carbon sequestration, waste water treatment and bio-fuel production. Int J Bio-Science Bio Technol 5(2):17–26
Prakash J, Pushparaj B, Carlozzi P, Torzillo G, Montaini E, Materassi R (1997) Microalgal biomass drying by a simple solar device. Int J Solar Energy 18(4):303–311
Rawat I, Kumar RR, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy 103:444–467
Sarimeseli A (2011) Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers Manag 52(2):1449–1453
Show KY, Lee DJ, Tay JH, Lee TM, Chang JS (2015) Microalgal drying and cell disruption—recent advances. Bioresour Technol 184:258–266
Singh A, Olsen SI (2011) A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl Energy 88:3548–3555
Soeder CJ, Pabst W (1975) Production, properties, preclinical and clinical testing of Scenedesmus 276-3a. The PAG Compendium, C-2, 2113. World Mark Press Ltd, New York
Soysal Y (2004) Microwave drying characteristics of parsley. Biosyst Eng 89(2):167–173
Soysal Y, Öztekin S, Eren Ö (2006) Microwave drying of parsley: modelling, kinetics, and energy aspects. Biosyst Eng 93(4):403–413
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35(2):171
Teo CL, Jamaluddin H, Zain NAM, Idris A (2014) Biodiesel production via lipase catalysed transesterification of microalgae lipids from Tetraselmis sp. Renew Energy 68:1–5
Toğrul İT, Pehlivan D (2002) Mathematical modelling of solar drying of apricots in thin layers. J Food Eng 55(3):209–216
Usub T, Lertsatitthankorn C, Poomsa-Ad N, Wiset L, Siriamornpun S, Soponronnarit S (2010) Thin layer solar drying characteristics of silkworm pupae. Food Bioprod Process 88(2):149–160
Varith J, Dijkanarukkul P, Achariyaviriya A, Achariyaviriya S (2007) Combined microwave-hot air drying of peeled longan. J Food Eng 81(2):459–468
Viswanathan T, Mani S, Das KC, Chinnasamy S, Bhatnagar A, Singh RK, Singh M (2012) Effect of cell rupturing methods on the drying characteristics and lipid compositions of microalgae. Bioresour Technol 126:131–136
Xu L, Brilman DWW, Withag JA, Brem G, Kersten S (2011) Assessment of a dry and a wet route for the production of biofuels from microalgae: energy balance analysis. Bioresour Technol 102(8):5113–5122
Yaldiz O, Ertekin C, Uzun HI (2001) Mathematical modeling of thin layer solar drying of sultana grapes. Energy 26(5):457–465
Yanfen L, Zehao H, Xiaoqian M (2012) Energy analysis and environmental impacts of Microalgal biofuel in China. Energy Policy 45:142–151
Zhu Liandong (2015) Biorefinery as a promising approach to promote microalgae industry: an innovative framework. Renew Sustain Energy Rev 41:1376–1384
Acknowledgments
This work is funded by the CHED-PHERNet Sustainability Studies Program of the Commission for Higher Education (CHED), Philippines. The authors are also grateful for the assistance of De La Salle University Mechanical Engineering Department and University of the Philippines Los Baños, Laguna. Parts of this work were carried out in the National Institute of Physics, University of the Philippines, Diliman, the Chemistry Department and the Physics Department of De La Salle University, and the School of Material Science Engineering of Universiti Malaysia Perlis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villagracia, A.R.C., Mayol, A.P., Ubando, A.T. et al. Microwave drying characteristics of microalgae (Chlorella vulgaris) for biofuel production. Clean Techn Environ Policy 18, 2441–2451 (2016). https://doi.org/10.1007/s10098-016-1169-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1169-0