Abstract
The complications from S. aureus bacteremia (SAB) and infective endocarditis (SAIE) are higher in patients with diabetes. We summarize the characteristics and outcome of diabetic patients enrolled in a multicenter trial of daptomycin vs. standard therapy for SAB and SAIE. Adult patients with SAB were randomized to daptomycin 6 mg/kg/day or standard therapy (vancomycin 1 g every 12 h or antistaphylococcal penicillin 2 g every 4 h, both with gentamicin 1 mg/kg every 8 h for 4 days). Clinical success was defined as survival, resolution of S. aureus infection, and clinical outcome of cure or improved 6 weeks after end of therapy. Diabetic patients (86/235) were older, more overweight, and were more likely to present with systemic inflammatory response syndrome (SIRS) and to have complicated SAB. Clinical success rates were similar (67.4% in diabetics and 70.5% in non-diabetics). The mortality rate was significantly higher among diabetic patients (22.1% vs. 11.4%, p = 0.038). In the diabetes subgroup, the clinical success and mortality rates were comparable between the daptomycin and the standard therapy arms. The presence of diabetes is associated with significantly higher mortality in patients with SAB and SAIE. Daptomycin is an alternative therapeutic option in diabetic patients with these serious staphylococcal infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although staphylococci are common causes of bacteremia in diabetic patients [1, 2], few studies have specifically evaluated the frequency of staphylococcal bacteremia in diabetic vs. non-diabetic patients. Serious infections such as bacteremia and endocarditis in patients suffering from diabetes mellitus (DM) represent a special concern for clinicians due to the increased risk for complications compared to patients without DM [2–6]. In a recent study, diabetic patients were 4.8 times more likely than non-diabetics to develop central-line related bloodstream infections following surgery [7]. Diabetes has also been recognized as an independent risk factor for recurrence of Staphylococcus aureus bacteremia (SAB) [8]. With diabetic patients constituting up to 30% of all patients with infective endocarditis (IE) [9–11], treating physicians are called upon to promptly institute optimal therapy in an attempt to prevent complications in this high-risk group of patients. However, the response of diabetic patients with bacteremia and IE to different antibiotic regimens has not been well studied.
Daptomycin is a lipopeptide agent with rapid bactericidal activity against S. aureus [12]. It is approved for the treatment of complicated skin and skin structure infections at a dose of 4 mg/kg/day [13]. In an international, prospective, randomized trial of daptomycin vs. standard therapy for SAB and S. aureus infective endocarditis (SAIE), daptomycin was efficacious and less nephrotoxic than standard therapy [14]. It subsequently gained approval for the treatment of SAB and right-sided endocarditis [15] at a dose of 6 mg/kg/day. In this analysis, we describe the clinical characteristics, responses to antibiotics, and outcomes of diabetic patients enrolled in the daptomycin SAB/SAIE trial.
Methods
Study design and patients
This was an open-label randomized active-control study that was conducted between August 28, 2002 and February 16, 2005 in 40 sites in the United States and eight sites in Western Europe. Patients were considered for enrolment into the study if they were at least 18 years of age and had at least one blood culture that was positive for S. aureus within two calendar days of initiating study medication. Exclusion criteria included creatinine clearance of less than 30 ml/min, known osteomyelitis, polymicrobial bacteremia, and pneumonia. Complete exclusion criteria are detailed in the initial publication [14].
Randomization, treatments, and outcomes
Eligible patients were randomized to receive either daptomycin at 6 mg/kg/day or standard therapy with either vancomycin at 1 g every 12 hours (for MRSA isolates) or an antistaphylococcal penicillin at 2 g every four hours (for methicillin-susceptible S. aureus [MSSA] isolates). All patients randomized to daptomycin with a high likelihood of left-sided infective endocarditis at randomization and all patients randomized to standard therapy were also to receive gentamicin at 1 mg/kg every eight hours for the first four days.
The primary efficacy measure was the success rate six weeks after the end of therapy in randomized patients who received at least one dose of study drug and who did not have a high likelihood of having LIE at entry (the modified intent-to-treat (MITT) population). A blinded external adjudication committee reviewed individual patient data to establish diagnoses and outcomes. In the original publication, success was defined as survival, clinical cure or improvement and a documented clearance of bacteremia [14]. Patients who had premature discontinuation of therapy, who received potentially effective non-study antibiotics, or who did not have blood cultures taken 6 weeks following completion of therapy were considered to be treatment failures. In this analysis, we instead describe a less regulatory (and more relevant) definition of “clinical success” based only on survival, clinical outcome of cure or improved, and resolution of S. aureus infection 6 weeks following the end of therapy.
Entry and final diagnoses and duration of therapy
Entry diagnoses were determined according to the modified Duke criteria for infective endocarditis [16]. Final diagnoses were based on standard clinical definitions as outlined in the initial publication [14].
Statistical analysis
Given the limited number of patients in each arm, descriptive statistics were performed for this subgroup analysis. In addition, differences in clinical characteristics between patients with and without DM were analyzed using Fisher’s exact test for categorical data and the Wilcoxon rank sum test for continuous data; p-values are two-sided. The 95% confidence interval for the difference in success rates was calculated using the normal approximation to the binomial distribution.
Results
Study population
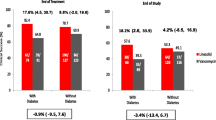
Of the 235 patients included in the MITT population, 86 patients (36.6%) had DM, of whom 68 (79.1%) were receiving insulin therapy. Forty-four patients with DM were randomized to daptomycin and 42 were randomized to standard therapy. As shown in Table 1, compared to patients without DM, patients with DM were older (median age 59.5 vs. 50.0 years, p = 0.007) and more overweight (median body-mass index [BMI] 28.6 vs. 25.4 kg/m2, p < 0.001). More patients with DM than those without DM presented with symptoms of systemic inflammatory response syndrome (SIRS) (81.4% vs. 71.1%; p = 0.088). There was no difference among the two groups with respect to risk factors for infection, except for injection drug use which was less common among diabetic patients (10.5% vs. 27.5%; p = 0.003). The proportion of infections caused by MRSA strains was similar in diabetic and non-diabetic patients. With respect to types of infection, more patients in the DM group had a final diagnosis of complicated bacteremia (61.6% vs. 45.6%; p = 0.021). Right-sided endocarditis was more commonly diagnosed in patients without DM (4.7% vs. 20.8%; p = 0.001), while LIE was similar in the DM and non-DM groups (5.8% vs. 8.7%). In the DM subgroup, there were no major differences between patients on daptomycin vs. standard therapy with regard to baseline patient characteristics and final diagnoses.
Outcome at test-of-cure
In the MITT population, clinical success rates were 67.4% in diabetic patients and 70.5% in non-diabetics (difference of −3.0; 95% CI around the difference −15.3 to 9.3) (Table 2).
Mortality rates were significantly higher among patients with DM (22.1% vs. 11.4%, p = 0.038) (Table 2). This difference was most pronounced in two subgroups of patients: those with uncomplicated bacteremia (20.8% vs. 5.4%), and those with IE (55.6% vs. 11.4%). There was no difference in deaths among patients treated with daptomycin compared to standard therapy. In the DM group, patients with IE had significantly higher mortality than those with SAB alone without IE (55.6% vs. 18.2%; p = 0.022).
Treatment failure in DM and non-DM patients
Failure rates were comparable in DM and non-DM patients (Table 3). A major difference between the two groups was failure due to patient death which was higher in DM patients (15.1% vs. 8.7%).
Safety
The percentages of patients with drug-related adverse events were similar in the DM and non-DM groups (41.9% vs. 36.7%). The percentages of patients with serious drug-related adverse events were also similar (5.8% vs. 2.7%).
Discussion
Diabetic patients constitute a sizable proportion of patients with SAB and SAIE. Recent data suggest that up to one third of patients with IE have a diagnosis of DM [9–11]. In addition, compared to non-diabetic patients, patients with DM often have worse outcomes [3–5, 9]. In an analysis of a large cohort of patients with IE included in the International Collaboration on Endocarditis-Merged Database (ICE-MD), patients with DM had a 30% in-hospital mortality rate, compared to 19% in patients without DM [17]. This and other studies have confirmed that DM is an independent predictor of early mortality in patients with IE [17, 18].
S. aureus has become a major cause of bloodstream infections, both in the hospital and the community settings [19, 20]. A similar trend is seen in IE where data from retrospective as well as prospective studies show that S. aureus is now the leading etiologic agent in IE [21, 22]. Diabetic patients have increased incidence and severity of a variety of infections [2, 3]. Consequently, the growing role of S. aureus in endovascular infections is particularly important to consider in diabetic patients because they are more likely than non-diabetics to be colonized and subsequently infected with S. aureus [1, 23–25].
The use of vancomycin in SAB and SAIE has been hampered by growing concern over its efficacy in treating serious life-threatening infections. In addition to published reports on failure of vancomycin treatment in the setting of MRSA bacteremia [26], there is presently evidence of an increasing prevalence of S. aureus isolates that exhibit heteroresistance to vancomycin [27–29]. Given the high burden of disease that DM represents, there is a need to evaluate the efficacy of new antimicrobial agents in diabetic patients.
We found that diabetic patients had more risk factors for infection at baseline than their non-diabetic counterparts (older and more obese). In the ICE-MD cohort, IE patients with DM underwent surgery less frequently for the index IE episode than patients without DM (32% vs. 45%) [17]. The authors attributed this finding to the greater underlying comorbid conditions among diabetic patients rather than to the absence of indications for surgery. An important finding in our analysis was that diabetic patients were more likely to have complicated SAB compared to non-diabetics. On the other hand, patients in the DM group had significantly less rates of injection drug use and right-sided endocarditis, which are conditions usually associated with better clinical outcomes than left-sided endocarditis [30].
Significantly more patients with DM had a fatal outcome from SAB or SAIE (22.1% vs. 11.4%), which is in agreement with previous studies showing that DM is an independent predictor of in-hospital mortality in patients with IE [17, 18]. DM patients with SAIE were at greatest risk of death, followed by DM patients with SAB alone without IE, then by non-DM patients regardless of the presence of IE. Although mortality rates were higher in the diabetic population across all types of infections, the largest differences in mortality between diabetics and non-diabetics were observed in patients with IE and those with uncomplicated bacteremia. Such high mortality rates in the setting of complex as well as relatively simple infections substantiate the independent contribution of diabetes as a comorbid condition to the poor outcome of patients with S. aureus infections.
We found no differences in clinical success at test-of-cure between patients with DM who received daptomycin and those who received standard therapy (68.2% and 66.7%, respectively). Daptomycin is therefore an alternative agent for use in diabetic patients having SAB with or without IE. The availability of new therapies is of great importance, since vancomycin failures are increasingly being reported [26].
This analysis has several limitations. First, it is a subgroup analysis, where the potential for over-interpretation exists [31]. Because of this, the results have been presented with no inference of statistical significance between the treatment arms. Second, the open-label nature of the trial constitutes a source of bias since investigators may have been influenced by knowledge of treatment assignment when deciding to initiate and discontinue treatment according to their clinical judgment [32]. However, the potential for bias was lessened by utilizing blood culture data in the endpoint and by employing an independent adjudication committee whose members were blinded to treatment assignment. Finally, DM was defined clinically by reviewing medical records rather than through the use of diagnostic criteria based on the glycometabolic state of patients.
In conclusion, this study has highlighted the worse outcome of diabetic patients with SAB and SAIE compared to non-diabetics, as evidenced by significantly higher mortality rates. Future research should be geared towards understanding the specific causes of the observed increased mortality risk in diabetics and ways to prevent such a poor outcome in this growing patient population (e.g. earlier surgical intervention, more aggressive attempts at earlier diagnosis of metastatic foci of infection, etc.) The results of this subgroup analysis also demonstrate that daptomycin at 6 mg/kg/day is an effective alternative to standard therapy for SAB and SAIE in patients with DM.
References
Breen JD, Karchmer AW (1995) Staphylococcus aureus infections in diabetic patients. Infect Dis Clin North Am 9:11–24
Joshi N, Caputo GM, Weitekamp MR, Karchmer AW (1999) Infections in patients with diabetes mellitus. N Engl J Med 341:1906–1912
Calvet HM, Yoshikawa TT (2001) Infections in diabetes. Infect Dis Clin North Am 15:407–421
Carton JA, Maradona JA, Nuno FJ, Fernandez-Alvarez R, Perez-Gonzalez F, Asensi V (1992) Diabetes mellitus and bacteraemia: a comparative study between diabetic and non-diabetic patients. Eur J Med 1:281–287
Jean G, Charra B, Chazot C et al (2002) Risk factor analysis for long-term tunneled dialysis catheter-related bacteremias. Nephron 91:399–405
Leibovici L, Samra Z, Konisberger H, Kalter-Leibovici O, Pitlik SD, Drucker M (1991) Bacteremia in adult diabetic patients. Diabetes Care 14:89–94
Sreeramoju PV, Tolentino J, Garcia-Houchins S, Weber SG (2008) Predictive factors for the development of central line-associated bloodstream infection due to gram-negative bacteria in intensive care unit patients after surgery. Infect Control Hosp Epidemiol 29:51–56
Kreisel K, Boyd K, Langenberg P, Roghmann MC (2006) Risk factors for recurrence in patients with Staphylococcus aureus infections complicated by bacteremia. Diagn Microbiol Infect Dis 55:179–184
Bishara J, Peled N, Samra Z, Sagie A, Leibovici L, Pitlik S (2004) Infective endocarditis in diabetic and non-diabetic patients. Scand J Infect Dis 36:795–798
Cabell CH, Jollis JG, Peterson GE et al (2002) Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med 162:90–94
Moreno R, Zamorano J, Almeria C et al (2002) Influence of diabetes mellitus on short- and long-term outcome in patients with active infective endocarditis. J Heart Valve Dis 11:651–659
Critchley IA, Draghi DC, Sahm DF, Thornsberry C, Jones ME, Karlowsky JA (2003) Activity of daptomycin against susceptible and multidrug-resistant Gram-positive pathogens collected in the SECURE study (Europe) during 2000–2001. J Antimicrob Chemother 51:639–649
Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI (2004) The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 38:1673–1681
Fowler VG Jr, Boucher HW, Corey GR et al (2006) Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 355:653–665
Khatib R, Saeed S, Sharma M, Riederer K, Fakih MG, Johnson LB (2006) Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 25:181–185
Li JS, Sexton DJ, Mick N et al (2000) Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638
Kourany WM, Miro JM, Moreno A et al (2006) Influence of diabetes mellitus on the clinical manifestations and prognosis of infective endocarditis: a report from the International Collaboration on Endocarditis-Merged Database. Scand J Infect Dis 38:613–619
Chu VH, Cabell CH, Benjamin DK Jr et al (2004) Early predictors of in-hospital death in infective endocarditis. Circulation 109:1745–1749
Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP (1999) Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis 29:239–244
Steinberg JP, Clark CC, Hackman BO (1996) Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin Infect Dis 23:255–259
Fowler VG Jr, Miro JM, Hoen B et al (2005) Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021
Miro JM, Anguera I, Cabell CH et al (2005) Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 41:507–514
Ahluwalia A, Sood A, Sood A, Lakshmy R, Kapil A, Pandey RM (2000) Nasal colonization with Staphylococcus aureus in patients with diabetes mellitus. Diabet Med 17:487–488
Berman DS, Schaefler S, Simberkoff MS, Rahal JJ (1987) Staphylococcus aureus colonization in intravenous drug abusers, dialysis patients, and diabetics. J Infect Dis 155:829–831
Canario DG, Idris MH, Cunha BA (2004) Methicillin-resistant and -sensitive Staphylococcus aureus nasal colonization of insulin-dependent children with juvenile onset diabetes mellitus. Am J Infect Control 32:371–372
Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC Jr, Eliopoulos GM (2004) Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 42:2398–2402
Nunes AP, Schuenck RP, Bastos CC et al (2007) Heterogeneous resistance to vancomycin and teicoplanin among Staphylococcus spp. isolated from bacteremia. Braz J Infect Dis 11:345–350
Plipat N, Livni G, Bertram H, Thomson RB Jr (2005) Unstable vancomycin heteroresistance is common among clinical isolates of methiciliin-resistant Staphylococcus aureus. J Clin Microbiol 43:2494–2496
Wang JL, Tseng SP, Hsueh PR, Hiramatsu K (2004) Vancomycin heteroresistance in methicillin-resistant Staphylococcus aureus, Taiwan. Emerg Infect Dis 10:1702–1704
Moss R, Munt B (2003) Injection drug use and right sided endocarditis. Heart 89:577–581
Lagakos SW (2006) The challenge of subgroup analyses-reporting without distorting. N Engl J Med 354:1667–1669
Boucher HW (2008) Is it possible to “blind” a trial of community-acquired pneumonia? Clin Infect Dis 47(Suppl 3):S210–S215
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanafani, Z.A., Kourany, W.M., Fowler, V.G. et al. Clinical characteristics and outcomes of diabetic patients with Staphylococcus aureus bacteremia and endocarditis. Eur J Clin Microbiol Infect Dis 28, 1477–1482 (2009). https://doi.org/10.1007/s10096-009-0808-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-009-0808-3