Abstract
Previous reports have associated hyperglycemia to poor outcome among aged and comorbid Staphylococcus aureus bacteraemia (SAB) patients. However, the prognostic impact of hyperglycemia in SAB irrespective of age and underlying conditions including a diagnosis of diabetes has received little attention. The objective here was to evaluate the prognostic relevance of hyperglycemia at onset of methicillin-sensitive SAB (MS-SAB). It was a retrospective study of MS-SAB patients. Blood glucose was measured within 24 h of positive blood cultures. The patient cohort was analyzed en bloc and by categorization according to age, underlying conditions and a diagnosis of diabetes. Altogether 161 patients were identified. High initial blood glucose levels were observed among diabetics (p < 0.001), patients with deep infections (p < 0.05) and poor outcome at 28- or 90-days (p < 0.05). Receiver operating characteristics presented the glucose cut-off level of 7.2 mmol/L as a significant predictor of mortality with an area under the curve of 0.63 (95% CI 0.52–0.75, p < 0.05). Blood glucose ≥7.2 mmol/L connected to higher 28- (9 vs. 20%, p < 0.05) and 90-day (14 vs. 29%, p < 0.01) mortality. In Cox proportional hazard regression the blood glucose cut-off value of 7.2 mmol/L significantly predicted 90-day mortality (HR, 2.12; 95% CI, 1.01–4.46; p < 0.05). Among young and healthy non-diabetics the negative prognostic impact of high glucose was further accentuated (HR 7.46, p < 0.05). High glucose levels had no prognostic impact among diabetics. Hyperglycemia at SAB onset may associate to poor outcome. The negative prognostic impact is accentuated among young and healthy non-diabetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is one of the leading bloodstream pathogens and a major cause of both community- and healthcare-associated bacteraemias (SAB) [1, 2]. Parameters such as severe sepsis [3], deep infection foci, e.g. endocarditis [4, 5] and methicillin-resistance [4, 6], may impair prognosis whereas infectious disease specialist consultation [4, 7, 8] is known to improve outcome. Previous studies have demonstrated a deep infection focus in up to 70% of SAB patients [5, 7]. However, despite identification of prognostic parameters and introduction of novel antibiotics [9] the mortality rates remain high and range from 12 to 35% in recent reports [4, 10, 11].
Severe infections may induce a hyper-metabolic state and hyperglycemia [12, 13] through mechanisms such as elevated cytokine and cortisol levels [14, 15] and insulin deficiency [16]. Hyperglycemia exerts various harmful effects including a pro-coagulant action [17] and altered polymorphonuclear leukocyte functions which may hamper leucocyte migration, adherence, phagocytosis and intracellular killing [18–20]. A negative prognostic impact of hyperglycemia has been observed in various infectious diseases such as pneumonia in diabetics [21] and non-diabetics [22, 23] as well as bacteraemia among intensive care unit (ICU) patients [13] and more specifically due to S. aureus [11, 24, 25], Pseudomonas aeruginosa [26] and other gram-negative bacteria [27] and candidemia [28].
Previous evaluations on the prognostic impact of hyperglycemia in SAB have included aged and comorbid patients with considerable 44–54% occurrence of methicillin-resistance [11, 24, 25]. Blood glucose has been recorded either as mean levels during the first 7 days after SAB onset [24, 25] or on the day of admission or on the closest day before the blood cultures were drawn [11]. Furthermore, the statistical analyses have been performed with breakpoints for blood glucose results determined in advance [11, 24, 25]. However, high age, comorbidity and MRSA are well known risk factors for poor outcome [2, 4, 6, 8]. To the best of our knowledge the relevance of hyperglycemia in MS-SAB patient cohorts irrespective of age and underlying conditions including a diagnosis of diabetes has not been previously evaluated.
The objective of the present study was to evaluate the impact of hyperglycemia, at onset of MS-SAB, on disease progression and prognosis. We applied receiver operating characteristics analysis to identify a statistically significant blood glucose cut-off value for outcome prediction. Lack of methicillin-resistant S. aureus (MRSA) enabled us to avoid the impact of differences in empirical antibiotic therapy.
Methods
Study population
This was a retrospective study recruiting all adult patients (n = 342) from Helsinki University Central Hospital in Finland with at least one positive blood culture for S. aureus during 2000–2002 and 2006–2007. Two time-periods were included to exclude any unknown temporary differences in personnel or treatment practices. Patients and corresponding S. aureus isolates were matched by using the unique personal number given to all residents of Finland. Patient data were retrieved from both electronic (2006–2007) and written (2000–2002) hospital archives. Any possible disadvantage with either information storage pattern was accounted for by including both paper and electronic hospital records. Five cases of MRSA bacteraemia were omitted. Patient records were followed for 90 days. Data collection included gender, age, bacteraemia acquisition, underlying diseases, and length and administration route of any antibiotic therapy. Furthermore, infection focus documentation was based on clinical suspicion or verified by radiological, bacteriological, or pathological investigations. Laboratory results and time to defervescence (axillary temperature below 37.5 °C) were recorded. Primary endpoint was mortality at 28 or 90 days. Secondary endpoints were prevalence of deep infection foci, time to defervescence and length of hospitalization.
Definitions
Community- and healthcare-associated SAB have been defined previously [7]. Modified Duke criteria were applied to define endocarditis [29]. Sepsis in connection with hypotension, hypo-perfusion, or organ failure was classified as severe sepsis whereas sepsis with arterial hypotension despite adequate fluid resuscitation was defined as septic shock [30]. McCabes’s criteria were used to classify severity of underlying diseases into healthy and nonfatal or ultimately and rapidly fatal [31]. Infectious disease specialist consultations within 7 days of the first positive blood cultures for S. aureus were documented and categorized into (1) formal bedside consultation, (2) informal telephone consultation or (3) no consultation [7].
Antibiotic therapy
Semisynthetic penicillin was defined as the standard antibiotic therapy. Cefuroxime, clindamycin or vancomycin was given to patients with contradictions for penicillin. Rifampicin and fluoroquinolone were provided as additional antibiotic therapy. Proper length of antibiotic therapy was defined as intravenous administration for at least 28 days for deep infection focus and at least 14 days in the absence of any deep infection. Antibiotic indications, dosage and administration routes have been provided in detail previously [32, 33].
Blood glucose
Blood glucose was measured in connection with, shortly before or shortly after blood culture collecting time-points such that all glucose samples were measured within 24 h of blood culture collection.
Statistical analysis
Data is presented either as absolute values and percentages or as median and interquartile ranges (IQR, 25th and 75th percentiles). Categorical variables are compared with Pearson’s X2 -test whereas non-parametric data is analyzed with Mann–Whitney u-test or Students t-test. Odds ratios (OR) with 95% confidence intervals (CI) were calculated. Discriminative power of blood glucose in predicting 90-day mortality was evaluated by receiver operating characteristic (ROC) curves. The Youden index was defined as the point on the ROC-curve maximizing both sensitivity and specificity values equally to locate the cut-off point. The ROC-curve derived glucose cut-off point was applied for the Kaplan-Meier estimator method and for Cox proportional hazard regression model predicting mortality. Univariate factors with p < 0.05 were allowed for the Cox proportional hazard regression model. All tests were two-tailed and p < 0.05 was considered as significant. SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Patient characteristics and blood glucose concentrations
Altogether 342 patients were identified for the study but due to missing blood glucose samples the results of 161 patients are shown. Starting from the day of positive blood cultures each patient was provided with an antibiotic effective in vitro against the S. aureus blood isolate. Most patients received a β-lactam antibiotic whereas a minority was provided with vancomycin (2.5%). The majority of patients received formal bedside infectious disease specialist consultation (66%) whereas only 22% had informal telephone consultation.
The mean blood glucose level close to the time point of positive blood culture drawing was 8.35 ± 4.5 (±SD) mmol/L and the median blood glucose was 7.00 mmol/L. The distribution of blood glucose levels among patients is presented in Fig. 1. Patient demographics, bacteraemia acquisition or underlying conditions had no significant impact on blood glucose with the exception of diabetes that associated to significantly higher blood glucose levels (p < 0.001) (Table 1). Severity of illness according to the parameters of severe sepsis and ICU treatment did not influence the blood glucose level. A deep infection focus was diagnosed in 73% of patients and it was associated to significantly higher blood glucose levels (p < 0.05), although this trend was not observed for endocarditis (Table 1).
Outcome, defervescence and hospitalization
No significant association between blood glucose and time to defervescence was observed (Table 1). The total case fatality in 161 patients at 28 days was 15% and at 90 days 22%. Mean blood glucose levels were significantly higher among patients who died within 28 or 90 days (Table 1). The mean (±SD) time of hospitalization for patients that survived was 36 (±32) days.
Cut-off values for glucose in predicting mortality
By ROC-analysis blood glucose at the time-point for positive blood cultures was a significant predictor for mortality. The area under the curve (AUC) in the ROC analysis was 0.63 (95% CI 0.52–0.75, p < 0.05) and produced a cut-off value of 7.2 mmol/L that predicted 90-day mortality (Fig. 2).
Receiver operating characteristic (ROC) curve for blood glucose concentration (mmol/L) at blood culture collecting time-point for predicting 90-day mortality in patients with Staphylococcus aureus bacteraemia (n = 161). The area under the curve (AUC) was 0.63 (95% CI 0.52–0.75) (p < 0.05) and the optimal cut-off value 7.2 mmol/L with sensitivity of 69% and specificity of 58%
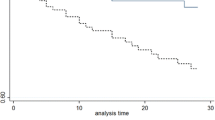
The whole patient cohort was stratified according to the blood glucose cut-off value of 7.2 mmol/L. A diagnosis of diabetes (OR 4.47, p < 0.001) was the only demographic parameter associated with blood glucose levels above the cut-off value (Table 2). The blood glucose cut-off level connected significantly to higher 28- (OR 3.01, p < 0.05) and 90-day (OR 3.12, p < 0.01) mortality. Moreover, patients with blood glucose < 7.2 mmol/L who survived had significantly shorter time of hospitalization as compared to those with blood glucose > 7.2 mmol/L (p < 0.05). Kaplan-Meier analysis for the whole patient cohort associated patients with blood glucose levels above the cut-off value of 7.2 mmol to significantly higher mortality (log-rank 0.005) (Fig. 3).
Parameters in univariate analysis with significantly elevated risk for 90-day mortality were severe sepsis (OR 3.35, p < 0.01), informal telephone infectious disease specialist consultation (OR 2.65, p < 0.05), blood glucose cut-off value of 7.2 mmol/L (OR 3.01, p < 0.01) and age > 60 years (OR 2.28, p < 0.05). In contrast, lack of severe underlying diseases (McCabe’s healthy or nonfatal classification) (OR 0.13, p < 0.001), formal bedside infectious disease specialist consultation (OR 0.15, p < 0.001) and rifampicin therapy for at least 14 days (OR 0.18, p < 0.001) were associated with better prognosis. In the Cox proportional hazard regression model, the independent prognostic parameters were age > 60 years (HR 3.78, p = 0.001), McCabe’s healthy or nonfatal classification (HR 0.26, p < 0.001), severe sepsis (HR 2.83, p < 0.01), rifampicin therapy for at least 14 days (HR 0.23, p = 0.001), formal bedside infectious disease specialist consultation (HR 0.26, p = 0.001) and a blood glucose cut-off value of 7.2 mmol/L (HR 2.12, p < 0.05) (Table 3).
Furthermore, the Cox proportional hazard regression model of Table 3 was re-performed with the patient cohort categorized according to age, underlying conditions and a diagnosis of diabetes. When including only previously healthy (McCabe’s healthy-nonfatal classification) non-diabetics (mean age 48.5 ± 17.5 years) the blood glucose cut-off value of 7.2 mmol/L associated with poor outcome in Cox proportional hazard regression model (HR 7.46, p < 0.05) (Table 4). However, when including only diabetic patients, irrespective of underlying conditions or age, the blood glucose cut-off value of 7.2 mmol/L had no prognostic impact.
Discussion
The main finding of the present study was a poor prognostic impact associated with hyperglycemia at the time of detection of SAB. During the 90-days follow-up patients with hyperglycemia within 24 h of the blood culture collection time-point had more than a 2-fold higher hazard ratio for a fatal outcome when adjusting for all other prognostic parameters. The negative prognostic impact of hyperglycemia was accentuated when including only young and previously healthy non-diabetics. However, hyperglycemia had no prognostic impact among patients with a diagnosis of diabetes.
Previous studies have identified parameters with indisputable prognostic impact in SAB such as old age and underlying conditions [2, 4, 6], severe sepsis [3] and infectious disease specialist guided therapy management [4, 7, 8]. Many studies report the occurrence of diabetes [2, 4, 5, 8, 24, 25, 33] or other factors that may influence glucose balance, e.g. corticosteroid therapy [2, 5, 8, 33]. However, few studies on SAB report separate laboratory tests, e.g. blood glucose, and to the best of our knowledge only three reports have evaluated the impact of glucose balance on outcome in SAB [11, 24, 25].
The results of the present study with a poor prognostic impact due to hyperglycemia at the initial phase of a severe infection are in line with many previous reports [11, 22–26, 28]. However, previous studies have applied various measurement time-points and different categorization patterns of blood glucose. This makes comparison of results challenging. Some authors report blood glucose levels at hospital admission or at the closest day before blood culture drawing [11, 22, 23] or report an average blood glucose concentration within 48 h of positive blood cultures [26]. In some reports the mean blood glucose levels were derived by averaging highest daily glucose values within the 7 days after infection onset [24, 25, 28] or blood glucose levels from one day prior until 5 days after onset of infection [13]. Most authors stratify results according to blood glucose levels exceeding 167–170 mg/dl (i.e. 9.3–9.4 mmol/L) [11, 24, 25] or the range of 6–13.9 mmol/L [23, 28]. The ROC analysis in the present study presented a glucose cut-off value of 7.2 mmol/L that is lower compared to the breakpoint values applied in previous reports [11, 24, 25, 28]; although one study applied an even lower value of 6.0 mmol/L [23]. To the best of our knowledge, the glucose breakpoint values in earlier reports have been determined a priori whereas one study applied a CART-analysis (classification and regression tree analysis) to define a breakpoint in the average concentration of blood glucose in the first 48 h after positive blood cultures [26]. Thus, as far as we know, the present study is the first to apply ROC analyses to identify a statistically significant blood glucose cut-off value for mortality in severe systemic infections such as SAB.
A recent report observed that hyperglycemia during the first 7 days among SAB patients resulted more often in discharge to a long-term care facility or inpatient rehabilitation [25]. The present study did not record discharge addresses. However, length of hospitalization among patients that survived was analyzed and hyperglycemia at the initial phase of SAB associated with significantly longer hospital stay. Furthermore, previous reports concluded that hyperglycemia may reflect infection severity and outcome in non-diabetic patients but not in diabetic patients [34, 35]. The results of the present study are in line with these two reports as the poor prognostic impact of hyperglycemia in SAB was accentuated among previously healthy non-diabetics whereas among diabetics the initial blood glucose levels had no influence on outcome.
To the best of our knowledge, this is the first study evaluating the connection between hyperglycemia determined within 24 h of positive blood cultures and prognosis among SAB patients. The patient cohort was stratified according to ROC analysis derived blood glucose cut-off value and attempts were made to control reasons for differences in outcome between the various groups. Many of the parameters with prognostic impact in the present study have been identified earlier, i.e. age and underlying conditions [2, 4, 6], severe sepsis [3], adjunctive rifampicin therapy [32] and infectious disease specialist consultation [7, 36].
Altogether 73% of patients had deep infection foci. This is in line with the studies presenting deep focus occurrence among 68–74% of SAB patients [5, 7]. Patients with a deep infection focus had significantly higher blood glucose levels, although this difference was not observed for patients with endocarditis nor when categorizing patients according to the ROC analysis related cut-off value of 7.2 mmol/L. Our study is not able to present any explanation for the association between hyperglycemia at the initial phase of SAB and presence of a deep infection focus, and diabetics were not overrepresented among patients with a diagnosed deep infection focus. Previous reports on hyperglycemia and SAB do not comment on any association between blood glucose and infection foci [11, 24, 25].
The present study deviates from previous reports on hyperglycemia and SAB with respect to age and comorbidity [11, 24, 25]. In the present study only one third of patients were aged over 60 years and almost three fourths had a healthy- or nonfatal McCabe’s classification regarding underlying conditions. However, in previous reports the mean age has ranged from 68 to 72 years with mean Charlson’s comorbidity index of 1.5–4.6 [11, 24, 25]. Thus, the results of the present study apply to a younger and less ill patient cohort as compared to previous reports.
The present study includes limitations that relate to its retrospective nature. First, parameters that may influence blood glucose were only partially documented. The retrospective study design did not enable documentation of any ongoing medications prior to blood culture collection. This is an evident weakness as it is well known that, in addition to diabetes related therapies like insulin, various other medications may cause blood glucose level fluctuations, e.g. non-steroidal anti-inflammatory drugs may alter insulin release from beta cells [37]. In addition, any infusion fluids administered in connection with blood culture collection were undocumented. Hence, as some infusion fluids contain glucose it is possible that the etiology of hyperglycemia during blood culture collection time-point in some patients may have been iatrogenic. Second, we recorded only one glucose measurement within 24 h of blood culture collection. The documentation of several glucose values would have enabled more precise analysis either (1) as an arithmetic mean of several values [26] or (2) as a time-weighted analysis [38]. Third, the blood glucose measurements, within 24 h of blood culture collection, were drawn irrespective of time and fasting, i.e. recent intake of food or liquids. Fourth, the magnitude of the n-number in the present study (161) is slightly higher than that of two previous reports (100–135) [24, 25], although lower than in a third study (340) [11] on hyperglycemia and SAB. Furthermore, the retrospective nature of the study did not allow for any glycemic control and hence further research is needed to determine whether continuous monitoring and control of elevated glucose levels influence the outcome of SAB.
In conclusion, despite the retrospective study design, low n-number and lack of information regarding background medication and fasting, the present study demonstrated a negative prognostic impact of hyperglycemia in the early phase of SAB. The negative prognostic impact was accentuated among young and healthy non-diabetics. Future prospective studies are needed to establish the relevance of underlying medication, fasting and glycemic control on outcome in SAB.
References
Laupland KB, Lyytikäinen O, Søgaard M, Kennedy KJ, Knudsen JD, Ostergaard C et al (2013) The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 19(5):465–471
Kaech C, Elzi L, Sendi P et al (2006) Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect 12(4):345–352
Ammerlaan H, Seifert H, Harbarth S et al (2009) Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis 49(7):997–1005
Rieg S, Peyerl-Hoffmann G, de With K et al (2009) Mortality of S. aureus bacteremia and infectious diseases specialist consultation — a study of 521 patients in Germany. J Infect 59(4):232–239
Fowler VG Jr, Olsen MK, Corey GR et al (2003) Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 163(17):2066–2072
Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y (2003) Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36(1):53–59
Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A (2012) Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus bacteraemia. Clin Infect Dis 56(4):527–535
Choi SH, Cho SY, Park JH, Chung JW (2011) Impact of infectious-disease specialist consultations on outcomes of Staphylococcus aureus bacteremia in a hospital with a low volume of patients with S. aureus bacteremia. J Infect 62(2):181–185
Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME et al (2011) Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis 11:208–222
Pragman AA, Kuskowski MA, Abraham JM et al (2012) Infectious disease consultation for staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract 20(4):261–267
Kobayashi D, Yokota K, Takahashi O, Arioka H, Fukui T (2014) A predictive rule for mortality of inpatients with Staphylococcus aureus bacteraemia: a classification and regression tree analysis. Eur J Intern Med 25(10):914–918
Robinson LE, van Soeren MH (2004) Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues 15:45–62
Vandijck DM, Oeyen SG, Buyle EM et al (2008) Hyperglycemia upon onset of ICU-acquired bloodstream infection is associated with adverse outcome in a mixed ICU population. Anaesth Intensive Care 36:25–29
Gearhart MM, Parbhoo SK (2006) Hyperglycemia in the critically ill patient. AACN Clin Issues 17:50–55
Marik PE, Raghavan M (2004) Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med 30:748–756
McCowen KC, Malhotra A, Bistrian BR (2001) Endocrine and metabolic dysfunction syndromes in the critically ill. Grit Gare Glin 17:107–124
Carr ME (2001) Diabetes mellitus: a hypercoagulable state. J Diabetes Complicat 15:44–54
Pozzilli P, Leslie R (1994) Infection and diabetes: mechanisms and prospects for prevention. Diabet Med 11:935–941
Stegenga ME, van der Crabben SN, Blümer RM et al (2008) Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood 112(1):82–89
Butler S, Btaiche I, Alaniz C (2005) Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy 25(7):963–976
Kornum JB, Thomsen RW, Riis A et al (2008) Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case–control study. Diabetes Care 31(8):1541–1545
McAlister FA, Majumdar SR, Blitz S et al (2005) The relation between hyperglycemia and outcomes in 2471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care 28(4):810–815
Lepper PM, Ott S, Nüesch E et al (2012) Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 344:e3397
Bader MS (2007) Hyperglycemia and mortality in elderly patients with Staphylococcus aureus bacteremia. South Med J 100(3):252–256
Bader MS (2015) Staphylococcus aureus bacteremia in community-dwelling older adults hyperglycemia, mortality, and discharge destination. Infect Dis Clin Pract 23:243–247
Patel TS, Cottreau JM, Hirsch EB, Tam VH (2016) Impact of hyperglycemia on outcomes of patients with Pseudomonas aeruginosa bacteremia. Diagn Microbiol Infect Dis 84(2):155–158
Peralta G, Sánchez MB, Garrido JC, Ceballos B, Mateos F, De Benito I et al (2010) Altered blood glucose concentration is associated with risk of death among patients with community-acquired Gram-negative rod bacteremia. BMC Infect Dis 10:181
Bader MS, Hinthorn D, Lai SM et al (2005) Hyperglycemia and mortality of diabetic patients with candidemia. Diabet Med 22:1252–1257
Li JS, Sexton DJ, Mick N et al. (2008) Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638
Levy MM, Fink MP, Marshall JC et al (2003) SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31(4):1250–1256
McCabe WR, Jackson GG (1962) Gram negative bacteraemia. Etiology and ecology. Arch Intern Med 110:847–855
Forsblom E, Ruotsalainen E, Järvinen A (2015) Improved outcome with early rifampicin combination treatment in methicillin-sensitive Staphylococcus aureus bacteraemia with a deep infection focus—a retrospective cohort study. PLoS One 10, e0122824
Ruotsalainen E, Järvinen A, Koivula I et al (2006) Finlevo Study Group: Levofloxacin does not decrease mortality in Staphylococcus aureus bacteraemia when added to the standard treatment: a prospective and randomized clinical trial of 381 patients. J Intern Med 259(2):179–190
Rueda AM, Ormond M, Gore M, Matloobi M, Giordano TP, Musher DM (2010) Hyperglycemia in diabetics and non-diabetics: effect on the risk for and severity of pneumococcal pneumonia. J Infect 60:99–105
Schuetz P, Kennedy M, Lucas JM, Howell MD, Aird WC, Yealy DM et al (2012) Initial management of septic patients with hyperglycemia in the noncritical care inpatient setting. Am J Med 125:670–678
Saunderson RB, Gouliouris T, Nickerson EK, Cartwright EJ, Kidney A, Aliyu SH et al (2015) Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteraemia in adults. Clin Microbiol Infect 21(8):779–785
Li J, Zhang N, Ye B, Ju W, Orser B, Fox JE et al (2007) Non-steroidal anti-inflammatory drugs increase insulin release from beta cells by inhibiting ATP-sensitive potassium channels. Br J Pharmacol 151:483–493
Vogelzang M, van der Horst ICC, Nijsten MWN (2004) Hyperglycaemic index as a tool to assess glucose control: a retrospective study. Crit Care 8:R122–R127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
The trial was approved by The Institutional Review Board of Helsinki University Central Hospital and The Ethical Committee of Helsinki University Central Hospital. A written informed consent was provided by each patient.
Funding
The study has been supported by grants from The Medical Society of Finland and the foundations Dorothea Olivia, Karl Walter och Jarl Walter Perkléns minne and Svenska Kulturfonden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Forsblom, E., Ruotsalainen, E. & Järvinen, A. Prognostic impact of hyperglycemia at onset of methicillin-sensitive Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 36, 1405–1413 (2017). https://doi.org/10.1007/s10096-017-2946-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2946-3