Abstract
Cognitive reserve (CR) is a construct that originates from the observation of poor correspondence between brain damage and clinical symptoms. The aim of the study was to investigate the association between cognitive reserve (CR), brain reserve (BR) and cognitive functions and to evaluate whether CR might attenuate/moderate the negative impact of brain atrophy and lesion load on cognitive functions in multiple sclerosis (MS). To achieve these aims, ninety-eight relapsing-remitting MS patients underwent the brief repeatable battery of neuropsychological tests and Stroop test (ST). CR was assessed by vocabulary-based estimate of lifetime intellectual enrichment. All patients underwent a 3T MRI to assess T2-lesion load and atrophy measures, including normalized gray matter and white matter (nWMV) volumes. The BR was evaluated by maximal lifetime brain volume expressed by intracranial volume (ICV). Hierarchical regressions were used to investigate whether higher BR and/or CR is related to better cognitive performances after controlling for potentially confounding factors. The ICV was not associated with any cognitive tests. Intellectual enrichment was positively associated with performance on tests assessing memory, attention and information processing speed, verbal fluency and inhibitory control. Significant relationship between nWMV and ST was moderated by intellectual enrichment. In conclusion, the findings suggested that CR seems to mitigate cognitive dysfunction in MS patients and can reduce the negative impact of brain atrophy on inhibitory control, relevant for integrity of instrumental activities of daily living.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive reserve (CR) is a construct that originates from the observation of poor correspondence between brain damage and clinical symptoms: patients with the same degree and extension of brain damage can show different clinical symptomatology [1]. Thus, individuals with higher CR appear to be healthier and have less severe clinical symptoms than those with low CR [1]. As for CR, brain reserve (BR) is considered to be a placeholder for understanding resilience mechanisms against neuropathology [2]. This passive form of reserve can be measured by the maximal lifetime brain volume (MLBV), estimated with head size or intracranial volume (ICV) [3].
MS is a chronic neurologic disease characterized by central nervous system white matter lesions and cerebral atrophy, thus resulting in sensorimotor symptoms and cognitive impairment (CI). CI occurs at both early and advanced stages of MS and its rates range from 43 to 70% [4]. CI has been related to lower patients’ quality of life (QoL) [5]; therefore, the identification of possible protective factors against cognitive decline in MS might be relevant to avoid reduced work capacity and QoL.
Previous cross-sectional and longitudinal studies on MS patients revealed that patients with greater intellectual enrichment (e.g., education, cognitive leisure) are able to withstand more severe brain disease [6,7,8,9,10] maintaining better cognitive performance than patients with lower CR, independently of clinical characteristics [3, 6, 11,12,13,14,15,16] and brain atrophy [16,17,18,19,20]. However, other longitudinal studies revealed a significant moderating effect of CR on the impact of brain atrophy on cognition only at baseline evaluation but not at follow-up [21]. It was suggested that the absence of such a moderating effect of CR might be limited or even suppressed by the severe or extended brain damage, when the disease progresses [17, 21].
Some studies found that CR also moderates the negative effect of brain atrophy on cognition [16, 18, 22, 23]. In addition to CR, larger MLBV, an indirect measure of BR, was found to protect against cognitive decline in MS patients [3, 19]. More recently, some authors found that cognitive leisure was related to larger hippocampal volume and better memory in a large but clinically and geographically heterogeneous sample of MS patients [24, 25]. The larger hippocampal volume would mediate the relationship between cognitive leisure and memory and would be a key component of the neuroanatomical basis of reserve against memory deficits in MS [24].
Although available cross-sectional and longitudinal studies suggested that CR and BR might play a role against CI associated with MS, they seem to be characterized by some methodological limitations: some studies included a small and/or clinically heterogeneous samples (e.g., including two or three different disease phenotypes), others did not evaluate the possible confounding effects of specific behavioral symptoms (e.g., fatigue or depression) which can negatively influence cognitive performance in MS patients. Taking into account the abovementioned limitations, we performed a cross-sectional study with a twofold aim: (1) to further explore the association between CR, BR and cognitive functions, (2) to evaluate whether CR might attenuate/moderate the negative impact of brain damage (lesion load and brain atrophy) on cognitive functions in MS, controlling for the possible contribution of depression, fatigue and disability to CI in MS patients. For these purposes, we enrolled a large and clinically homogeneous sample of relapsing-remitting MS (RR-MS) patients, who underwent: (1) a 3T magnetic resonance imaging (MRI) to assess T2 lesion load (T2-LL) and brain atrophy measures, (2) the vocabulary subtest, to measure intellectual enrichment [4, 6] and (3) a neuropsychological evaluation to assess verbal and visual memory, attention, concentration, processing speed, and inhibitory control. Taking into account previous reports on the protective effect of CR and BR on cognitive status, we speculated that (1) a high level of both intellectual enrichment and BR was related to better cognitive performances; (2) CR might attenuate/moderate the negative impact of lesion load and/or brain atrophy on cognitive status in MS, independently on depression, fatigue and disability.
Materials and methods
Subjects
We screened 115 MS outpatients consecutively admitted to the MS center of I Division of Neurology, University of Campania “Luigi Vanvitelli”, Naples, Italy. To be included in the present study, each patient had to meet the following inclusion criteria: (1) diagnosis of RR-MS [26], (2) no relapses and steroid therapy for at least 3 months prior to the study, (3) the absence of other neurological diseases, (4) no assumption of psychoactive drugs potentially interfering with neuropsychological examination.
Standard protocol approvals, registrations, and patient consents
The Local Ethics Board approved the study. We obtained signed written informed consent from all participants.
Procedures
All patients underwent clinical and neuropsychological testings to assess cognitive reserve and cognitive status, and a 3T MRI examination.
Clinical and neuropsychological testing
Disease duration (expressed in years) was recorded for each patient. Disability was measured by the Expanded Disability Status Scale (EDSS). Depressive symptoms and fatigue was measured by Chicago Multiscale Depression Inventory (CMDI) and Fatigue Severity Scale (FSS), respectively.
Intellectual enrichment was evaluated by means of vocabulary (VOC) subtest of Wechsler Adult Intelligence Scale-Revised. This subtest measures vocabulary knowledge, which is considered as an estimate of lifetime intellectual enrichment in cognitive research, since it is strongly correlated with enriching life activities (e.g., occupation, reading and education) [27].
To assess cognitive functioning, all MS patients underwent the Italian version of the Rao’s brief repeatable battery (BRB) [28]. It consists of tests assessing verbal memory (Selective Reminding Test [SRT]), visual memory (10/36 Spatial Recall Test [SPART]), attention, concentration and speed of information processing (Paced Auditory Serial Addition Test 3″ and 2″ [PASAT 3″ and 2″], Symbol Digit Modalities Test [SDMT]), and verbal fluency (Word List Generation). Moreover, we also administered Stroop test (ST) to assess inhibitory control. Performance on each test of the BRB and on ST was evaluated in reference to available Italian normative values [28]. Neuropsychological evaluations were performed by a trained neuropsychologist, blinded to both clinical and MR data.
MRI acquisition
Brain MRI scans were acquired on a 3T GE Medical System (Milwaukee, WI) scanner equipped with an 8-channel parallel head coil. The following images were acquired: (1) DP/T2 weighted (dual-echo (DE) fast spin echo (FSE), repetition time [TR] = 3080 ms, echo time [TE] 1/TE2 = 24/127.5 ms, axial slices = 44/44, matrix = 256 × 384, field of view [FOV] = 240 mm, slice thickness = 3 mm, interslice gap = 0 mm); (2) high-resolution 3D-T1 (magnetization prepared Fast Spoiled Gradient echo, TR = 6,988 ms, TI = 650 ms, TE = 2,85 ms, matrix = 256 × 256, slice number = 166, sagittal slices, flip angle = 8°, voxel size = 1 × 1 × 1.2 mm3, FOV = 256, sagittal).
T2 lesion load (T2-LL) and atrophy measures
The identification of T2 hyperintense lesions in MS patients was conducted on DP/T2 images by a single experienced observer blinded to patients’ clinical characteristics. The MIPAV software (Medical Image Processing, Analysis and Visualization; version 4.2.2; http://mipav.cit.nih.gov) was used to contour lesions and to compute T2-LL for each patient. Normalized WM (nWMV) and gray matter (GM) (nGMV) volumes were measured on 3D-T1 images using the SIENAx software after T1-hypointense lesion refilling [29].
BR measure
As in a previous study [19] MLBV was measured by ICV, adjusted for gender. Specifically, SIENAx volume scaling factor, a proxy of ICV resulting from the transformation that matches the extracted brain and skull to standard space brain and skull images (derived from Montreal Neurological Institute [MNI] 152 standard image) [29].
Statistical analysis
Clinical, cognitive, and demographic variables were reported as counts and percentages, for categorical variables, or mean, standard deviation (SD), median, and range for continuous variables. For VOC test, raw scores were converted to residual scores (RES-VOC) by regression where age, educational level, and gender were entered as independent variables and VOC score as dependent variable. All MRI parameters were normalized using logarithmic transformation.
Correlational analysis (Pearson) was performed to evaluate the association between CR, BR and MRI parameters.
Brain reserve
To determine the possible effect of demographic and clinical variables, brain damage measures and ICV on cognitive functions, we performed separate hierarchical linear regressions in which z-score of each cognitive test of BRB and of ST was entered as the dependent variable. In detail, for SRT, SPART, PASAT 3″ and 2″, SDMT we entered z-score based on education [28] as dependent variable and age, gender, EDSS, FSS, and CMDI in block 1; T2-LL, nGMV and nWMV in block 2 and ICV in block 3. Finally, three interaction terms between ICV and T2-LL, nGMV and nWMV were entered in block 4, to evaluate whether BR moderates the effect of brain atrophy and lesion load on cognition. For both WLG and ST, we entered z-score based on education and gender as dependent variable and age, EDSS, FSS, and CMDI in block 1; T2-LL, nGMV and nWMV in block 2 and ICV in block 3. Finally, three interaction terms between ICV and T2-LL, nGMV and nWMV were entered in block 4 to evaluate whether BR moderates the effect of brain atrophy and lesion load on cognition.
All variables were entered in the block in a stepwise fashion. To lessen the correlation between the three interaction terms and their component variables, predictor variables were centered.
Cognitive reserve
To determine the possible effect of clinical variables, brain atrophy measures and CR on cognitive functions, we performed separate hierarchical linear regressions including z-score for the cognitive outcome variables. In detail, for SRT, SPART, PASAT 3″ and 2″, SDMT we entered z-score based on education [28] as dependent variable and age, gender, EDSS, FSS, and CMDI in block 1; T2-LL, nGMV and nWMV in block 2; ICV in block 3; RES-VOC in block 4. Finally, three interaction terms between RES-VOC and T2-LL, nGMV and nWMV were entered in block 5 to evaluate whether CR moderates the effect of brain atrophy and lesion load on cognition. For both WLG and ST, we entered z-score based on education and gender as dependent variable and age, EDSS, FSS, and CMDI in block 1; T2-LL, nGMV and nWMV in block 2; ICV in block 3; RES-VOC in block 4. Finally, three interaction terms between RES-VOC and T2-LL, nGMV and nWMV were entered in block 5 to evaluate whether CR moderates the effect of brain atrophy and lesion load on cognition.
All variables were entered in the block in a stepwise fashion. To lessen the correlation between the three interaction terms and their component variables, predictor variables were centered.
Probability values lower than the 0.05 level were considered statistically significant. The SPSS software version 20 (SPSS Inc., Chicago, IL) was used for the statistical analysis.
Results
Out of 115 MS patients, 98 patients (70 females and 28 males) having RR-MS were enrolled in the study; 4 patients with clinical isolated syndrome and 13 patients with secondary progressive MS were excluded. Demographic and clinical features, MRI findings, and neuropsychological data of the study sample are shown in Table 1.
Brain reserve
The ICV did not correlate with T2-LL (r = − 0.004, p = 0.971), nGMV (r = 0.002, p = 0.986), nWMV (r = − 0.195, p = 0.055).
Table 2 reports significant predictors of performance on each neuropsychological test obtained from multiple hierarchical regressions.
Memory
Regression analysis showed that poor performance on two verbal memory tasks (SRT-LTS and CLTR) was associated with higher T2-LL. The third test (SRT-D) scores were instead associated with nGMV. The SRT-LTS and CLTR scores were associated with age. Gender, clinical variables, ICV interaction terms between ICV and brain atrophy measures did not contribute to verbal memory scores.
As for visual memory, poor performance on SPART was associated with high T2-LL and EDSS. The remaining demographic and clinical parameters, nGMV, nWMV or interaction terms between ICV and brain atrophy measures did not contribute to the score significantly.
Attention and information processing speed
A poor performance on SDMT was associated with high age, EDSS, T2-LL, and reduced nWMV, whereas gender, clinical variables, nGMV, ICV or interaction terms between ICV and brain atrophy measures did not contribute to the score significantly.
Regarding PASAT tasks, performance was associated with nGMV, whereas demographic and remaining variables, T2-LL, nWMV, ICV or interaction terms between ICV and brain atrophy measures did not contribute to the score significantly.
Verbal fluency and inhibitory control
A poor performance on WLG and ST was associated with reduced nWMV. Demographic, clinical parameters, the remaining brain atrophy measures, and interaction between ICV and all MRI parameters were not associated with cognitive score.
Cognitive reserve
RES-VOC did not correlate with any MRI measure (T2-LL: r = − 0.169, p = 0.097; nGMv: r = 0.054 p = 0.598; nWMV: r = 0.198, p = 0.052).
Table 3 reports significant predictors of performance on each neuropsychological test obtained from multiple hierarchical regressions.
Memory
Performance on SRT-LTS, SRT-CLTR and SRT-D was associated with RES-VOC; score on SRT-LTS and SRT-CLTR was related to age. Poor performance on SRT-LTS was associated with higher T2-LL, whereas gender, clinical parameters, nGMV, nWMV, ICV or interaction terms between RES-VOC and brain atrophy measures did not contribute to the scores significantly. The performance on SRT-D was significantly associated with reduced nGMV, whereas gender, clinical parameters, T2-LL, nWMV, ICV or interaction terms between RES-VOC and brain atrophy measures did not contribute to the score significantly. As regards spatial memory, higher score on SPART tests was significantly associated with high RES-VOC, low EDSS and low T2-LL. Age, gender, clinical variables, nGMV, nWMV, or interaction terms between RES-VOC and brain atrophy measures did not contribute to the score significantly.
Attention and information processing speed
A better performance on PASAT 3″ and 2″ was associated with higher RES-VOC and nGMV. PASAT 2″ was associated with gender. PASAT tests were not associated with the other demographic, clinical T2-LL, nWMV, ICV or interaction terms between RES-VOC and brain atrophy measures did not contribute to the score significantly.
As for SDMT, better performance was associated with higher RES-VOC, higher nWMV and lower age, EDSS and T2-LL score. Gender, other clinical variables, nGMV, ICV or interaction terms between RES-VOC and brain atrophy measures did not contribute to the score significantly.
Verbal fluency and inhibitory control
We also found that better performance on WLG was associated with higher RES-VOC and nWMV scores, whereas demographic and clinical variables, T2-LL, nGMV, ICV or interaction between RES-VOC and brain atrophy measures was unrelated with cognitive score.
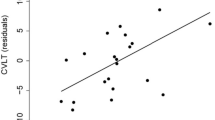
Finally, a better performance on interference task of ST was associated with lower disease duration, high RES-VOC and nWMV. The T2-LL or nGMV was not associated with cognitive score. Moreover, we found an interaction between RES-VOC and nWMV indicating that higher performance on RES-VOC moderates the relationship between WM atrophy and performance on ST. The interaction between RES-VOC and other brain atrophy measures was not associated with performance at ST.
Discussion
The present study explored the relationships of both CR and BR with cognitive functions in RRMS patients, after controlling for a large number of factors affecting cognitive performance in such a disease [4]. Moreover, we assessed the possible moderating role of CR and BR in the association between MRI-measured pathology/damage and cognitive outcomes.
The importance of controlling several key factors in studies on the effects of CR and BR is confirmed by the observation that in MS exists a significant link between cognitive functions and clinico-demographic parameters as well as measures of structural damage (T2-LL, GM and WM atrophy, etc.) [30,31,32,33]. For instance, while some studies demonstrated a significant association of focal WM lesions with poor cognitive performance [31], others suggested a more prominent contribution of WM atrophy on some specific cognitive domains [32], and others pointed to the relevance of the GM atrophy on cognitive deterioration in MS [33, 34].
In the present paper, we found that reduced nWM volume contributes to poor performance on SDMT, further supporting previous finding about a role of WM atrophy on reduced attention [30] and information-processing speed [31, 32]. Moreover, a positive relationship between nWMV, WLG and Stroop test suggested that WM damage contributed to reduced verbal fluency and altered inhibitory control. The negative association between T2-LL and SPART score confirmed that higher lesion load contributed to poor performance on spatial memory tasks [31, 33]. In agreement with some previous studies [30, 32, 33], the reduction of nGMV contributed to poor performance on verbal long-term memory and attention tests (assessed by SRT and PASAT). Taken together, our findings confirmed that GM and WM MS-related damage could negatively affect several cognitive domains such as memory, executive functions, attention and information-processing speed.
We did not find any relationship between BR and cognitive performance, thus suggesting that BR (or at least the surrogate MRI-derived measure we used) does not protect against deficits of memory, attention, and executive functioning. Our results partially diverge from previous studies [3, 19] in which BR did not protect against memory problems, but protected against disease-related cognitive inefficiency and decline in verbal fluency. The divergence in results might also reflect some methodological differences between previous studies [3, 19] and ours: Sumowski and colleagues [3, 19], indeed, enrolled a small sample of MS patients including two different forms of disease (RR-MS and secondary progressive MS) and evaluated cognitive efficiency and memory domains using composite z-scores. We, instead, enrolled a clinically homogeneous and large sample of MS patients and entered the z-score for each task of BRB and ST as dependent variables in the regression analysis.
The most important finding of the present study was that a higher CR was significantly related to a better performance on all cognitive tests of the BRB in a large sample of RRMS patients, after controlling for patients’ demographic, clinical, psychological and MRI features/variables. In this respect, it is worth mentioning that the lack of significant association between RES-VOC and brain atrophy confirmed that vocabulary knowledge does not relate to MS pathology and can be used as an easy, brief and standardized tool to estimate premorbid intellectual status/enrichment in MS patients [8, 11].
Our results demonstrate that CR strongly contributes to efficiency of cognitive abilities such as verbal and spatial memory, attention and information processing speed, in line with the idea that CR protects against the development of cognitive defects in MS. Therefore, our finding confirmed the strong association between higher CR and better performance on memory [3, 7, 13, 17,18,19,20,21, 24, 25], attention and information processing speed [7, 12, 13, 15,16,17, 20, 22,23,24], verbal fluency [17, 18, 21] tests reported in some studies on small or clinically heterogeneous samples of MS patients. As for executive functioning, we observed that CR has a protective role on impairment of inhibitory control, assessed by means of the ST as previously reported [10]. In addition, it is noteworthy that the relationship between nWMV and ST was moderate by RES-VOC, suggesting that intellectual enrichment can significantly reduce the negative impact of brain atrophy on this ability. Inhibitory control is a cognitive ability which allows to suppress, interrupt or delay an active behavior or cognitive course of action; it is related to social competence and emotional regulation and thus is essential for effective interaction with environment [35]. Dysfunctions of inhibitory control occur in a variety of clinical disorders and are associated with manifestation of behavioral problems such as impulsivity, irritability, compulsivity in psychiatric and neurological diseases. Moreover, among the components of executive functioning (e.g., planning, working memory, cognitive flexibility, sequencing), inhibition seems to be the most strongly related to instrumental activity daily living (IADL) integrity [36]. Our results, therefore, suggest that patients with low intellectual enrichment may be particularly at risk for developing difficulties in inhibitory control and functional impairments in performing IADL. However, since we found only one significant effect of the interaction between CR and white matter atrophy on ST performance, this result might be spurious and therefore it should deserve to be cautiously interpreted and better investigated in future studies.
Since CR seems to protect cognitive functions in MS directly and indirectly, through attenuating the negative impact of brain atrophy on cognition, in particular on inhibitory control, we suggest to routinely evaluate intellectual enrichment to identify MS patients at high risk for CI and difficulties in daily living. The early identification of these patients can be relevant to address them to early intervention cognitive training to build up or improve their cognitive reserve.
The main limitation of the present study is related to the cross-sectional nature of the study that did not allow us to shed light on the relationship between CR or BR and cognitive change over the course of the disease.
In conclusion, the present study evidences a protective role of CR on negative influence of MS-related brain damage on cognitive functioning, in particular on inhibitory control. These results encourage MS research to further investigate the potential role/utility of simple measures of CR, such the VOC, for early identification of MS patients at high risk for developing cognitive dysfunctions [4].
References
Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8(3):448–460
Valenzuela MJ, Sachdev P (2006) Brain reserve and dementia: a systematic review. Psychol Med 36(4):441–454
Sumowski JF, Rocca MA, Leavitt VM et al (2013) Brain reserve and cognitive reserve in multiple sclerosis: what you’ve got and how you use it. Neurology 80(24):2186–2193
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7(12):1139–1151
Benedict RH, Wahlig E, Bakshi R et al (2005) Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci 231(1–2):29–34
Sumowski JF, Chiaravalloti N, Deluca J (2009) Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol 31(8):913–926
Bonnet MC, Deloire MS, Salort E, Dousset V, Petry KG, Brochet B, AQUISEP Study Group (2006) Evidence of cognitive compensation associated with educational level in early relapsing-remitting multiple sclerosis. J Neurol Sci 251(1–2):23–28
Sumowski JF, Chiaravalloti N, Wylie GR, DeLuca J (2009) Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsych Soc 15(4):606–612
Sumowski JF, Chiaravalloti N, Leavitt VM, Deluca J (2012) Cognitive reserve in secondary progressive multiple sclerosis. Mult Scler 18(10):1454–1548
Chillemi G, Scalera C, Terranova C et al (2015) Cognitive processes and cognitive reserve in multiple sclerosis. Arch Ital Biol 153(1):19–24
Sumowski JF, Wylie GR, Gonnella A, Chiaravalloti N, Deluca J (2010) Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology 75(16):1428–1431
Benedict RH, Morrow SA, Weinstock Guttman B, Cookfair D, Schretlen DJ (2010) Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc 16(5):829–835
Martins Da Silva A, Cavaco S, Moreira I et al (2015) Cognitive reserve in multiple sclerosis: protective effects of education. Mult Scler 21(10):1312–1321
Feinstein A, Lapshin H, O’Connor P, Lanctôt KL (2013) Sub-threshold cognitive impairment in multiple sclerosis: the association with cognitive reserve. J Neurol 260(9):2256–2261
Luerding R, Gebel S, Gebel EM, Schwab-Malek S, Weissert R (2016) Influence of formal education on cognitive reserve in patients with multiple sclerosis. Front Neurol 7:46
Modica CM, Bergsland N, Dwyer MG et al (2016) Cognitive reserve moderates the impact of subcortical gray matter atrophy on neuropsychological status in multiple sclerosis. Mult Scler 22(1):36–42
Amato MP, Razzolini L, Goretti B et al (2013) Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurology 80(19):1728–1733
Sumowski JF, Wylie GR, Chiaravalloti N, DeLuca J (2010) Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology 74(24):1942–1945
Sumowski JF, Rocca MA, Leavitt VM et al (2014) Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology 82(20):1776–1783
Nunnari D, De Cola MC, Costa A, Rifici C, Bramanti P, Marino S (2016) Exploring cognitive reserve in multiple sclerosis: new findings from a cross-sectional study. J Clin Exp Neuropsychol 38(10):1158–1167
Rocca MA, Riccitelli GC, Meani A, Pagani E, Del Sette P, Martinelli V, Comi G, Falini A, Filippi M (2018) Cognitive reserve, cognition, and regional brain damage in MS: a 2-year longitudinal study. Mult Scler. https://doi.org/10.1177/1352458517750767
Booth AJ, Rodgers JD, Schwartz CE et al (2013) Active cognitive reserve influences the regional atrophy to cognition link in multiple sclerosis. J Int Neuropsychol Soc 19(10):1128–1133
Pinter D, Sumowski J, DeLuca J et al (2014) Higher education moderates the effect of T2 lesion load and third ventricle width on cognition in multiple sclerosis. PLoS One 9(1):e87567
Sumowski JF, Rocca MA, Leavitt VM et al (2016) Searching for the neural basis of reserve against memory decline: intellectual enrichment linked to larger hippocampal volume in multiple sclerosis. Eur J Neurol 23(1):39–44
Sumowski JF, Rocca MA, Leavitt VM, Riccitelli G, Meani A, Comi G, Filippi M (2016) Reading, writing, and reserve: literacy activities are linked to hippocampal volume and memory in multiple sclerosis. Mult Scler 22(12):1621–1625
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Stern Y (2009) Cognitive reserve. Neuropsychologia 47(10):2015–2028
Amato MP, Portaccio E, Goretti B et al (2006) The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12(6):787–793
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62(2):782–790
Preziosa P, Rocca MA, Pagani E et al (2016) Structural MRI correlates of cognitive impairment in patients with multiple sclerosis: a multicenter study. Hum Brain Mapp 37(4):1627–1644
Sacco R, Bisecco A, Corbo D et al (2015) Cognitive impairment and memory disorders in relapsing–remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. J Neurol 262(7):1691–1697
Sanfilipo MP, Benedict RH, Weinstock-Guttman B, Bakshi R (2006) Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 66(5):685–692
Damjanovic D, Valsasina P, Rocca MA et al (2017) Hippocampal and deep gray matter nuclei atrophy is relevant for explaining cognitive impairment in MS: a multicenter study. AJNR Am J Neuroradiol 38(1):18–24
Amato M, Portaccio E, Goretti B et al (2007) Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 64(8):1157–1161
Aron AR, Robbins TW, Poldrack RA (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18(4):177–185
Jefferson AL, Paul RH, Ozonoff A, Cohen RA (2006) Evaluating elements of executive functioning as predictors of instrumental activities of daily living (IADLs). Arch Clin Neuropsychol 21(4):311–320
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study was approved by the appropriate ethics committee and therefore was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Santangelo, G., Bisecco, A., Trojano, L. et al. Cognitive performance in multiple sclerosis: the contribution of intellectual enrichment and brain MRI measures. J Neurol 265, 1772–1779 (2018). https://doi.org/10.1007/s00415-018-8905-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8905-9