Abstract
Cognitive reserve (CR) contributes to preserve cognition despite brain damage. This theory has been applied to multiple sclerosis (MS) to explain the partial relationship between cognition and MRI markers of brain pathology. Our aim was to determine the relationship between two measures of CR and cognition in MS. One hundred and forty-seven MS patients were enrolled. Cognition was assessed using the Rao’s Brief Repeatable Battery and the Stroop Test. CR was measured as the vocabulary subtest of the WAIS-R score (VOC) and the number of years of formal education (EDU). Regression analysis included raw score data on each neuropsychological (NP) test as dependent variables and demographic/clinical parameters, VOC, and EDU as independent predictors. A binary logistic regression analysis including clinical/CR parameters as covariates and absence/presence of cognitive deficits as dependent variables was performed too. VOC, but not EDU, was strongly correlated with performances at all ten NP tests. EDU was correlated with executive performances. The binary logistic regression showed that only the Expanded Disability Status Scale (EDSS) and VOC were independently correlated with the presence/absence of CD. The lower the VOC and/or the higher the EDSS, the higher the frequency of CD. In conclusion, our study supports the relevance of CR in subtending cognitive performances and the presence of CD in MS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive deficits (CD) affect up to 70% of multiple sclerosis (MS) patients. Information processing speed and visual and verbal memory are the most frequently involved cognitive domains [1]. CD have a negative impact on daily living activities, social functioning, and gaining and maintaining employment [2]. They may start in the early stages of the disease and generally worsen over time, but their relationship with clinical variables [2] (e.g., disease duration, physical disability) and magnetic resonance imaging (MRI) measures of brain tissue damage is still debated. Indeed, MS patients with a severe MRI disease burden (estimated by T2-lesion load and brain atrophy) may show no CD [3]. An incomplete association between cognitive status and structural findings (the so-called cognitive-radiologic dissociation) has also been reported in elder age [4] and in some other neurological diseases, such as Alzheimer’s disease (AD) [4], traumatic brain injury [5], and stroke [6].
These observations led to the cognitive reserve (CR) theory that try to explain the individual differences (in brain structure and/or task processing) that may allow some people to cope the same brain pathology degree without disease-related CD than others who develop CD [7]. A growing body of research extended the CR hypothesis also to MS showing that higher CR softens the negative effect of the disease on cognitive status [8,9,10,11,12,13,14,15].
Stern defined two models of CR: (I) passive reserve (or brain reserve) that is largely genetically determined and it refers to quantitative/structural measures such as intracranial volume, maximal lifetime brain growth, or neuronal count and (II) active reserve (or cognitive reserve) that refers instead to how effectively each individual can take advantage of the available brain reserve, remaining preserved by CD [7].
In our study, we focused our attention on the active component of CR (from now simply CR).
CR cannot be measured directly, so during the years, several proxies have been used.
CR has been usually estimated from premorbid verbal intelligence [16], education [17], cognitive leisures [18], occupational attainment [19], and/or vocabulary knowledge [8]. Some studies have used complex CR index resulting from the combination of multiple proxies [15]. However no “gold standard” is currently being identified. There have been decades of research speculating what measures are good proxies for CR, even in MS [20].
Within the range of all CR measures, we have focused our attention on two variables: the vocabulary knowledge measured as vocabulary subtest score (VOC) of the Wechsler Abbreviated Scale of Intelligence-Revised (WAIS-R) and the number of years of formal/academic education (EDU). We have chosen these two variables because they are easily retrievable in the course of a clinical visit.
VOC is an accepted method of evaluating premorbid verbal intelligence [21] and assesses the extent of acquired vocabulary knowledge, which is greatly influenced by some enriching life activities such as educational attainment, occupational experiences, and frequent reading [22]. This test has been previously used [8, 11, 23] and is considered a good proxy of CR in MS, given its robustness and resistance to age-related decline, independent of neurological damage [24].
EDU is considered an advantageous proxy of CR also given that MS onset is typically during the 20s and 30s, largely after one’s schooling is complete [25].
The aim of the present study was to separately assess the relationship of VOC and EDU with cognitive performances after controlling for multiple clinical and demographic variables in a large group of MS patients.
Materials and methods
Study population
One hundred and fifty-six patients with MS (according to the revised McDonald criteria) [26] were consecutively screened for the present study at the MS Center of the University of Campania “L. Vanvitelli”, Italy.
All patients were relapse and steroid therapy-free for at least 3 months before the enrollment and had no history of other neurological and psychiatric conditions, learning disability, and assumption of psychoactive drugs.
Nine MS patients (six female, three male) were not enrolled in the study because they declined participation or did not complete the neuropsychological assessment. The final study sample included 147 MS patients.
The study was approved by the local Ethics Committee. A written informed consent was obtained from all subjects prior to participation.
Clinical and neuropsychological assessment
Age, gender, disease duration, and EDU were recorded for each patient.
All patients underwent the following: (i) a complete neurological examination, including the assessment of disability by the Expanded Disability Status Scale (EDSS) [27]; (ii) a neuropsychological (NP) evaluation assessing cognitive functioning (see below for details); (iii) an assessment of depressive symptoms by the Chicago Multiscale Depression Inventory (CMDI) [28]; (iv) an evaluation of fatigue by the Fatigue Severity Scale (FSS) [29].
Cognitive functioning was assessed using the version A of the Rao’s brief repeatable battery (BRB) [30]. BRB includes subtests which explore verbal memory and delayed recall [Selective Reminding Test (SRT) and SRT-delayed recall (D-SRT)], visuospatial memory and delayed recall [10/36 Spatial Recall Test (10/36-SPART) and 10/36 SPART-delayed recall (10/36 SPART-D)], concentration, sustained attention, and information processing speed [Paced Auditory Serial Addition Test at 3 s (PASAT 3″) and at 2 s (PASAT 2″), Symbol Digit Modalities Test (SDMT)], and verbal fluency on semantic stimulus [Word List Generation (WLG)]. Executive functions were also assessed with the Stroop Color-Word Interference Test (SCWIT), which mainly investigates inhibitory control.
Raw scores of each subtest were converted to z-scores based on published Italian normative data [31]. Failure at a subtest was defined by a performance with a z-score 2 standard deviations (SD) below normal.
Patients who failed at two subtests or more were defined cognitively impaired (CI). Those who failed none or only one subtest were considered as cognitively preserved (CP) [32].
All NP examinations were performed by the same trained neuropsychologist, blinded to clinical data.
CR assessment
CR was assessed by two measures: (I) the VOC of the Italian version of the WAIS-R and (II) number of years of formal/academic education (EDU).
VOC is obtained using 35 words (verbs, nouns, and adjectives) of increasing difficulties (e.g., bed, ship, hide, wear out). Each answer is evaluated on a 3-point scale (0–2), depending on the comprehensiveness of the response. Total score ranges from 0 to 70: the higher the score, the greater the vocabulary knowledge is. The test was administrated by a trained neuropsychologist, following precisely the instructions relating to the scoring allocation for each item. The time necessary for the administration is about 20 min.
Statistical analysis
The Shapiro-Wilk test and graphical methods were used to assess the normal distribution of data. In case of normal distribution, data were presented as mean and SD; in case of deviation from the normal distribution, data were presented as median.
A linear multivariate regression analysis, including demographic (i.e., age and sex) and clinical parameters (i.e., disease duration, EDSS, FSS, CMDI), two measures of CR (VOC and EDU) as independent predictors, and raw scores on each cognitive task as dependent variables was performed in order to evaluate the predictive role of VOC and/or EDU on each cognitive domain functioning (as raw score on each test).
A binary logistic regression analysis including disease duration, EDSS, FSS, CMDI, and VOC score as covariates, and absence (0) or presence (1) of CD as dependent variables was performed in order to assess predictors of cognitive status of MS patients.
Age, sex, and education were not included in the logistic regression analysis because these were already considered, when necessary, as correction factors for the z-score assignment (used to define presence/absence of CD) [31]. All data were analyzed using the SPSS version 20 software package (SPSS Inc., Chicago, IL).
Results
Patients’ main demographic, clinical, and NP features are summarized in Table 1.
One hundred and seven were women; the mean age was 37.76 ± 10.96, the mean EDU was 12.46 ± 3.75, and the disease duration was 10.06 ± 9.41 (Table 1).
According to their current disease phenotype, 128 patients were classified as relapsing-remitting and 19 as secondary progressive.
Depending on the NP performances, 35 patients were CI, 112 were CP. Table 2 includes the scores on each test for the two MS subgroups (CI and CP) separately. There were no significant differences in demographic and clinical characteristics between the two subgroups of patients (Table 2).
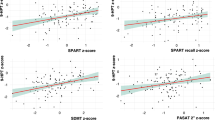
In the linear regression analysis, only VOC resulted correlated with (0.007 < p < 0.001) NP performances at all ten NP tests (Table 3). An older age was significantly associated with worse cognitive performances on verbal memory [SRT-LTS (β – 0.312; p 0.003), SRT-CLTR (β – 0.307; p 0.005), D-SRT (β – 0.305; p 0.004)], visuospatial memory [10/36-SPART-D (β – 0.244; p 0.026)], and sustained attention [SDMT (β – 0.201; p 0.022)] tests (Table 3).
Female gender significantly contributed to better performance on verbal memory [SRT-LTS (β 0.183; p 0.022)], information processing speed and sustained attention [SDMT (β – 0.328; p 0.001)], and verbal fluency [WLG (β 0.285; p 0.002)] tests (Table 3).
Higher EDSS score was significantly associated with worse sustained attention [SDMT (β – 0.328; p 0.001), verbal memory [D-SRT (β – 0.380; p 0.001)], and executive function [SCWIT (β 0.468; p < 0.001)] performances (Table 3).
EDU was correlated with SCWIT performance (Table 3).
The binary logistic regression showed that only EDSS and VOC were independently correlated with the presence/absence of CD (Table 4). The lower the VOC and/or the higher the EDSS, the higher the frequency of CD.
Discussion
CD are very common in MS [1], mostly affecting information processing speed, visual, and verbal memory. Cognitive integrity is fundamental for most daily activities as well as social and work functioning; moreover, it is crucial for therapy adherence [2]. As it occurs in other neurological diseases, there is only a partial relationship between MRI measures of MS brain pathology and cognitive functioning. The CR theory might contribute to explain this incomplete association [3].
Our main purpose was to determine the relationship between two easily obtainable measures of CR, VOC and EDU, and cognitive functioning in MS patients.
VOC estimates semantic knowledge acquired through enriching activities actively carried out by the subject such as education, occupational attainment, and frequent reading [24]. It is generally resistant to CD related to aging and it has the advantage to be a fast-running test (it takes about 20 min). Therefore, VOC might be retained a useful measure of CR [20].
Low levels of EDU in MS patients (less than 12 years) have been associated with low performances at NP tests compared to MS patients with higher levels of EDU (who conversely performed similarly to the matched controls) [33].
Most of previous studies on CR in MS have demonstrated that CR is correlated with cognitive performances [8,9,10,11,12,13,14,15]. However, some of these studies have investigated only a few cognitive domains or self-perception of wellness [9, 11, 14]. In the present study, we used BRB [30], a comprehensive, standardized, and specifically approved study for MS research NP battery. We also added the SCWIT to better explore executive functions.
Therefore, we thoroughly evaluated the cognitive profile of MS patients to gain more insight on the possible association of CR with specific cognitive domain impairment affecting daily living, working activities, and social functioning.
Our primary result was that, in MS, VOC is the sole clinical variable independently associated with cognitive performances in all the cognitive domains explored. Contrariwise, EDU is not related to the performances at most of the tests: we could speculate that EDU is more influenced than VOC by factors not related to the individual life experiences, such as socioeconomic status or parental education. EDU alone might not sufficiently capture the complex construct of CR because of cultural differences, as compulsory education requirements vary across cultures, countries, and school [34, 35]. In our opinion, the early onset of MS may limit the usefulness of EDU and employment associated-CR measures in MS research because patients may have delayed or prematurely interrupted their schooling and, even more so, may have been unable to achieve occupational employment [36]. Moreover, the quality of EDU, which we have not measured, might contribute to CR too.
In our study, EDU showed a significant correlation with SCWIT performance.
Only a few previous studies have explored the association between cognitive reserve and executive functions, demonstrating a correlation with EDU (in only one study) and reading activity [12, 37].
It has already been demonstrated that a higher cognitive reserve (measured as the mean sample-based z-scores for VOC and EDU) reduced the negative effect of MS on cognitive efficiency assessed with the SDMT and PASAT [8]. Other studies have shown that persons with MS with greater vocabulary knowledge and/or greater early-life participation in cognitive leisure activities are more capable to cope with MS without CD [38]. Moreover, MS patients with higher CR, assessed using measures of childhood cultural and educational enrichment, occupational attainment, and leisure activities, are more likely to report lower levels of perceived disability, higher levels of functional health, and higher levels of well-being (based entirely on patients’ self-perception) [14].
In order to overcome some limitations of previous studies that have been carried out on relatively small samples [9, 11,12,13,14], we included a large single-center group of MS patients.
We also found a significant negative association between EDSS score and cognitive performance on subtests that explore verbal memory, sustained attention, and executive functions. These results are in agreement with previous studies in which the EDSS score was significantly associated with CD [39] and with poor performance on memory tests and verbal fluency [8].
Another important finding is that only EDSS and VOC, among the clinical variables examined, are independently associated with the presence/absence of CD. This outcome confirms the results of a previous study conducted in a consecutive sample of 144 MS patients, in which a marker of premorbid verbal IQ and EDSS resulted highly independent predictors of CD [39].
There are some limitations in our study. First, in our sample, only 22% were CI, a bit low based on current literature; so this might be a limitation in generalizing to the broader MS population. Moreover, we did not assess cognitive leisure activities (e.g., reading books, magazines or newspapers, producing art or non-fiction writing, playing a musical instrument, playing structured games, participating in hobbies) [18], due to the lack of an Italian version of specific questionnaires.
Moreover, although the VOC is a subtest of the Italian version of the WAIS-R, a cut-off score has not been provided yet in the Italian population. Due to the lack of such cut-off value, we could not dichotomize this variable entering in regression analysis as proxy of CR; this issue might represent another limitation of the present study. Thus, future studies should be designed to validate VOC subtest in MS providing a specific cut-off score useful to screen individuals with low or high CR.
The availability of a tool to measure CR in MS patients might allow us to identify the individual capacity to cope with the disease before developing CD. Patients with lower CR could benefit from early cognitive rehabilitation [40] as they could learn and apply cognitive strategies into their life before clinical onset of CD. They also might be encouraged to carry out intellectually enriching leisure activities. Due to the lack of longitudinal studies demonstrating the protective effects of a higher VOC alone on CD in persons with MS, VOC cannot yet be considered a gold standard proxy of CR.
Given the impact of CD on the quality of life of MS patients, it is desirable to identify a simple and reliable test correlated with cognitive status in the course of the disease. Two small size sample longitudinal studies have shown that a higher CR (also estimated as VOC) is associated with less decline in cognitive efficiency over a follow-up period from 1.6 to 4.5 years, in MS patients [10, 15]. Future multi-center studies with a longitudinal design and a longer follow-up period might further assess the strength and utility of VOC as a clinically meaningful predictor of cognitive performance in MS patients. It would be very fascinating to extend the investigation of CR in the pediatric MS population too [41].
References
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7(12):1139–1151
Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F (1991) Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 41:692–696
Filippi M, Rocca MA, Benedict RH et al (2010) The contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology 75:2121–2128
Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11:1006–1012
Kesler SR, Adams HF, Blasey CM, Bigler ED (2003) Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl Neuropsychol 10(3):153–162
Elkins JS, Longstreth WT Jr, Manolio TA, Newman AB, Bhadelia RA, Johnston SC (2006) Education and the cognitive decline associated with MRI-defined brain infarct. Neurology 67(3):435–440
Stern Y (2009) Cognitive reserve. Neuropsychologia 47(10):2015–2028.4
Sumowski JF, Chiaravalloti N, Leavitt VM, DeLuca J (2012) Cognitive reserve in secondary progressive multiple sclerosis. Mul Scler 18(10):1454–1458
Sumowski JF, Wylie GR, Chiaravalloti N, DeLuca J (2010) Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology 74(24):1942–1945
Sumowski JF, Rocca MA, Leavitt VM, Dackovic J, Mesaros S, Drulovic J, DeLuca J, Filippi M (2014) Brain reserve and cognitive reserve against cognitive decline over 4.5 years in MS. Neurology 82(29):1776–1783
Benedict RH, Morrow SA, Weinstock Guttman B et al (2010) Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc 16(5):829–835
Ghaffar O, Fiati M, Feinstein A et al (2012) Occupational attainment as a marker of cognitive reserve in multiple sclerosis. PLoS One 7(10):e47206
Sumowski JF, Rocca MA, Leavitt VM, Riccitelli G, Comi G, DeLuca J, Filippi M (2013) Brain reserve and cognitive reserve in multiple sclerosis: what you’ve got and how you use it. Neurology 80(24):2186–2193
Schwartz CE, Snook E, Quaranto B, Benedict RHB, Vollmer T (2013) Cognitive reserve and patient-reported outcomes in multiple sclerosis. Mult Scler 19(1):87–105
Amato MP, Razzolini L, Goretti B, Stromillo ML, Rossi F, Giorgio A, Hakiki B, Giannini M, Pasto L, Portaccio E, de Stefano N (2013) Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurology 80(19):1728–1733
Alexander GE, Furey ML, Grady CL et al (1997) Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry 154(2):165–172
Stern Y, Alexander GE, Prohovnik I, Mayeux R (1992) Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 32:371–375
Scarmeas N, Levy G, Tang MX, Manly J, Stern Y (2001) Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57(12):2236–2242
Richards M, Sacker A (2003) Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol 25(5):614–624
Sandroff BM, Schwartz CE, DeLuca J (2016) Measurement and maintenance of reserve in multiple sclerosis. J Neurol 263(11):2158–2169
Strauss E, Sherman E MS, Spreen O. (2006) A compendium of neuropsychological tests. 3rd ed. Oxford: Oxford University Press
Stanovich KE, Cunningham AE (1992) Studying the consequences of literacy within a literate society: the cognitive correlates of print exposure. Mem Cogn 20:51–68
Sumowski JF, Chiaravalloti N, DeLuca J (2009) Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol 31:913–926
Lezak M (2004) Neuropsychological assessment, 4th edn. Oxford University Press. New York
Da Silva A, Cavaco S et al (2015) Cognitive reserve in multiple sclerosis: protective effect of education. Mult Scler 21(10):1312–1321
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Solari A, Motta A, Mendozzi L, Aridon P, Bergamaschi R, Ghezzi A, Mancardi GL, Milanese C, Montanari E, Pucci E (2004) Italian version of the Chicago Multiscale Depression Inventory: translation, adaptation and testing in people with multiple sclerosis. Neurol Sci 24(6):375–383
Krupp LB, La Rocca NG, Muir-Nash J et al (1989) The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46(10):1121–1123
Rao SM, Leo GJ, Berardin L et al (1991) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41(5):685–691
Amato MP, Portaccio E, Goretti B, Zipoli V, Ricchiuti L, de Caro MF, Patti F, Vecchio R, Sorbi S, Trojano M (2006) The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12(6):787–793
Bonavita S, Gallo A, Sacco R, Corte MD, Bisecco A, Docimo R, Lavorgna L, Corbo D, Costanzo AD, Tortora F, Cirillo M, Esposito F, Tedeschi G (2011) Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler 17(4):411–422
Bonnet MC, Deloire MSA, Salort E, Dousset V, Petry KG, Brochet B (2006) Evidence of cognitive compensation associated with educational level in early relapsing-remitting multiple sclerosis. J Neurol Sci 251:23–28
Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y (2011) Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc 17(4):593–601
Sandroff BM, Schwartz CE, DeLuca J (2016) Measurement and maintenance of reserve in multiple sclerosis. J Neurol 263:2158–2169
Amato MP, Goretti B, Ghezzi A, Lori S, Zipoli V, Portaccio E, Moiola L, Falautano M, de Caro MF, Lopez M, Patti F, Vecchio R, Pozzilli C, Bianchi V, Roscio M, Comi G, Trojano M, For the Multiple Sclerosis Study Group of the Italian Neurological Society (2008) Cognitive and psychosocial features of childhood and juvenile MS. Neurology 70(20):1891–1897
Luerding R, Gebel S, Gebel EM et al (2016) Influence of formal education on cognitive reserve in patients with multiple sclerosis. Front Neurol 7:46
Sumowski JF, Wylie GR, Gonnella A, Chiaravalloti N, DeLuca J (2010) Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology 75(16):1428–1431
Feinstein A, Lapshin H, O’Connor P et al (2013) Sub-threshold cognitive impairment in multiple sclerosis: the association with cognitive reserve. J Neurol 260(9):2256–2261
Bonavita S, Sacco R, Della Corte M, Esposito S, Sparaco M, d’Ambrosio A, Docimo R, Bisecco A, Lavorgna L, Corbo D, Cirillo S, Gallo A, Esposito F, Tedeschi G (2015) Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study. J Neurol 262(1):91–100
Pastò L, Portaccio E, Goretti B, Ghezzi A, Lori S, Hakiki B, Giannini M, Righini I, Razzolini L, Niccolai C, Moiola L, Falautano M, Simone M, Viterbo RG, Patti F, Cilia S, Pozzilli C, Bianchi V, Roscio M, Martinelli V, Comi G, Trojano M, Amato MP, on behalf of the MS Study Group of the Italian Neurological Society (2016) The cognitive reserve theory in the setting of pediatric-onset multiple sclerosis. Mult Scler 22(13):1741–1749
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Della Corte, M., Santangelo, G., Bisecco, A. et al. A simple measure of cognitive reserve is relevant for cognitive performance in MS patients. Neurol Sci 39, 1267–1273 (2018). https://doi.org/10.1007/s10072-018-3422-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3422-2