Abstract
This paper addresses the design and techno-economic analysis of an integrated system for the production of biodiesel from algal oil produced via the sequestration of carbon dioxide from the flue gas of a power plant. The proposed system provides an efficient way to the reduction in greenhouse gas emissions and yields algae as a potential alternative to edible oils currently used for biodiesel production. Algae can be processed into algal oil by various pathways. The algal oil can then be used to produce biodiesel. A flowsheet of the integrated system is synthesized. Then, process simulation using ASPEN Plus is carried out to model a two-stage alkali catalyzed transesterification reaction for converting microalgal oil of Chlorella species to biodiesel. Cost estimation is carried out with the aid of ICARUS software. Further economic analysis is performed to determine profitability of the algal oil to biodiesel process. The results suggest that, for the algal oil to biodiesel process analyzed in this study, factors such as choosing the right algal species, using the appropriate pathway for converting algae to algal oil, selling the resulting biodiesel and glycerol at a favorable market selling prices, and attaining high levels of process integration can collectively render algal oil to be a competitive alternative to food-based plant oils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel is a transportation fuel that has grown immensely in popularity over the past decade. With the dwindling reserves of fossil fuels, it is now more important than ever to search for transportation fuels that can serve as alternatives to crude oil-based fuels such as gasoline and diesel fuel. Common sources for biodiesel feedstock include soy, sunflower, safflower, canola, and palm. Lately there has been growing controversy about the use of potential food sources for the production of fuel. In attempt to address these concerns, researchers have turned their focus from the popular feedstock and are currently investigating the use of alternative, non-food related feedstock such as oil from algae.
Algae are a large and diverse group of simple plant-like organisms, ranging from unicellar to multicellar forms. These cells have the ability to convert carbon dioxide to biomass that can further be processed downstream to produce biodiesel, fertilizer and other useful products. Photosynthetic growth of algae requires carbon dioxide, water and sunlight. Temperature should be in the range of 20–30°C in order to have good growing conditions. Algae also need other inorganic nutrients like phosphorus and nitrogen in order to grow. The fact that micro algae grow in aqueous suspensions, allows for more efficient access to H2O, CO2 and other nutrients which explains the potential for the production of more oil per unit area than other crops currently used. The chemical composition of algae differs based on species. Algae have several characteristics that cause them to be a candidate biodiesel feedstock that deserves serious investigation.
The advantages of using algae for biodiesel production include:
-
No competition for land with crops
-
No competition with the food market
-
Ability to grow in water with high levels of salt so there is no additional demand of fresh water. Also, areas with saline ground water that has no other useful applications can be targeted
-
Overall use less water than oilseeds
-
High oil yield: algae (of the aquatic species) require less land for growth than biodiesel feedstock from terrestrial plants because they are capable of producing more oil per hectare (Chisti 2008a). Table 1 shows the potential gallons of oil per acre per year from different crops. Furthermore, the oil content in algae (per dry weight) can reach as high as 80% (Chisti 2008a). It is worth noting that the oil from microalgae can be extracted with yields up to 80–90% (Grima et al. 1994; Fajardo et al. 2007; Belarbi et al. 2000).
Table 1 Gallons of oil per acre per year (Chisti 2008a) -
Efficient sequestration of CO2: another reason why microalgae are attractive is that CO2 (of about half of the of dry algae weight) is needed for growth (Chisti 2008a). CO2 is a common industrial pollutant, thus microalgae can contribute to reducing atmospheric CO2 by consuming CO2 wastes from industrial sources such as power plants.
There are nine major groups of algae which are cyanobacteria (Cyanophyceae), green algae (Chlorophyceae), diatoms (Bacillariophyceae), yellow-green algae (Xantophyceae), golden algae (Chrysophyceae), red algae (Rhodophyceae), brown algae (Phaeophyceae), dinoflagellates (Dinophyceae) and ‘pico-plankton’ (Prasinophyceae and Eustigmatophyceae) (Hu et al., 2008). Of these nine groups, the green algae are the largest taxonomic group. Microalgae have been known to survive under a wide range of conditions. Under optimal conditions, microalgae have lipid content between 5 and 20% dry weight while under unfavorable conditions lipid content increases between 20 and 50% (Hu et al. 2008). Hence, it is ideal to cultivate microalgae under optimal conditions and later expose them to unfavorable conditions in order to increase lipid content.
Laboratory experiments utilizing green algae, diatoms, and oleaginous species from other eukaryotic taxa show that the microalgae have oil content of 26, 23, and 27% dry weight, respectively, under optimal conditions and 46, 38, and 45% dry weight, respectively under stress conditions (Hu et al. 2008). Depending on the species of microalgae, oil content can be further increased by limiting certain nutrients such as nitrogen, phosphorus or sulfur. For example, limiting sulfur content can increase lipid content in Chlorella sp. (Otsuka 1961).
With the growing interest in growing algae for energy applications, different opinions have been expressed. The opinions range from concerns to skepticism about the energy efficiency, scaleup, and economic feasibility of microalgal use for transportation fuels and other energy needs (e.g., Anslow 2008; Sweeney 2008; Reijnders 2008) to positive assessment of its efficiency and future industrial applications in producing biodiesel meeting ASTM standards (e.g., Chisti 2007, 2008a, b; Miao and Wu 2006).
The growth of algae requires carbon dioxide as one of the main nutrients needed. There is an opportunity to sequester CO2 by using flue gas emissions from industrial sources as the CO2 feed for algae cultivation. The objective of this paper is to develop a techno-economic analysis of a process for sequestering CO2 from flue gas into growing algae which provides lipids that are processed to produce biodiesel. A combination of system synthesis, simulation, integration, and analysis is used to assess the technical and economic performance of the process. A case study is solved to discuss the various metrics of the process.
Problem statement
The problem to be addressed in the paper may be stated as follows:
Given an industrial source (e.g., power plant) which produces flue gas (flowrate M and composition Z), it is desired to sequester CO2 from the flue gas to grow algae which is to be processed to produce biodiesel. The paper will develop a systems approach for the alternative process paths and perform a techno-economic analysis to determine the optimal design of a flue gas to biodiesel system through the cultivation of algae. The paper will also provide an analysis of the technical and economic metrics of the aforementioned steps.
System overview
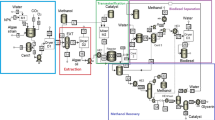
The overall system is composed of two main sections: an upstream processing section which is aimed at sequestering the CO2, growing the algae, and producing the lipids and a downstream processing section which includes the pretreatment of the lipids followed by transesterification then separation and finishing to yield the biodiesel. Figure 1 illustrates these key steps.
Algae selection
The choice of algae species should address specific characteristics that allow the use of flue gas as the CO2 source. Much research has been done on the tolerance of different species to flue gases. Several species were found to be suitable for the growth of algae using flue gas. One of these many species is Chlorella species. Hanagata et al. (1992) found that Chlorella is tolerant to CO2 concentrations of up to 40% by volume. Sung et al. (1999) reported that chlorella grew in conditions of up to 40°C. These results indicate that Chlorella is a good choice for this study.
In this work, the Chlorella species is chosen. The oil content of Chlorella typically ranges between 28 and 32% dry weight (Chisti 2007) but can reach 46% dry weight under stress conditions (Hu et al. 2008) and 55% dry weight when grown heterotrophically (Miao and Wu 2006). Information about the fatty acid compositions of various microalgae (namely the green algae in the classes Chlorophyceae and Prasinophyceae) was published in 1992 (Dunstan et al. 1992). Chlorella is in the class Chlorophycea and the fatty acid compositions of three Chlorella species were listed. Chlorella sp. (CS-195) was used in this analysis because of its potential ease for use in simulation. It is interesting to note that the Chlorella protothecoides (CS-41) composition includes the same fatty acids present in the Chlorella sp. chosen (Dunstan et al. 1992) but in slightly different proportions. Another reason why Chlorella sp. was chosen is the availability of information about its growth, harvesting and extraction.
Feedstock production
Algae can be cultivated via an open system or a closed system. Raceway ponds are the most commercially used open system for growing algae. Photobioreactors are a closed system for algae cultivation. Both raceway ponds and photobioreactors are described by Chisti (2007). Two systems are considered in this work: the use of an open pond system versus the Bio-King system (Clean Tech 2008) that uses a reactor to cultivate algae. The Bio-King process is utilized by a company in The Netherlands.
Methods for harvesting include centrifugation, filtration, and flocculation. Centrifugation is expensive but also one of the most effective ways to harvest algae. The Alfa Laval PX series centrifuges will be used to harvest the algae. Centrifugation will result in the algae being 30% solid with 70% moisture content. As a result, further drying is required.
Drying is considered to be the most energy intensive part of this process. There are many ways to dry the wet paste slurry that comes out of the centrifuge. As mentioned the slurry contains 30% solids with the remaining 70% water. To try and save on energy costs for this process, the drying will be done using excess flue gas.
Extraction is the final step in the processing of algae for use in biodiesel production. Algal oil can be extracted either physically, chemically or both. An expeller/press can be used to physically extract algal oil. The BK-oil press is capable of processing 20 kg/h and will be used for this study.
Chlorella is an algal species that contains anywhere from 29 to 32% lipids (oil content). For this study it is assumed that Chlorella is about 30% oil and for sensitivity analysis purposes, the cost of producing algal oil assuming 50% oil content will also be evaluated.
Cost of producing algal oil
An analysis was conducted assuming a process that utilized the Bio-King Bioreactor for growing the algae, centrifugation for harvesting, excess flue gas for drying, and the Bio-King oil press for extraction. Economic and sensitivity analyses were conducted for this process and used for estimating a range of costs for producing oil from algae. Specifically, two factors were varied: oil content in the algae and performance. Two oil contents are considered: 30 and 50% (dry basis oil in algae). The second factor is the performance of the drying and extraction units. For the high-performance case, low cost of electricity ($0.05/kWh), high production (100 ton/day plant), and the use of heat integration in drying using the hot flue gases were assumed. For the low-performance case, high cost of electricity ($0.20/kWh), low production (1 ton/day), and no heat integration for drying was assumed. The cost estimates are presented in Table 2. These costs are used later in the economic analysis of the production of biodiesel.
Biodiesel process description
Miao and Wu (2006) have shown that a species of Chlorella (Chlorella protothecoides) can be used to produce biodiesel that meets ASTM standards. The microalgae were grown heterotrophically to increase the oil content from 14.6% dry weight to 55.2% dry weight. Acid transesterification was used since the acid value for the algal oil was reported as 8.97 mg KOH/g. The biodiesel yield was approximately 70% at 50°C and conditions of 60% H2SO4 catalyst, 5 h reaction time, 160 rpm, 9.12 g microalgal oil, and 30–1 methanol to oil ratio (Miao and Wu 2006).
The design, integration, and economic assessment of the process are based on the procedure shown in Fig. 2. This approach is based on well-established procedures in the areas of process synthesis, simulation, and integration.
In this work the algal oil is transesterified to biodiesel in a continuous process. Biodiesel can be produced by one of three common routes. They are: acid catalyzed transesterification, base catalyzed transesterification or acid catalyzed esterification of feedstock to fatty acids and then to alkyl esters (NBB 2008). Base catalyzed transesterification is the well-established means of processing biodiesel and the overwhelming option used in industry for economic and technical reasons. Rashid et al. (2008) produced methyl esters from sunflower oil utilizing NaOH catalyst at 1 wt% concentration in a 6:1 methanol to oil ratio at 60°C at yields of 97.1%. Georgogianni et al. (2008) reported methyl ester yields from the processing of sunflower oil of 90% for conditions of 60°C, 7:1 methanol to oil molar ratio and 1 wt% NaOH as catalyst. In the results and discussion it was later stated that “the highest conversion to ester (93–98%) was observed at a ratio of 6:1” (Georgogianni et al. 2008). Rashid and Anwar (2008) found that biodiesel could be produced from safflower oil with yields up to 98% for base-catalyzed transesterification utilizing sodium methoxide catalyst at 1 wt% concentration, 60°C, and 6:1 methanol to oil ratio and yields of above 90% could be achieved for the same conditions with the exception of the use of NaOH as a catalyst. Meka et al. (2007) also synthesized biodiesel from safflower oil and found that at 60°C for 6:1 methanol to oil ratio and 1 wt% NaOH catalyst, yield of 96% could be obtained. Leung and Guo (2006) performed experiments utilizing neat canola oil and used frying oil and found that for experiments exploring different parameters, a temperature of 60°C was optimum for a reaction time of 20 min used frying oil, that ester content was highest (98%) for canola oil for an alcohol to oil ratio of 6:1 (corresponding to a yield of 94%) and that for the three alkali catalyst explored sodium hydroxide was the cheapest and had an optimum concentration of 1.0 for neat canola oil and 1.1 wt%for used frying oil. Foon et al. (2004) in exploring the kinetics of the transesterification of palm oil, performed experiments utilizing base-catalyzed transesterification and found that formation of methyl esters was fastest for NaOH at 60°C for the parameters explored. Conversions above 97% were reported. Leevijit et al. (2008) utilized alkali catalyzed transesterification in a 6-stage reactor to a fatty acid methyl ester product from palm oil. NaOH was used at 60°C in a methanol to oil ratio of 6:1. Gerpen et al. (2004) describes the various possible routes for biodiesel production including alkali-transesterification.
For this reason, base-catalyzed transesterification is used in this investigation. Pretreatment is required for feedstock with high free fatty acid (FFA) content (i.e. greater than 1%, such as waste cooking oils) as well as feedstock with substantial amounts of impurities (such as some algal oils). Feedstock with FFA content of 1 wt% or less are generally required for base-catalyzed transesterification. The algal oil used for the processing of biodiesel in this work is assumed to have only trace amounts of impurities and to have FFA content of 0.05 wt% therefore no pretreatment is required.
In general, the biodiesel process in this work consists of seven sections:
-
Feedstock composition
-
Two-stage transesterification
-
FAME and glycerol separation
-
Methanol recovery
-
Alkali removal
-
Water washing (FAME purification)
-
Glycerol purification
Feedstock composition
The feedstock in this work is algal oil from Chlorella sp. and is characterized in terms of the composition of the individual fatty acids and triglycerides. The FFA content is assumed to be 0.05 wt% and thus pretreatment is not necessary. Based on the data presented by Dunstan et al. (1992) for Chlorella sp. (CS-195), the fatty acid composition is distributed such that the total weight percent is 0.05. For the remaining 99.95 wt%, the same data (Dunstan et al. 1992) are distributed such that it encompasses the triglyceride composition. For simplification, each triglyceride is represented as containing three identical component fatty acids, although in reality numerous possible combinations exist for the fatty acids comprising each triglyceride.
Since the ASPEN Plus simulation software only has the thermodynamic data and other information for a limited number of fatty acids and the corresponding triglycerides and methyl esters that are found in plant oils, fats and algal oils (Myint and El-Halwagi 2009), most of the components of the algal oil feedstock were entered manually using the user-defined method and the structures of each compound were constructed using ISIS software.
Two-stage transesterification
The overall reaction between the triglycerides (algal oil) and methanol is given by:

Consequently, one molecule of each triglyceride in the algal oil reacts with three molecules of methanol to produce three molecules of methyl esters, the biodiesel product, and one molecule of glycerol (Gerpen et al. 2004).
Based on several studies of alkali-catalyzed transesterification, the reaction will be carried out at the temperature near the boiling point of the alcohol (60°C for methanol). A molar ratio of 6:1, alcohol:oil, is also confirmed to be the optimal ratio by numerous studies (Ma and Hanna 1999; Tapasvi et al. 2005; Meher et al. 2006; Myint and El-Halwagi 2009). In this study, the temperature of 60°C, methanol as the alcohol, a molar ratio of 6:1 methanol to oil, and NaOH as the base catalyst are the conditions used as a result of comprehensive literature review mentioned above “Biodiesel process description”.
In the first reactor, sodium hydroxide with a concentration of 1.0 wt% of the feed algal oil was used. The concentration of NaOH for the unreacted oil suggested in patent documents by Wimmer (1995) and Tanaka et al. (1981) for the second reactor is 0.2 wt% of inlet oils. For the process where 97.7% conversion is assumed through each reactor, no additional NaOH is needed in the second reactor. For the process where 70% conversion is assumed through each reactor, additional NaOH that is only 0.14 wt% of inlet oils is needed in the second reactor as a result of mass balance calculations, to bring the total NaOH to 1.0 wt% of the inlet to the second reactor. The purity of algal oil is assumed to be 99.95 wt% while the FFA content was assumed to be 0.05 wt%.
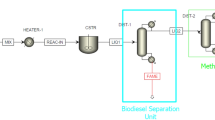
In order to increase the conversion of the algal oil, two transesterification reactions are conducted in sequence. Conversion of feedstock have been documented by Tanaka et al. (1981) to reach up to 99.5 wt% using this two-step process. In this work, the conversion is set to the same percent in each reactor. In the first scenario, the conversion through each reactor is set at 97.7% and in the second scenario the conversion through each reactor is set at 70%. The reaction products biodiesel and glycerol from the first reactor (REACT1) are separated in a decanter (DECANT4) with the byproduct glycerol sent to a distillation column (MET-DIST2) for purification. The unreacted triglycerides (algal oil) is transesterified in the second reactor (REACT2), followed by a further separation of glycerol from biodiesel in another decanter (DECANT1).
FAME and glycerol separation
The transesterification products (fatty acid methyl esters or FAME, and glycerol) are cooled to 92 F (33.3°C) in the first reactor from 60°C, and pumped to a decanter (DECANT4) where FAME and byproduct glycerol are separated. The biodiesel and glycerol from the second reactor are further separated in another decanter (DECANT1). FAME and glycerol are separated at a temperature below the reaction temperature and at atmospheric pressure simply because of their immiscibility and gravity difference. Since the glycerol phase is much denser than biodiesel phase, the two can be gravity separated. The glycerol is drawn off the bottom of the settling vessel.
Methanol recovery
FAME or biodiesel is the main component of the lighter products. It is separated in a decanter (DECANT1), is heated to 60°C and then sent to a distillation column (MET-DIST1) with theoretical stages of 6, a total condenser and a kettle reboiler. In this distillation column, methanol is separated and recovered from the biodiesel phase through the overhead as a vapor. The reflux ratio is set at 1.5 in order to obtain a good separation between methanol and other components.
Alkali removal
The bottom effluents from the distillation column (MET-DIST1) are cooled to 25°C and then sent to another decanter (DECANT2), where the excess sodium hydroxide is neutralized with hydrogen chloride. Hydrogen chloride is added not only to remove residual sodium hydroxide catalyst but also to split any soap that may form during the reverse saponification reaction given by:

Neutralizing the stream before the water washing step aids in reducing the water required for purifying the FAME and in minimizing the chances of emulsion formation when the wash water is added to the FAME.
Water washing (FAME purification)
Once separated from other components such as sodium hydroxide and triglycerides in the decanter (DECANT2), FAME (the biodiesel) is purified by washing gently with warm water to remove residual catalyst, salts, methanol, free glycerol and soaps. A biodiesel purity of 99.65 wt% is required to meet ASTM D 6751 of biodiesel specification. Waste water exiting the water washing unit can then be recycled.
Glycerol purification
After separation in decanter (DECANT4), the glycerol stream is heated to 60°C and then sent to glycerol distillation column (MET-DIST2) with five theoretical stages, a total condenser and a kettle reboiler. Residual FAME exits via the overhead column in terms of vapor, while the glycerol exits through the bottoms, is cooled and can be used commercially.
Process simulation and design of biodiesel production
In this simulation, NRTL and RK-Soave thermodynamic properties were used. Although the thermodynamic data for some triglycerides, fatty acids and methyl esters are available in ASPEN Plus, certain crucial thermodynamic properties (i.e. ideal gas heat capacity) for the components available in the ASPEN Plus databanks are not present. Most of the components were not available in the ASPEN Plus databanks at all. Thermodynamic properties not available in ASPEN Plus must be either entered by a user-defined method or estimated by ASPEN Plus upon providing the molecular structure of the compounds (Myint and El-Halwagi 2009). The molecular structure of all the triglycerides, fatty acids and methyl esters were created using ISIS draw software and exported as .mol files and then imported to ASPEN Plus. Furthermore, ASPEN Plus UNIFAC group contribution method was used based on the molecular structures. Since the component database in ASPEN Plus does not automatically distinguish between cis and trans compounds, there are some expected deviations between the actual thermodynamic data and the data estimated from the imported molecular structure (Myint and El-Halwagi 2009).
Na+ and OH− ions were used instead of solid NaOH because the simulation does not support the use of the solid form in the reaction (Myint and El-Halwagi 2009). Similarly, H+ and Cl− ions were used instead of the HCl in the ASPEN Plus databank. Electrolyte properties must be defined for the Na+, OH−, H+ and Cl− ions.
Feed wash water amount was determined by performing the water sensitivity analysis in order to achieve methyl esters purity higher than 99.65 wt% as required by the ASTM D 6751 standards for biodiesel purity. In addition, a sensitivity analysis was performed of distillate mass flow rate in distillation column (MET-DIST2) in order to guarantee that the purity of glycerol is higher than 90% the temperature of glycerol is lower than its decomposition temperature 554 F (290°C given by Material Safety Data Sheet of glycerol, available at http://avogadro.chem.iastate.edu/MSDS/glycerine.htm). Similarly, sensitivity analysis of distillate mass flow rate in column (MET-DIST1) was performed as well in order to keep the biodiesel stream temperature below its decomposition temperature 482 F (250°C).
Results and discussion
For the processing of the algal oil to biodiesel, two simulations were conducted. Figures 3 and 4 depict the processes. In the first simulation, there was 70% conversion through each reactor and an overall conversion of 90%. In the second simulation, 97.7% conversion was assumed through each reactor and an overall conversion of 99.7% was achieved. Both simulations used the same initial amount of methanol and sodium hydroxide. Also both simulations have the same initial FFA and triglyceride composition.
In the simulation with 70% conversion through each reactor, there is a substantial amount of unreacted triglycerides (7,920 lb/h), and much additional methanol (1,901 lb/h) and additional sodium hydroxide (80 lb/h) required. The overall conversion is 90%. Some of the methanol that is utilized cannot be recovered. In contrast, the simulation with 97.7% conversion through each reactor has much less unreacted triglycerides (607 lb/h) and needs no additional methanol or sodium hydroxide in the second reactor. The overall conversion is 99.7%. For both cases, the resulting biodiesel is 99.7% pure and the density is calculated to be 6.4 lb/gal.
Heat integration via pinch analysis
Data for the hot and cold streams for the cases of 90 and 99.7% overall conversions are presented in Tables 3 and 4. Next, the thermal pinch analysis (e.g., Linnhoff and Hindmarsh 1983; Papoulias and Grossmann 1983) is used to carry out heat integration and to determine minimum heating and cooling utility requirements. The grand composite curves (Dhole and Linnhoff 1993) are illustrated in Figs. 5 and 6 and show the utility saving for 90 and 99.7% overall conversion, respectively. The results are summarized in Tables 5 and 6. The second simulation (99.7% overall conversion) has greater utility savings (63% for heating and 53% cooling) than the first simulation (90% overall conversion) (39% for heating and 37% for cooling). Additionally, mass integration for recycling methanol and water was carried out using source–sink mapping techniques (e.g., El-Halwagi 1997, 2006).
Economics
ASPEN ICARUS has been used to compute project capital and equipment cost. Once the simulation has been completed and ASPEN Plus is run, the information from ASPEN Plus can be exported into ICARUS. For the algal oil to biodiesel process, the total capital investment is $10.9 million for 99.7% overall conversion and $10.5 million for 90% overall conversion). The total direct equipment costs estimated from ASPEN ICARUS are $2.6 million for the 90% overall conversion process and $2.8 million for the 99.7% overall conversion process. Raw material costs are presented in Table 7 for the 90% overall conversion case and in Table 8 for the 99.7% overall conversion case. Cost of algal oil comprises the bulk of the raw material costs in each case.
Profit (P), payback period (PP), return on investment (ROI) and breakeven (BE) analysis calculations were done for the low-performance and high-performance scenarios for the cost of producing algal oil and for the assumptions of 30 and 50% oil content of algae for both the 90% and the 99.7% overall conversion processes. The same for calculations (P, PP, ROI, BE) were repeated for the assumptions of 30 and 50% oil content but this time for the cost of producing algal oil that is an average of the low-performance and high-performance values. This was done for both the 90% and the 99.7% overall conversion processes. In addition, for all of these calculations, a low selling price of $3.69/gal and a high selling price of $4.20/gal for biodiesel were assumed for all the calculations (P, PP, ROI, BE) mentioned previously. The selling prices were obtained or assumed based on data from the Clean Cities Alternative Fuel Price Report (2008) from January to October 2008. The assumptions of glycerol selling price ($0.60/gal and $0.88/gal) were made based on information from ICIS Pricing (2008) for the same period.
Based on the results of the economic and sensitivity analysis, the most profitable results where those utilizing process integration. For the cases assuming oil content of 50%, the high performance and average cases were profitable and the low performance case was not highly profitable or unprofitable in some cases. When assuming 30% oil content, only the high performance cases were profitable. Return on investment results are presented in Figs. 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, and 18 for the cases assuming 50% oil content with heat integration and in Figs. 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, and 30 for the cases assuming 30% oil content with heat integration.
Conclusion
In this study, the use of microalgal oil from the Chlorella species to produce biodiesel via a two-step alkali-catalyzed reaction was investigated and simulated in ASPEN Plus. The oil content of the algae was assumed as 30 and 50%. Using a previously analyzed cultivation process, it deemed possible to obtain algal oil from high-performance and low-performance alternatives that could be analyzed for both the 30 and 50% oil content cases. The transesterification reaction simulation was performed twice for two cases: 70% conversion through each reactor and 97.7% conversion through each reactor. Economic analysis was performed on ASPEN ICARUS utilizing both simulation results. The economic analysis included sensitivity analyses incorporating the various aspects that were investigated (30 and 50% oil content, high-performance and low-performance cases, an average of the high and low performance cases, and the two simulation results). As an outcome of analyzing the results of the calculations for profit, payback period, return on investment and breakeven analyses, it was found that the most profitable scenarios for producing biodiesel from Chlorella species microalgal oil are those assuming 50% oil content and incorporating heat integration. These results reveal that the production of biodiesel from microalgal oil will indeed prove profitable and will be a competitive alternative to food derived plant oils under the appropriate conditions (selection of algae, algal growth and processing, selection of a high-yield biodiesel process, and achieving high levels of process integration).
References
Anslow M (2008) Slippery territory: biofuels created from algae aren’t as clean as they seem. Ecologist 38:3–12
Belarbi E-H, Molina G, Chisti Y (2000) A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzyme Microb Technol 26:516–529
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2008a) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131. doi:10.1016/j.tibtech.2007.12.002
Chisti Y (2008b) Response to Reijnders: do biofuels from microalgae beat biofuels from terrestrial plants? Trends Biotechnol. doi:10.1016/j.tibtech.2008.04.002
Clean Cities Alternative Fuel Price Report (2008) Alternative Fuels and Advanced Vehicles Data Center. http://www.afdc.energy.gov/afdc/price_report.html. Accessed 14 July 2008
Clean Tech (2008) BioKing commercializes small algae bioreactors. http://media.cleantech.com/node/1073. Accessed 10 July 2008.
Dhole VR, Linnhoff B (1993) Distillation column targets. Comput Chem Eng 17(5–6):549–560
Dunstan GA, Volkman JK, Jeffrey SW, Barrett SM (1992) Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 2. Lipid classes and fatty acids. J Exp Mar Biol Ecol 161:115–134
El-Halwagi MM (1997) Pollution prevention through process integration. Academic Press, San Diego
El-Halwagi MM (2006) Process Integration. Academic Press, New York
Fajardo AR, Cerdán LE, Medina AR, Frenández FGA, Moreno PAG, Grima EM (2007) Lipid extraction from the microalgae Phaeodactylum tricornutum. Eur J Lipid Sci Technol 109:120–126
Foon, Cheng S, May, Choo Y, Ngan, Ma A, Hock, Chuah C (2004) Kinetics study on transesterification of palm oil. J Oil Palm Res 16(2):19–29
Georgogianni KG, Kontominas MG, Pomonis PJ, Avlonitis D, Gergis V (2008) Conventional and in situ transesterification of sunflower seed oil for the production of biodiesel. Fuel Process Technol 89:503–509
Gerpen JV, Shanks B, Pruszko R (2004) Biodiesel production technology. National Renewable Energy Laboratory. Subcontractor report NREL/SR-510-36244
Grima EM, Medina AR, Giménez AG, Pérez JAS, Camacho FG, Sánchez JLG (1994) Comparison between extraction of lipids and fatty acids from microalgal biomass. J Am Oil Chem Soc 71(9):955–959
Hanagata N, Takeuchi T, Fukuju Y, Barnes DJ, Karube I (1992) Tolerance of microalgae to high CO2 and high temperature. Phytochemistry 31(10):3345–3348
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
ICIS Pricing. http://www.icispricing.com. Accessed 14 July 2008
Leevijit T, Tongurai C, Prateepchaikul G, Wisutmethangoon W (2008) Performance test of a 6-stage continuous reactor for palm methyl ester production. Bioresour Technol 99:214–221
Leung DYC, Guo Y (2006) Transesterification of neat and used frying oil: optimization for biodiesel production. Fuel Process Technol 87:883–890
Linnhoff B, Hindmarsh E (1983) The pinch design method for heat exchanger networks. Chem Eng Sci 38(5):745–763
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70:1–15
Meher LC, Sagar DV, Naik SN (2006) Technical aspects of biodiesel production by transesterification—a review. Renew Sustain Energy Rev 10:248–268
Meka PK, Tripathi V, Singh RP (2007) Synthesis of biodiesel fuel from safflower oil using various reaction parameters. J Oleo Sci 56(1):9–12
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Myint LL, El-Halwagi MM (2009) Process analysis and optimization of biodiesel production from soybean oil. J Clean Tech Environ Policies. ISSN 1618-954X (Print). doi: 10.1007/s10098-008-0156-5
National Biodiesel Board (NBB) (2008) http://www.biodiesel.org/pdf_files/fuelfactsheets/production.pdf. Accessed 18 April 2008
Otsuka H (1961) Changes of lipid and carbohydrate contents of Chlorella cells during the sulfur starvation, as studied by the technique of synchronous culture. J Gen Appl Microbiol 7:72–77
Papoulias SA, Grossmann IE (1983) A structural optimization approach in process synthesis. II. Heat recovery networks. Comput Chem Eng 7(6):707–721
Rashid U, Anwar F (2008) Production of biodiesel through base-catalyzed transesterification of safflower oil using an optimized protocol. Energy Fuels 22:1306–1312
Rashid U, Anwar F, Moser BR, Ashraf S (2008) Production of sunflower oil methyl esters by optimized alkali-catalyzed methanolysis. Biomass Bioenergy. doi:10.1016/j.biombioe.2008.03.001
Reijnder L (2008) Do biofuels from microalgae beat biofuels from terrestrial plants? Trends Biotechnol. doi:10.1016/j.tibtech.2008.04.001
Sung KD, Lee JS, Shin CS, Park SC, Choi MJ (1999) CO2 fixation by Chlorella sp. KR-1 and its cultural characteristics. Bioresour Technol 68:269–273
Sweeney D (2008) Algae biofuel mania. http://www.stockhouse.com/Columnists/2008/June/11/Algae-biofuel-mania. Accessed 12 June 2008
Tanaka Y, Okabe A, Ando S (1981) Method for the preparation of a lower alkyl ester of fatty acids. Patent number: 4303590
Tapasvi D, Wiesenborn D, Gustafson C (2005) Process model for biodiesel production from various feedstocks. Am Soc Agric Eng 48:2215–2221
Wimmer T (1995) Process for the production of fatty acid esters of lower alcohols. Patent number: 5399731
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pokoo-Aikins, G., Nadim, A., El-Halwagi, M.M. et al. Design and analysis of biodiesel production from algae grown through carbon sequestration. Clean Techn Environ Policy 12, 239–254 (2010). https://doi.org/10.1007/s10098-009-0215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-009-0215-6