Abstract
Purpose
Increasing evidence has suggested that metformin may play positive roles in a wide range of infectious diseases. This study aimed to investigate the clinical impact of metformin exposure during Staphylococcus aureus bacteremia (SAB) in patients with diabetes.
Methods
A 3-year observational cohort study of 452 patients (aged ≥ 16 years) with SAB was performed at a tertiary care hospital. Metformin exposure was defined as receiving metformin during SAB, regardless of metformin use before the onset of bacteremia.
Results
Of 452 patients, 51 (11.3%) were classified in Group A (diabetes with metformin exposure), 115 (25.4%) in Group B (diabetes without metformin exposure), and 286 (63.3%) in Group C (no diabetes). The 30-day mortality rate in Group A was significantly lower than that in Group B (3.9% [2/51] versus 14.8% [17/115]; p = 0.04) and lower than that in Group C (3.9% [2/51] versus 17.1% [49/286]; p = 0.02). The mortality rates did not differ between Group B and Group C (14.8% [17/115] versus 17.1% [49/286]; p = 0.57). The rates of persistent and recurrent bacteremia were comparable among the three groups. Multivariate analysis indicated that metformin exposure was significantly associated with reduced mortality (adjusted odds ratio, 0.20; 95% confidence interval, 0.04–0.88; p = 0.03).
Conclusions
Metformin exposure during SAB appears to be an independent predictor of survival in patients with diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus remains a leading cause of bloodstream infections in both community and healthcare settings, and S. aureus bacteremia (SAB) is associated with significant morbidity and mortality [1]. Patients with diabetes mellitus (DM) may have increased susceptibility to S. aureus colonization and infection compared with the general population, and DM has been identified as an independent risk factor for developing SAB [2, 3]. Several cohort studies have shown the prevalence of diabetes in patients with SAB to be substantial, with varying rates (20–40%) among populations [4].
Metformin, a widely used first-line oral antidiabetic drug for type 2 DM, has recently received increasing attention as a potential anti-infective agent [5]. Although the mechanisms underlying the beneficial effects of metformin beyond its glucose-lowering action are not fully understood, a number of laboratory and clinical studies have suggested that metformin may have protective and therapeutic roles in a wide range of infectious diseases [5]. A recent meta-analysis of five retrospective cohort studies indicated that metformin use might be associated with lower mortality in patients with sepsis [6].
There are few in vitro and animal studies that have investigated the effects of metformin on the pathogenesis of S. aureus infection, showing that metformin inhibits glucose-mediated bacterial growth in airway epithelium [7]. However, to our knowledge, no human studies evaluating the association between metformin and SAB have been published. Therefore, we conducted a cohort study to evaluate the clinical impact of metformin exposure during SAB in patients with DM.
Materials and methods
Study design and patient selection
This observational cohort study was performed at the Asan Medical Center, a 2700-bed tertiary care teaching hospital in Seoul, Republic of Korea. From January 2016 through December 2018, patients aged ≥ 16 years with SAB were enrolled and followed up according to the study protocol over 12 weeks. Only the first episode of SAB in each patient was included in the analysis. Patients with polymicrobial bacteremia were excluded. The study was approved by the Asan Medical Center Institutional Review Board.
Data collection and definitions
The study data were derived from a prospective registry-based SAB cohort. All medical records were reviewed using standardized study protocols. Demographic characteristics, underlying diseases or conditions, laboratory results, site of infection, patient management, and clinical outcomes were evaluated. Patient-reported information about antidiabetic medication history during the previous month in electronic medical records was collected retrospectively. Additionally, we used primary care prescription records provided by referring physicians.
Metformin exposure was defined as receiving metformin or metformin-containing drugs during SAB, regardless of metformin use before the onset of bacteremia. Diabetic patients who received metformin during SAB were classified as diabetes with metformin exposure group. Diabetic patients who received metformin only before the onset of SAB or those who did not receive metformin were classified as diabetes without metformin exposure group. Site of acquisition was classified as community-onset (community-associated or healthcare-associated) and nosocomial, as previously described [8]. Site of infection was determined based on clinical, radiological, and microbiological investigations. Empirical antibiotic therapy was considered inappropriate if an antibiotic given within 24 h of the index blood culture was not active against the isolated organism. Persistent bacteremia was defined as bacteremia for ≥ 7 days while receiving appropriate antibiotic therapy. Recurrent bacteremia was defined as a subsequent episode of bacteremia within 30 days after discontinuation of antibiotic therapy. The primary outcome was 30-day all-cause mortality.
Microbiological data
All S. aureus isolates were identified using standard methods. Antimicrobial susceptibilities were determined using the MicroScan system (Dade Behring, West Sacramento, CA, USA) and the standard criteria of the Clinical and Laboratory Standards Institute. Methicillin resistance was confirmed by polymerase chain reaction detection of the mecA gene. The minimum inhibitory concentration (MIC) of vancomycin was determined using the Etest (AB Biodisk, Solna, Sweden) according to the manufacturer’s instructions. Staphylococcal cassette chromosome mec (SCCmec) type, multilocus sequence type (MLST), and agr genotype were identified using previously described methods [9,10,11]. Clonal complexes (CCs) were assigned to groups of isolates sharing six of seven alleles by use of eBURST (http://eburst.mlst.net).
Statistical analysis
Categorical variables were analysed using the chi-square or Fisher’s exact test, and continuous variables were analysed using Student’s t-test, the Mann–Whitney U test, or Kruskal–Wallis test, as appropriate. Risk factors associated with mortality were assessed using multivariate logistic regression analysis. All variables with statistical significance in the univariate analysis were included in the multivariate analysis. The final model was constructed using the backward elimination method. Survival analysis was conducted using the Kaplan–Meier method, and 30-day cumulative survival was compared using the log-rank test. All statistical analyses were performed using SPSS for Windows, version 25.0 (IBM Corp., Armonk, NY, USA), with p < 0.05 considered statistically significant.
Results
Study population and patient characteristics
A total of 452 patients with SAB were identified during the study period. Of these, 51 (11.3%) were classified in Group A (diabetes with metformin exposure), 115 (25.4%) in Group B (diabetes without metformin exposure), and 286 (63.3%) in Group C (no diabetes) (Fig. 1).
The baseline characteristics of these patients are shown in Table 1. The median age was 64 years (interquartile range [IQR], 54–72 years), and 262 (58.0%) were male. The site of acquisition of bacteremia was classified as community-associated (n = 87 [19.2%]), healthcare-associated (n = 179 [39.6%]), and nosocomial (n = 186 [41.2%]). Hypertension (n = 192 [42.5%]) was the most common underlying comorbidity, followed by cancer (n = 182 [40.3%]), diabetes (n = 166 [36.7%]), immunosuppressive therapy (n = 123 [27.2%]), liver cirrhosis (n = 73 [16.2%]), chronic kidney disease (n = 59 [13.1%]), hematologic malignancy (n = 32 [7.1%]), heart failure (n = 30 [6.6%]), alcoholism (n = 28 [6.2%]), solid organ transplantation (n = 27 [6.0%]), and neutropenia (n = 20 [4.4%]). The primary sites of infection were as follows: catheter-associated (24.3%), osteoarticular (11.7%), skin and soft tissue (10.0%), endovascular (9.5%), and unknown (17.5%). Age, gender distribution, Charlson Comorbidity Index, APACHE II score, as well as rates of methicillin resistance, hypertension, liver cirrhosis, chronic kidney disease, heart failure, septic shock, and skin and soft tissue infection, were significantly different among the three groups. Compared with Group A, the rates of alcoholism were higher in Group B (0% [0/51] versus 7.8% [9/115]; p = 0.06) and Group C (0% [0/51] versus 6.6% [19/286]; p = 0.09), although these differences did not reach statistical significance. Body mass index (BMI) and serum lactate concentration at the onset of bacteremia were comparable among the three groups. The median serum glucose level at the onset of bacteremia was significantly higher in diabetic patients than in non-diabetic patients (185 mg/dL versus 122 mg/dL, p < 0.001), with no statistically significant difference between diabetic patients with metformin exposure and those without metformin exposure (199 mg/dL versus 169 mg/dL, p = 0.48). The mean glycosylated hemoglobin (HbA1c) level was significantly higher in Group A than in Group B (8.1% versus 7.0%, p = 0.03). The rates of insulin use during SAB were similar between Group A and Group B (58.8% versus 70.4%, p = 0.14).
Clinical outcomes
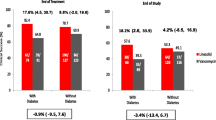
Figure 2 shows the 30-day mortality, persistent bacteremia, and recurrent bacteremia rates of 452 patients with SAB. A total of 68 patients died, resulting in a crude mortality rate of 15.0%. The mortality rate in Group A was significantly lower than that in Group B (3.9% [2/51] versus 14.8% [17/115]; p = 0.04) as well as that in Group C (3.9% [2/51] versus 17.1% [49/286]; p = 0.02). The mortality rates did not differ between Group B and Group C (14.8% [17/115] versus 17.1% [49/286]; p = 0.57). The rates of persistent and recurrent bacteremia were comparable among the three groups.
Clinical outcomes of 452 patients with Staphylococcus aureus bacteremia stratified by diabetes mellitus status and metformin exposure. Note: For persistent bacteremia, 8 patients (1 in the Group A, 1 in the Group B, and 6 in the Group C) were excluded from the analysis due to inadequate follow-up blood cultures
The Kaplan–Meier survival analysis showed significant differences in 30-day cumulative survival between Group A and Group B (p = 0.046) and between Group A and Group C (p = 0.02) (Fig. 3).
Risk factors associated with 30-day mortality
The risk factors associated with 30-day mortality in 452 patients with SAB are shown in Table 2. In the univariate analysis, age, cancer, liver cirrhosis, Charlson Comorbidity Index, APACHE II score, septic shock, serum lactate concentration, and metformin exposure were identified as significant variables associated with mortality. The methicillin resistance rates were not different between patients who survived and those who died (46.9% [180/384] versus 47.1% [32/68]; p = 0.98). DM was not associated with an increased risk of death. Multivariate analysis indicated that Charlson Comorbidity Index (adjusted odds ratio [aOR], 1.23; 95% confidence interval [CI], 1.08–1.40; p = 0.001), APACHE II score (aOR, 1.06; 95% CI, 1.02–1.10; p = 0.004), and metformin exposure (aOR, 0.20; 95% CI, 0.04–0.88; p = 0.03) were significantly associated with mortality.
Microbiological findings
The microbiological characteristics of the 452 S. aureus isolates are summarized in Table 3. Six CCs accounted for 93.2% of isolates (CC8 [42.0%], CC5 [30.1%], CC1 [10.6%], CC15 [4.6%], CC30 [3.5%], and CC97 [2.4%]). ST72 (34.1%) and ST5 (23.2%) were the major clonal types. Of the 208 isolates (46.0%) that were available for SCCmec data, the rates of SCCmec type III were significantly different among the three groups. The rates of agr subgroup and agr dysfunction were comparable among the three groups. The overall vancomycin MIC distribution was as follows: MIC ≤ 1.0 mg/L, 185 isolates (40.9%); MIC of 1.5 mg/L, 216 isolates (47.8%), and MIC ≥ 2.0 mg/L, 51 isolates (11.3%). The antimicrobial susceptibility testing to non-β-lactam antibiotics showed no differences among the three groups except for ciprofloxacin.
Discussion
There are several reports of the possible benefits of metformin use for patients with sepsis and bacteremia [6]. However, to our knowledge, this was the first study evaluating the impact of metformin exposure during SAB on clinical outcomes in patients with DM. In our single-center observational cohort study, the overall 30-day mortality of SAB was 15.0%. More than one-third of patients had diabetes, and DM itself was not associated with an increased risk of death. Among diabetic patients, 30.7% (51/166) received metformin therapy during SAB. Most importantly, we found that metformin exposure during SAB was an independent factor for predicting survival in patients with diabetes. The mortality benefit was observed despite higher serum HbA1c levels at the onset of bacteremia in the metformin-exposed group.
Metformin, a biguanide oral hypoglycemic agent, is thought to exert its metabolic action primarily through the inhibition of hepatic gluconeogenesis [12]. Although the precise mechanisms remain ill-defined, respiratory complex I and glycerophosphate dehydrogenase are proposed as key molecular targets of metformin in mitochondria [13, 14]. More recently, the intestinal tract has been implicated as an important metformin’s extrahepatic target. Metformin-induced alterations of gut microbiota composition and function may contribute to improved glucose metabolism [15]. Furthermore, accumulating evidence suggests that metformin possesses anti-infective and/or anti-inflammatory properties beyond its glucose-lowering action. The observed pleiotropic effects of metformin on various infectious and non-infectious conditions have prompted active investigations into its therapeutic applications for diabetic and non-diabetic populations [5, 16, 17].
There are still areas of uncertainty about how metformin acts against microorganisms, and the relative contributions of glycemic control, immunomodulatory effect, and direct antimicrobial activity are not yet clear [5]. Metformin reduces the hyperglycemia-induced proliferation of bacteria, such as S. aureus or Pseudomonas aeruginosa, in airway epithelium [7, 18]. Additionally, metformin can inhibit the expression of pro-inflammatory mediators and ameliorate endotoxin-induced tissue damage associated with sepsis [5]. However, clinical studies have shown inconsistent results regarding the association between preadmission metformin use and mortality among septic patients with DM [6]. Retrospective study designs, small sample sizes, and confounding variables were considered as limitations of those studies.
Compared with sepsis studies, our study focused on the association between metformin and SAB, and only 11.3% (51/452) of patients had septic shock. We defined metformin exposure as receiving metformin during SAB, regardless of previous metformin use, thereby assessing the benefits of metformin therapy during bacteremia. Thus, it is not clear whether metformin use before the onset of SAB can affect outcomes. Nevertheless, we believe that our findings, combined with those of previous studies, add further evidence supporting the positive roles of metformin in bacterial bloodstream infections.
Metformin is contraindicated for patients with moderately to severely impaired kidney function because of concerns about lactic acidosis [19]. It is thus possible that the metformin non-exposed groups in our study may represent patients with risk factors for poor outcomes, such as more advanced diabetes with multiple comorbidities. However, when subgroup analysis excluding the patients (n = 96) with estimated glomerular filtration rates < 30 mL/min/1.73 m2 was performed, metformin exposure (aOR, 0.19; 95% CI, 0.04–0.87; p = 0.03) remained significantly associated with reduced mortality (data not shown). This indicates that the association between metformin and SAB was not likely confounded by differences in renal function among the groups.
Interestingly, there was no significant link between DM and the risk of death from SAB in our cohort. It is generally believed that infections are increasing in frequency and severity in diabetic patients, and these patients may have an excess risk of death owing to infection-related causes when compared with those without diabetes [20, 21]. Population-based studies have revealed DM to be associated with about a two- to three-fold increased risk of developing SAB, and the odds vary according to the type, duration, and severity of DM [2, 3]. By contrast, whether DM can contribute to poor SAB outcomes has been debated [3, 4]. A Danish group reported an increased risk of mortality after SAB in association with DM without complications but not in association with DM with complications [3]. In a pooled analysis of prospective cohort studies, DM was not associated with poorer SAB outcomes [4]. Although the reasons for the discrepant findings are not clear, our data emphasize a possible influence of metformin on SAB-associated mortality, which should be accounted for in future research.
This study had several limitations. First, it was conducted at a single tertiary care hospital in South Korea, meaning that our findings may not be generalizable to different institutions or population groups. Second, some retrospective data about previous antidiabetic medication history were included. However, as we defined metformin exposure as receiving metformin during SAB, misclassification of metformin-exposed patients as metformin non-exposed was unlikely. Third, we were unable to collect prescription data about classes of oral antidiabetic drugs other than metformin; neither were we able to collect data about co-medications with immunomodulatory effects, such as statins. Therefore, an assessment of the influence of those potentially significant covariates on clinical outcomes could not be performed. Fourth, our data were limited by the lack of detailed information about various DM-related characteristics (type, duration, complication, etc.), and the reasons for no metformin use or the discontinuation of metformin during SAB (lack of efficacy, side effects, concomitant illnesses, etc.) in the diabetes without metformin exposure group could not be specified. Fifth, some missing values regarding patients’ BMI, serum lactate, serum glucose, and serum HbA1c data might be sources of bias. Sixth, apart from the presence of comorbidities, pre-hospital self-care could affect in-hospital outcomes. Health behaviors such as tobacco and alcohol use might be markers of self-care status. Unfortunately, smoking data were unavailable in our study. The higher rates of alcoholism in the metformin non-exposed groups might partly reflect poor self-care in these groups. In contrast, serum HbA1c levels were higher in the diabetes with metformin exposure group, suggesting relatively decreased treatment adherence in this group, although serum glucose levels were not different between diabetic patients with or without metformin exposure. Assuming that defining self-care status is complex, there might remain the possibility of unmeasured confounders. Seventh, if metformin use and other unrelated risk factors (which are also related to mortality) each have influence on developing SAB, then a spurious association between metformin use and mortality could be created (i.e., collider bias). Taken together, with other unidentified confounding factors not listed above, our findings must be interpreted with caution, and additional high-quality research is required to validate the association between metformin and SAB.
In conclusion, metformin exposure during SAB appears to be an independent factor for predicting survival among patients with diabetes. Given the novel immunomodulatory roles of metformin as well as its well-established efficacy, good safety profile, and relatively low cost, further exploration is warranted to repurpose metformin as a host-directed therapy.
Data availability
The datasets generated during the current study are not publicly available as they contain health related data but limited datasets (without any identifiable, person-related data) are available from the corresponding author upon reasonable request.
References
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. https://doi.org/10.1128/CMR.00134-14
Smit J, Sogaard M, Schonheyder HC, Nielsen H, Froslev T, Thomsen RW (2016) Diabetes and risk of community-acquired Staphylococcus aureus bacteremia: a population-based case-control study. Eur J Endocrinol 174:631–639. https://doi.org/10.1530/EJE-16-0023
Hansen MU, Gotland N, Mejer N, Petersen A, Larsen AR, Benfield T, Danish Staphylococcal Bacteremia Study Group (2017) Diabetes increases the risk of disease and death due to Staphylococcus aureus bacteremia. A matched case-control and cohort study. Infect Dis (Lond) 49:689–697. https://doi.org/10.1080/23744235.2017.1331463
Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG, Hellmich M, Hopkins S, Kern WV et al (2014) Staphylococcus aureus bloodstream infection: A pooled analysis of five prospective, observational studies. J Infect 68:242–251. https://doi.org/10.1016/j.jinf.2013.10.015
Malik F, Mehdi SF, Ali H, Patel P, Basharat A, Kumar A, Ashok F et al (2018) Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev 34:e2975. https://doi.org/10.1002/dmrr.2975
Liang H, Ding X, Li L, Wang T, Kan Q, Wang L, Sun T (2019) Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care 23:50. https://doi.org/10.1186/s13054-019-2346-4
Garnett JP, Baker EH, Naik S, Lindsay JA, Knight GM, Gill S, Tregoning JS et al (2013) Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax 68:835–845. https://doi.org/10.1136/thoraxjnl-2012-203178
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W et al (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. https://doi.org/10.7326/0003-4819-137-10-200211190-00007
Oliveira DC, de Lencastre H (2002) Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 46:2155–2161. https://doi.org/10.1128/AAC.46.7.2155-2161.2002
Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG (2000) Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38:1008–1015. https://doi.org/10.1128/JCM.38.3.1008-1015.2000
Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, Nesme X et al (2002) Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun 70:631–641. https://doi.org/10.1128/IAI.70.2.631-641.2002
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia 60:1577–1585. https://doi.org/10.1007/s00125-017-4342-z
Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348:607–614. https://doi.org/10.1042/bj3480607
Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ et al (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510:542–546. https://doi.org/10.1038/nature13270
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, Stahlman M et al (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23:850–858. https://doi.org/10.1038/nm.4345
Schuiveling M, Vazirpanah N, Radstake TRDJ, Zimmermann M, Broen JCA (2018) Metformin, a new era for an old drug in the treatment of immune mediated disease? Curr Drug Targets 19:945–959. https://doi.org/10.2174/1389450118666170613081730
Lv Z, Guo Y (2020) Metformin and its benefits for various diseases. Front Endocrinol (Lausanne) 11:191. https://doi.org/10.3389/fendo.2020.00191
Patkee WRA, Carr G, Baker EH, Baines DL, Garnett JP (2016) Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth. J Cell Mol Med 20:758–764. https://doi.org/10.1111/jcmm.12784
Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK (2014) Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 312:2668–2675. https://doi.org/10.1001/jama.2014.15298
Magliano DJ, Harding JL, Cohen K, Huxley RR, Davis WA, Shaw JE (2015) Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care 38:1274–1280. https://doi.org/10.2337/dc14-2820
Zoppini G, Fedeli U, Schievano E, Dauriz M, Targher G, Bonora E, Corti MC (2018) Mortality from infectious diseases in diabetes. Nutr Metab Cardiovasc Dis 28:444–450. https://doi.org/10.1016/j.numecd.2017.12.007
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C1234). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
This study was conceived and designed by JYL and YSK. JYL, ESK, EC, SB, and JJ acquired the data. YJL, MJK, and YPC analysed and interpreted the data. The study was supervised by SHK, SHC, SOL, and YSK. YJL drafted the article, which was critically revised by SHK, SHC, SOL, and YSK.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Asan Medical Center Institutional Review Board.
Consent to participate
Not applicable.
Consent to publish
All authors gave their consent for publication.
Competing interests
There are no potential conflicts of interest for any authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J.Y., Kim, E.S., Chang, E. et al. Clinical impact of metformin exposure during Staphylococcus aureus bacteremia in patients with diabetes mellitus. Eur J Clin Microbiol Infect Dis 42, 1439–1447 (2023). https://doi.org/10.1007/s10096-023-04679-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04679-6