Abstract
The aim of this study was to describe the epidemiology of methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia in a diabetic and a non-diabetic population of the University Hospital of Charleroi and to analyze medical outcomes, including risk of metastatic infection and mortality. Descriptive and multivariable analyses were performed using MedCalc 18.9 (MedCalc Software bvba, Ostend, Belgium). A total of 248 patients with MSSA bacteremia were identified between 1st January 2012 and 28th June 2017 out of which 32.7% were diabetic. Within the diabetic patients, we observed more prolonged hospital duration of stay (p = 0.034), more secondary bacteremia of cutaneous sources (including cellulitis, diabetic foot and ulcer) (p = 0.037), and more metastatic infection (p = 0.002). The overall 30-day mortality was 24.2% with no difference between the two groups. With a logistic regression analysis, it was demonstrated that age ≥ 60 years (odds ratio (OR), 2.20 (95% CI, 1.03–4.67)) and Charlson Comorbidity Index (CCI) ≥ 3 (OR, 2.95 (95% CI, 1.51–5.79)) were the only independent risk factors of mortality, while removal of the primary site of infection was a protective factor (OR, 0.27 (95% CI, 0.12–0.62)). Risk of developing metastatic infection was increased with diabetes (OR, 2.08 (95% CI, 1.12–3.90)), while early empirical antibiotic therapy (OR, 0.38 (95% CI, 0.20–0.71)) decreased this risk. Diabetes was not associated with increased 30-day mortality after MSSA bacteremia. However, diabetes increased significantly the risk of metastatic infection. An aggressive treatment of MSSA bacteremia seems crucial to improve the outcome of diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is a major cause of bacteremia, with a 30-day mortality of 20 to 40% [1,2,3,4]. Five percent of the Belgian population suffer from diabetes, which can increased susceptibility to Staphylococcus aureus bacteremia because of coexisting comorbidities and complications, tissue hyperglycemia, and decreased oxygenation, which generally lead to reduced immunity [5, 6].
Data regarding the prognostic factors of MSSA (methicillin-sensitive Staphylococcus aureus) bacteremia are conflicting, and to our knowledge, only few studies have investigated the impact of diabetes as a primary objective. Therefore, we conducted a retrospective observational study to describe the epidemiology of MSSA bacteremia in both diabetic and non-diabetic patients, and we investigated the impact of diabetes on the outcome and mortality of MSSA bacteremia.
Materials and methods
Study population
The study included all patients hospitalized between 1st January 2012 and 28th June 2017 in the University Hospital of Charleroi (Belgium) with a first episode of bacteremia with methicillin-sensitive Staphylococcus aureus. This multisite hospital has 1321 beds, including 44 ICU beds, with approximately 125 000 admissions per year. We limited inclusions to patients aged ≥ 15 years, hospitalized with complete medical records available, and who received a treatment.
Patients were identified in the database of microbiology laboratory with Infectio.Global® 3.0.1 (partner4lab, Nancy, France) and bacteremia characteristics were retrospectively collected.
Definitions
We defined an episode of MSSA bacteremia as a bacteremia in a patient who has had at least one MSSA-positive blood culture. Bacteremia was considered as community-acquired (CA) if it occurred ≤ 2 days after admission and as nosocomial thereafter [7].
Patients with community-acquired MSSA bacteremia and healthcare contact within 30 days of the current admission were sub-classified as healthcare-associated MSSA bacteremia (e.g., hospitalization, surgery, dialysis).
Source of bacteremia was determined according to the definitions of the National Surveillance Program for Hospital Infection (NSIH) [8]. Secondary bacteremia was sub-classified as cutaneous, postoperative wound, pulmonary, urinary, or joint-related. In our study, we defined diabetes as the presence of antidiabetic drugs on admission (oral antidiabetics and/or subcutaneous insulin), or new-onset diabetes defined according to usual biological criteria. The level of glycemic control was ascertained using the most recent glycated hemoglobin A1c (HbA1c) measurement within 1 year of the current hospitalization [1].
We used the Charlson Comorbidity Index (CCI) to identify the severity of previous comorbidity, which is a validated comorbidity scoring system [1, 9, 10]. We removed the diabetes from the CCI and designated the index as a modified CCI (m-CCI), according to Smit [1]. Level of comorbidity was defined as “high” if m-CCI was ≥ 3 [1].
We defined severe presentation as ICU admission, high APACHE II score, and/or lactatemia ≥ 2 mmol/L. Early empirical treatment was defined as an antibiotic therapy started before the result of the blood cultures, whether adapted to the antibiotic susceptibility test or not. Final treatment was considered as adequate when the antibiotic treatment included at least 14 days of intravenous treatment and was appropriate for the antibiotic susceptibility test.
Success was defined as negative blood culture and/or no sign of infection within 7 days of initiation of antibiotic therapy.
Bacteremia was considered as persistent when blood cultures remain positive after 48 h of appropriate antibiotic therapy.
We assessed all-cause 30-day mortality, intra-hospital mortality and bacteremia-related mortality.
Statistical analyses
Continuous variables were described using mean ± SD or median (interquartile range (IQR)) as appropriate. Discrete variables were reported using absolute values and percentages. Comparisons between two independent groups were performed using Student’s t tests (or Welch’s test for unequal variances) or the Mann-Whitney U tests as appropriate. Comparisons between proportions were assessed using chi-squared tests or exact Fisher’s tests as appropriate. Binary logistical regression analysis was performed to assess the relationship between binary outcomes and exposure variables using a stepwise approach. A p value < 0.05 was considered as statistically significant. All calculations were performed with IBM® SPSS® Statistics 22.0 and MedCalc® 18.6 (MedCalc Software bvba, Ostend, Belgium).
Results
Demographic data and characteristics of MSSA bacteremia are summarized and compared between diabetic and non-diabetic patients in Table 1. From 2012 to 2017, after withdrawal of multiple episodes and relapses, we identified 248 patients with first episode of MSSA bacteremia, of whom 81 (32.7%) had diabetes. Median HbA1c level was 7.1% (54 mmol/mol) (IQR, 6.5–7.9).
12.5% of patients were sent to the hospital after being examined by a general practitioner and 16.5% by a hospital clinician and 46% were admitted by the emergency department (25% was unknown—data not shown). We identified 70% of secondary bacteremia, whereas 50% of them were from soft-tissue infections such as cellulitis, diabetic foot, or ulcer.
Management and outcomes of MSSA bacteremia are represented in Table 2. Eradicable foci were removed in a majority of diabetic patients, by catheter removal or by surgery. Amputation was performed in more diabetic than non-diabetic patients (27.3% vs 3.7%; p = 0.008). We observed no difference between the 2 groups of patients in other type of surgeries (e.g., debridement, cardiac surgery, device removal) (data not shown).
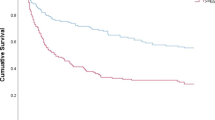
The 30-day mortality is 24.2% with no statically significant difference between the two groups.
Table 3 shows the multivariate analysis of 30-day mortality. An age ≥ 60 years and a m-CCI ≥ 3 increase the 30-day mortality risk. Neither diabetes nor HbA1c levels significantly influence 30-day mortality. The analysis was also conducted for attributable mortality and hospital mortality, showing identical results (data not shown). Table 4 presents the results of the multivariate analysis of factors influencing the risk of metastatic infection. Unlike mortality, age does not influence the risk of metastatic infection. Diabetes doubles the risk of metastatic infection (OR, 2.08 (1.12–3.90)); thus, the HbA1c level does not increase it.
Discussion
Our study including 248 episodes of MSSA bacteremia was conducted in the town of Charleroi, one of the most deprived strata of the region of Hainaut (Belgium) [11]. The rate of unemployment in the Hainaut in general (15.6%) and particularly in the area of the hospital (20.3%) is above the national mean (9.3%) and the mean annual income is also lower in the Hainaut (26 530€) than in Belgium in general (31 655€) [11]. This socio-economic situation (which is known to impact patient’s infection risk [12]) could explain that our patients are younger than patients included in other similar studies [7, 13,14,15] and that we have 32.7% of diabetic patients, about 10% more than the fraction reported in other studies (23 to 30%) [7,8,9,10].
The polypathological profile of patients with diabetes could explain the proportion of long hospital stays for these patients and the fact that around 30% of episodes are nosocomial [7, 8, 13, 16, 17].
In our study, nosocomial acquired infection has no influence on 30-day mortality. Kaech et al. showed that the nosocomial bacteremia is associated independently with a better prognosis (OR 0.4 (0.2–0.7)), and that the main reason for the better outcome is the earlier detection of bacteremia in hospital than in the community [3].
We observe 70% of secondary bacteremia and more than 30% of cutaneous source of infection, which is higher than other results found in literature (respectively 26 to 50–60% and 9.3 to 20%) [8, 14,15,16, 18,19,20,21]. Patients could have poorly treated cutaneous infections sources, in a context of precarious hygiene, and could have only come to hospital when the symptomatology was severe.
Interestingly, patients with diabetes did not have significantly higher ICU admission rates, APACHE II Score, or lactatemia, a marker of septic shock. These data show that diabetic patients do not have a more severe clinical presentation of MSSA bacteremia.
The rate of metastatic infection is significantly higher in our diabetic population, as reported by Kanafani et al. [9]. Diabetes promotes infection and therefore metastatic spread by altering immune defenses in hyperglycemic environment and by breaking the skin barrier, via diabetic foot ulcers and vascular disorder [1, 19]. Osteoarticular complications are well described in diabetic patients, with a 4-fold higher risk of osteomyelitis than in the non-diabetic patients [21].
We only found one study about the prognostic factors of metastatic infection, and it contradicts our findings. According to Fowler et al., neither diabetes nor empirical antibiotic therapy influences the risk of complications [17]. Our data suggests that early empirical antibiotic therapy protects from developing metastatic infection.
Removal of the original source of infection reduces the risk of death to 30 days. In our study, aggressive surgical treatment (such as amputation or surgical debridement of all devitalized tissues) is crucial in decreasing mortality.
Thirty-day mortality is 24.2% and attributable mortality 17.7%; these data are consistent with those found in literature [3, 15, 16]. However, compared with a Belgian study of 2007 and with Fowler et al., we observe a much lower attributable mortality (respectively 17.7% vs 32% and 17.7% vs 28%) [13, 17]. This difference in our study could be explained by the initial presentation of our patients directly in a hospital center (46% of patients arrived from the emergency department and 16.5% from a specialist consult) and not from general practitioner (which represents 12.5% of patients in our cohort), but also by the inclusion of MRSA bacteremia in the 2 other studies. In our study, age ≥ 60 years doubles the risk of 30-day mortality, which is well described in the literature [2, 10, 20].
Mortality is not higher in diabetic patients [15, 16, 18]. Unlike our finding, other studies show a significant difference in mortality in diabetic patients, including Kanafani et al. (22.1% vs 11.4%) [9, 20]. Moreover, diabetes is not an independent prognostic factor for mortality in the general population [2, 22]. But Mylotte et al. describe a 2.5 times greater risk of death at 30 days in diabetic patients (OR 2.4 (1.2–4.7)) [20], which is confirmed by Hansen et al. in 2017 (OR 1.61 (1.00–2.57)) [4].
In our study, the absence of correlation between diabetes and mortality may come from the fact that the patients, often for financial reasons, diabetic or not, attend hospital emergency departments from the outset, resulting in rapid intravenous antibiotic therapy. Moreover, in this impoverished population, diabetic patients are more medicalized than non-diabetic patients (e.g., free consultations organized for the diabetic patients by the Belgian health insurance system), which is proven by the relatively low level of HbA1c in our diabetics patients.
Our study might have some limitations. We have not identified all the characteristics of diabetes, such as the duration of disease, the differentiation between type 1 and type 2, and the associated complications. However, Smit et al. showed in 2016 that none of these characteristics influence the prognosis of MSSA bacteremia [15].
We focused on the clinical aspect of MSSA bacteremia and did not collect microbiological data regarding MSSA such as the detailed antibiotic susceptibility test or the presence of some virulence factors. The retrospective and monocentric characteristics of our study are also a weakness, but it is a long-term study (over 5 years) including a large number of episodes of MSSA bacteremia.
Conclusion
Our study of 258 episodes of Staphylococcus aureus methicillin-sensitive bacteremia at the University Hospital of Charleroi (Belgium) demonstrated that diabetic patients had statistically more cutaneous secondary bacteremia and a more frequent prolonged (≥ 15 days) hospital stay and developed more metastatic infection than non-diabetic patients, although those diabetic patients did not have more severe clinical presentations upon admission.
Diabetes did not appear to be an independent predictor of mortality; however, this could be explained by the free access to the hospital emergency department and with the free consultations provided to the diabetic patients by the Belgian healthcare insurance system, which enabled rapid diagnosis of infection and provided intravenous antibiotic therapy. Our study also highlighted that 30-day mortality risk factors are age ≥ 60 years and presence of comorbidities, shown by a m-CCI ≥ 3. In contrast, removal of the original source of infection reduced the risk of mortality. MSSA bacteremia was associated with an increased risk of metastatic infection in patients with diabetes.
Finally, our study showed that early initiation of empirical antibiotic therapy and eradication of the site of infection seemed capital to reduce mortality and metastatic infection complications.
References
Smit J (2017) Community-acquired Staphylococcus aureus bacteremia: studies of risk and prognosis with special attention to diabetes mellitus and chronic heart failure. Dan Med J 64:5
Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG, Hellmich M, Hopkins S et al (2014) Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Inf Secur 68(3):242–251
Kaech C, Elzi L, Sendi P, Frei R, Laifer G, Bassetti S et al (2006) Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect 12(4):345–352
Hansen M-LU, Gotland N, Mejer N, Petersen A, Larsen AR, Benfield T et al (2017) Diabetes increases the risk of disease and death due to Staphylococcus aureus bacteremia. A matched case-control and cohort study. Infect Dis (Lond) 49(9):689–697
Smit J, Søgaard M, Schønheyder HC, Nielsen H, Frøslev T, Thomsen RW (2016) Diabetes and risk of community-acquired Staphylococcus aureus bacteremia: a population-based case-control study. Eur J Endocrinol 174(5):631–639
User S. IDF diabetes atlas - across the globe [Internet]. [cited 2018 Feb 24]. Available from: http://www.diabetesatlas.org/across-the-globe.html
Thwaites GE, Scarborough M, Szubert A, Nsutebu E, Tilley R, Greig J et al (2018) Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 391(10121):668–678
Bassetti M, Trecarichi EM, Mesini A, Spanu T, Giacobbe DR, Rossi M et al (2012) Risk factors and mortality of healthcare-associated and community-acquired Staphylococcus aureus bacteraemia. Clin Microbiol Infect 18(9):862–869
Kanafani ZA, Kourany WM, Fowler VG, Levine DP, Vigliani GA, Campion M et al (2009) Clinical characteristics and outcomes of diabetic patients with Staphylococcus aureus bacteremia and endocarditis. Eur J Clin Microbiol Infect Dis 28(12):1477–1482
Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR et al (2009) Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust 191(7):368–373
Altas socio-économique de Charleroi et Sud Hainaut (3ème édition, 2017), Intercommunal pour la Gestion et la Réalisation d’Etudes Techniques et Economiques - IGRETEC
van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB (2012) Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 25(2):362–386
Libert M, Elkholti M, Massaut J, Karmali R, Mascart G, Cherifi S (2008) Risk factors for meticillin resistance and outcome of Staphylococcus aureus bloodstream infection in a Belgian university hospital. J Hosp Infect 68(1):17–24
del Rio A, Cervera C, Moreno A, Moreillon P, Miró JM (2009) Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin Infect Dis 48(Suppl 4):S246–S253
Smit J, Thomsen RW, Schønheyder HC, Nielsen H, Frøslev T, Søgaard M (2016) Outcome of community-acquired Staphylococcus aureus bacteraemia in patients with diabetes: a historical population-based cohort study. PLoS One 11(4):e0153766
Rieg S, Peyerl-Hoffmann G, de With K, Theilacker C, Wagner D, Hübner J et al (2009) Mortality of S. aureus bacteremia and infectious diseases specialist consultation--a study of 521 patients in Germany. J Inf Secur 59(4):232–239
Fowler VG, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB et al (2003) Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 163(17):2066–2072
Lesens O, Methlin C, Hansmann Y, Remy V, Martinot M, Bergin C et al (2003) Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: a prospective study using the Charlson weighted index of comorbidity. Infect Control Hosp Epidemiol 24(12):890–896
Bertoni AG, Saydah S, Brancati FL (2001) Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care 24(6):1044–1049
Mylotte JM, Tayara A (2000) Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis 31(5):1170–1174
Shah BR, Hux JE (2003) Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 26(2):510–513
Big C, Malani PN (2010) Staphylococcus aureus bloodstream infections in older adults: clinical outcomes and risk factors for in-hospital mortality. J Am Geriatr Soc 58(2):300–305
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The study was approved by the ethic committee of the University Hospital of Charleroi on 27th September 2017 (CCB: B325201733405).
Informed consent
There was no informed consent because the author conducted a retrospective study with de-identified data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vanderschelden, A., Lelubre, C., Richard, T. et al. Outcome of methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia: impact of diabetes. Eur J Clin Microbiol Infect Dis 38, 2215–2220 (2019). https://doi.org/10.1007/s10096-019-03659-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03659-z