Abstract
The objective of this paper is to evaluate the association between physical disability in multiple sclerosis (MS) patients, the thickness of the retinal nerve fibre layer (RNFL) and corpus callosum volumes, as expressed by the corpus callosum index (CCI). This study was based on a cohort of 212 MS patients and 52 healthy control subjects, who were age and gender matched. The MS patients included 144 women and 177 relapsing-remitting MS (RRMS) patients. Peripapillary and volumetric optical coherence tomography (OCT) scans of the macula were performed using spectral-domain OCT technology. All magnetic resonance imaging (MRI) scans were performed using 1.5-T systems. CCI and RNFL were lower in MS than healthy control subjects (0.341 versus 0.386, p < 0.01 and 92.1 versus 105.0, p < 0.01). In addition, CCI correlated with RNFL (r = 0.464, p < 0.01). This was also true for the subgroup of patients with no history of optic neuritis (ON). There is a correlation between the thickness of the RNFL and CCI values in MS patients with no history of ON, which suggests that OCT might be a suitable marker for neurodegeneration in MS clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS), an inflammatory demyelinating and neurodegenerative disease of the central nervous system, is the most common, non-traumatic disabling neurological disease in young adults [1, 2]. Axonal loss is considered to be the predominant cause of ongoing disability in MS.
The retinal nerve fibre layer (RNFL) is composed predominantly of unmyelinated axons of retinal ganglion cells, which coalesce at the optic discs to form the optic nerves. Measurements of the peripapillary RNFL and macular thickness can be quantified objectively by optical coherence tomography (OCT), a high-resolution, quantitative retinal imaging technology [3]. The anterior visual pathway is an attractive model for understanding the relationship between inflammation, demyelination and neurodegeneration in MS. In vivo studies of the inner retina are particularly appropriate for the assessment and quantification of unmyelinated central nervous system axons. Histopathological studies have reported thinning of the RNFL in MS patients, and these changes have been correlated with disease activity and white matter lesion volume via magnetic resource imaging (MRI) [4, 5].
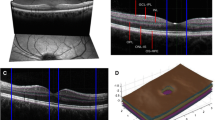
The corpus callosum (CC) is the largest compact white matter fibre bundle in the brain, connecting both hemispheres [6]. Previous MRI and histopathological studies have consistently revealed the CC to be one of the sites most commonly affected by demyelination and axonal loss [7, 8]. There are different ways to measure the CC, but the most practical method is the corpus callosum index (CCI), a normalised linear parameter that can be easily measured; lower CCI values indicate a lower CC volume [9] (Fig. 1). CC is a white matter tract that can be discretely identified by conventional MRI, having sharply demarcated two-dimensional limits on mid-sagittal T1-weighted imaging. The CCI has been suggested as a marker for brain atrophy in MS patients, which is easy to apply in the context of practical bedside parameters [9, 10].
According to Petzolt et al. and Frohman et al., the decrease in RNFL thickness (RNFLT) may reflect neurodegeneration, cerebral atrophy and progressive disease in MS patients [11, 12]. Investigating the associations amongst neurodegenerative changes for anatomically and physiologically unrelated structures of the brain would provide the opportunity to find clues concerning the global spreading of neurodegeneration in MS. The focus of this study is to assess the relationships between RNFLT, CC volume and clinical status in MS patients. We used CCI to represent CC volume. Our working hypothesis was that thinning of the RNFL is associated with CC atrophy in the MS population.
Methods
Subjects
We included 212 MS patients who had been admitted to our outpatient clinic (Department of Neurology, Yuzuncu Yıl University Medical Centre in Van, Turkey). The study was approved by the local Medical Ethics Committee (protocol number 2014/238) of the Yuzuncu Yıl University’s Faculty of Medicine. Written informed consent was obtained from all participants.
Inclusion criteria were being aged between 18 and 65 years with a definite diagnosis of MS, based on the McDonald criteria 2005 [13]. Patients were excluded from the study if they had suffered from optic neuritis (ON) in the previous 3 months, had steroid therapy less than 30 days before examination, were pregnant, had a refractive error of more than ±5.0 dioptres or any history of eye disease that could impact OCT measurements (e.g. age-related macular degeneration, diabetic retinopathy, uveitis, glaucoma or difficulty maintaining fixation). Disability was assessed using the Expanded Disability Status Scale (EDSS) [14]. Disease duration was defined to be the time from the first onset of MS symptoms. Healthy control subjects were included where there was no history of neurological or ophthalmic disease, where subjects were not pregnant and where they had no familial relation (first or second degree) to a MS patient.
Clinical and visual assessment
A detailed medical history, in particular of visual symptoms, was obtained from all study participants by face-to-face interviews with an experienced neurologist. EDSS scores were determined by the same neurologist to assess disability levels. The ophthalmologic assessment included best-corrected Snellen visual acuity, visual field analysis and direct ophthalmoscopy through dilated pupils. A history of ON was diagnosed by the treating physician and was cross-checked by medical records. Any previous incidence of ON had to be clearly determined either by patient self-reporting or via existing medical records. OCT scans and clinical examinations were performed in the same week. Brain MRI was performed within a month of the clinical examination.
OCT
OCT measurements of RNFLT were obtained on a high-resolution, spectral-domain OCT device (Heidelberg Spectralis-OCT, Heidelberg Engineering, Heidelberg, Germany). Measurements were taken by the same experienced operator for all participants. Three scans were taken without pupil dilation, and the best was chosen for analysis. All participants were examined using the peripapillary ring scan, which measures RNFLT around the optic nerve head in a circle with an angle of 12°. This resulted in a diameter of 3.4 mm, averaged to provide the RNFLT at 256 points along the circumference of the circular scan in each eye (see Fig. 2). The mean overall and temporal quadrant RNFLT measurements (nasal, temporal, superior and inferior) were obtained. OCT scans with signal strength equal to or greater than 7 (out of 10) were included in the study.
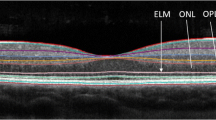
a Single frame of spectral-domain OCT images through the fovea and macular region of the right eye with retinal layers labelled from a MS patient. The nerve fibres of the retinal nerve fibre layer (RNFL) originate from the ganglion cells in the ganglion cell layer. The optic nerve is formed by the fibres of the RNFL which exit the retina at the optic disc. b Representative OCT images from the ON-unaffected left eye of the same patient. The upper panel shows the raw OCT retinal thickness map with RNFL segmentation between coloured lines and corresponding fundus image, the lower panel shows the RNFL thickness as a function of angle and the central circle shows the mean RNFL thickness for specific sectors. Colour indicates the relationship to normative data. NS superior nasal, N nasal, NI inferior nasal, TI inferior temporal, T temporal, TS superior temporal, G global
MRI analysis
All MRI scans were carried out in the Radiological Institute of the Yuzuncu Yıl University Medical Centre using 1.5-T systems (Siemens Magnetom Symphony, Erlangen, Germany) according to the recommendations of the MRI Working Group of the Consortium of Multiple Sclerosis Centres. Scans included axial pre- and post-gadolinium T1-weighted imaging, a sagittal fluid-attenuated inversion recovery (FLAIR) sequence and axial T2-weighted and axial FLAIR sequences in addition to sagittal T1-weighted imaging [15]. MRI acquisition parameters are the following: FLAIR using repetition time (TR)/echo time (TE)/inversion time (TI) = 8000/109/2370 ms, field of view (FOV) = 240 × 230 mm and slice thickness = 5 mm; sagittal T1-weighted spin-echo imaging using TR/TE = 489/9 ms, FOV = 230 × 230 mm and slice thickness = 5 mm; axial T2-weighted turbo spin-echo imaging using TR/TE = 3800/99 ms, FOV = 230 × 230 mm and slice thickness = 5 mm; axial T1-weighted spin-echo imaging using TR/TE = 489/9 ms, FOV = 230 × 190 mm and slice thickness = 5 mm; axial FLAIR imaging using TR/TE/TI = 8000/109/2370 ms, FOV = 230 × 200 mm and slice thickness = 5 mm; and axial diffusion-weighted imaging using b-values of 0 and 1000 s/mm2, TR/TE = 3200/100 ms and FOV = 230 × 230 mm. The contrast material (gadoteric acid or gadodiamide) was injected at 0.1 mmol/kg and coronal post-contrast T1-weighted spin-echo imaging performed using TR/TE = 489/9 ms, FOV = 230 × 190 mm and slice thickness = 5 mm.
All MRI scans were analysed by the same examiner (D) who was blind to patients’ clinical data. Seventy-five MRIs (35.3%) were evaluated by a second rater (AB), blind to both the results of D and the clinical data. The concordance rate was 0.92. For CC measurements, T1-weighted images were chosen because of their higher signal and contrast-to-noise ratio [9]. The measurements were made on a picture archiving and communication system workstation. The best mid-sagittal image was found by identifying the middle part of the fornix. After determining the widest anteroposterior diameter of the CC, we measured the anterior, posterior and middle segments of the CC. We then normalised the sum of these three segments to the anteroposterior diameter using a computerised measurement tool on screen, as illustrated in Fig. 1 and as described by Figueira et al. [9]. Higher CCI values indicate a higher CC volume.
Statistical analysis
Descriptive statistics for the continuous variables were presented as mean, standard deviation, minimum and maximum values, with count and percentages for categorical variables. Reliability was assessed as Cronbach’s alpha. CCI and overall and temporal quadrant RNFLT measurements were tested for significant differences. One-way analysis of variance (ANOVA) was used to compare group means. In the patient group, age and disease duration were considered as covariate variables for the OCT parameters. The Duncan multiple comparison test was also used to identify different group means, followed by ANOVA. The Shapiro-Wilk test was used for checking normality of the data. Spearman’s correlation analysis was carried out when normality assumption did not meet to determine linear relationships between the variables. In addition, the chi-squared test was performed to determine the relationship between categorical variables. The statistical significance level was measured as 5%; the Statistical Package for the Social Sciences (SPSS) was used for all statistical computations (version 22, IBM SPSS, Armonk, NY, USA).
Ethics statement
The study was approved by the Yuzuncu Yil University Faculty of Medicine Ethical Committee, and patients and controls were enrolled only after they signed the informed consent.
Results
Patient characteristics
A total of 212 MS patients and 52 healthy control subjects (17 male and 35 female) were enrolled in this study. Of the MS patients, 177 had relapsing-remitting MS (RRMS), 15 had primary progressive MS (PPMS) and 20 had secondary progressive MS (SPMS). The demographic data are summarised in Table 1. The mean disease duration of MS patients was 7.1 years. A hundred and forty-four patients (67.95%) were female. PPMS patients were older than SPMS patients with a mean age of 41.8 and 39.3 years, respectively. SPMS patients were older than RRMS (34.7 years) patients (p = 0.02). PPMS patients showed a shorter disease duration than SPMS and RRMS patients. A total of 85 patients (40.1%) had a history of ON, 16 had bilateral ON and 127 patients had no ON history. The median EDSS score was 2.5 (2.0–4.5), and mean EDSS was lower in female patients than in male patients (2.91 ± 1.86 in women versus 3.67 ± 2.19 in men, p = 0.01). The mean global and temporal quadrant RNFL was thinner and the CCI value was lower in the MS patients than in the control subjects (p < 0.01), as shown in Table 1. Eyes with a history of ON had a thinner temporal and global RNFL than those with no ON history (p < 0.01). Overall and temporal quadrant RNFLT values are shown in Table 2. In the same way, we found that the average RNFLT scores of unaffected contralateral eyes in patients with a history of ON were also subclinically abnormal when compared with the control group (see Table 1).
Group comparisons were carried out between MS subtypes. RRMS patients had higher CCI values and higher temporal and global RNFLT values than PPMS and SPMS patients (p < 0.01). There was no statistically significant difference between mean CCI and RNFLT values between PPMS and SPMS patients (p = 0.625).
Age and disease duration vary amongst MS subtypes; therefore, we used these variables for covariate analysis. We found that age did not have a statistically significant effect on the results of the covariate analysis in contrast to disease duration. After the covariate analysis, OCT parameters showed no statistically significant difference amongst MS subtypes (see Table 3).
Correlation analysis was carried out between RNFLT and CCI values in the control and patient groups. In contrast to the control group, the mean global and temporal quadrant RNFLT values in the patient group had a correlation with CCI values. Right-eye temporal quadrant and average RNFLT values had a correlation with CCI values (r 0.457, p < 0.01 and r 0.429, p < 0.01) as did left-eye temporal quadrant and average RNFLT values (r 0.463, p < 0.01 and r 0.464, p < 0.01).
The patient group was then divided according to ON history. In a correlation analysis of RNFLT, CCI and EDSS values in patients with no history of ON, the mean RNFLT had a positive correlation with the CCI and an inverse correlation with the EDSS score (Spearman rho = 0.533, p < 0.01 and Spearman rho = −0.539, p < 0.01, respectively). For details, refer to Fig. 3. In all patients, mean CCI values had a correlation with EDSS values (Spearman rho = −0.683, p < 0.01). For details, refer to Fig. 4.
Association of retinal nerve fibre layer thickness with corpus callosum index. Black dots represent the corpus callosum index and RNFL measures in eyes without history of optic neuritis. Long lines are from linear regression models without any corrections. Short lines are the 95% confidence intervals to the mean from this fit. CCI corpus callosum index, right ARNFL right eye average retinal nerve fibre layer thickness (μm), right TRNFL right eye temporal retinal nerve fibre layer thickness (μm), left TRNFL left eye temporal retinal nerve fibre layer thickness (μm), left ARNFL left eye average retinal nerve fibre layer thickness (μm)
Association of corpus callosum index and physical disability of patient group. Black dots represent the corpus callosum index and EDSS values of patients. Long lines are from the linear regression models without any corrections. Short lines are the 95% confidence intervals to the mean from this fit. CCI corpus callosum index, EDSS Expanded Disability Scale score (r −0.679, p < 0.01)
In the patient group, global and temporal quadrant RNFLT values were negatively associated with EDSS scores (Spearman rho = −0.435, p < 0.05). CCI values did not differ between patients with or without ON (p = 0.150).
Discussion
With neurodegeneration considered to be the principal pathological substrate underlying disability in MS, objectively assessing neurodegeneration in vivo in MS patients has long been a major priority. OCT enables the quantification of retinal structures at the optic disc, such as the RNFL, on a micro-metre scale. OCT offers a potential tool for monitoring axonal loss around the optic disc as retinal axonal measurements are not affected by myelin, making them ideal for investigating and tracking neurodegeneration. OCT has been consistently observed in MS. Besides MS, OCT is a well-established method for the quantification of neurodegeneration in several neurological diseases such as CADASIL [16], Alzheimer’s disease [17, 18] and Parkinson’s disease [19].
Non-conventional MRI techniques such as diffusion tensor imaging, magnetic transfer and myelin water imaging are not used in clinical practice due to the relatively complex and time-consuming post-processing needed to obtain these measurements. Therefore, our particular interest was CC measured via CCI values because the CCI is an easy and short-lasting method for clinicians to use routinely in outpatient clinics.
The RNFL and CC are anatomically and physiologically different structures whose neurodegenerative changes can be quantified separately. This fact makes them ideal for investigating possible associations. Does the spreading disease affect different areas to the same degree? The answer to this question will give us a great deal of information from a neurodegenerative perspective. The association between RNFLT and CC has not been much studied, whilst the association between CCI values and RNFLT in MS has been unexplored. A study reported a positive correlation between the volume of the central part of the CC and RNFL thickness in the literature [20].
We analysed the correlation between RNFLT and CCI values in a MS patient cohort. Notably, we found that lower RNFLT values in MS patients were associated with lower CCI values. Lower global and temporal quadrant RNFLT values had a correlation with lower CCI values, whereas there was no association between CCI and RNFL measurements in the control group. We also found this correlation in patients with no history of ON.
CC is normally resistant to age-related changes in healthy individuals [21], but it has been shown that CC atrophy emerges in MS patients over time. CC volume, CCI values and regional changes correlate well with disability in MS patients [22, 23]. Research has found that the thinning of the RNFL in MS is associated with the atrophy of whole-brain white matter and total deep-grey matter and with thalamus volumes [4, 24, 25].
In our study, the CCI values of the patient group were smaller than in the control group. Lower CCI values were associated with longer disease duration. The decrease in CC volumes may be the consequence of both demyelination and axonal loss. However, as the RNFL does not involve myelin, this can be read as an axonal pathology. The association between CCI and RNFLT values in the eyes of subjects with no history of ON is the most significant result from our study. It may indicate a possible global collective pathophysiologic background between non-anatomically related parts of brain.
As the mean disease duration of our patients was not long, the retinal pathology in eyes that were unaffected by ON may also suggest that neurodegeneration is not, in fact, limited to advanced disease stages but can begin early in disease development. Our results are corroborated by data from studies searching experimental autoimmune encephalomyelitis [26], clinically isolated syndromes [27] and brain histopathology [28].
The RNFL comprises axons of retinal ganglion cells (RGCs). Studies have reported depletion of the RGC layer in patients with MS with or without a history of ON [29–32]. This depletion may result from not only retrograde degeneration of damaged optic nerve axons as reported in most studies [29, 30] but also degeneration of primary RGCs in patients with MS [31, 32]. However, RNFL thinning may be caused by subpial degeneration of the optic nerve, leading to Wallerian degeneration, as Jehna et al. have suggested in their very recent study. They show a correlation between periventricular lesion burden and decreased cortical mean thickness, which they interpret as being indicative of subpial cortical pathology. This supports the concept that factors mediated by common cerebro-spinal fluid may play a role in accumulated damage to grey and white brain matter in MS [33]. In our study, the correlation between RNFLT and CCI values in patients not affected by ON is significant when we take into account the EDSS score and disease duration of the patient group. These findings may support subpial pathology.
Temporal-predominant peripapillary RNFL thinning is characteristic in MS [31, 34]. Although thinning of the RNFL is more severe in ON-affected eyes, RNFLT values in eyes that are not affected by ON are generally lower than in the control group [12]. Numerous studies have evaluated RNFL thickness in patients with MS with no history of ON, most of which reported RNFL thinning when compared to healthy control groups [35–37]. Our results are concurrent to those of these studies. The mean RNFLT of the control group is higher than that of the patient group (Table 1). As expected, eyes with a confirmed history of ON show an even more pronounced thinning of the RNFL (Table 2.) A total of 254 eyes that were not affected by ON had thicker RNFL values than the 170 ON-affected eyes. When we assessed average RNFLT values for unaffected contralateral eyes in patients with a history of ON, we found that these eyes were also subclinically abnormal when compared with the control group.
In our study, lower CCI values were associated with longer disease duration. We also found an association between EDSS scores and CCI values and between EDSS scores and RNFLT values. This association was also present in patients with no ON history (Table 3).
The findings of studies investigating RNFLT in MS subgroups are contradictory. Some studies show RNFL thinning in PPMS patients in contrast to RRMS patients. However, others have reported no difference [38–41]. In our study, RNFLT values were lower in PPMS patients than in RRMS patients before covariate analysis. However, after covariate analysis for the potential confounders of age and disease duration, we found no statistically significant difference between MS subtypes in terms of OCT parameters (Table 3). As expected, disease duration was longer in the SPMS group when compared with the RRMS and PPMS groups. There was no difference between the SPMS and PPMS groups in terms of RNFLT and CCI values.
Over time, RNFL thinning continues to develop in MS patients even in the absence of clinical activity. This enables the monitoring of subclinical disease progression [42]. Our study found that RNFLT has a correlation with CC structures in MS patients, which is not anatomically related. It is possible to elicit information about the process of global central nervous system (CNS) disease by combining the use of RNFLT and CCI measures. Assessing these measures in conjunction may also be highly informative and corroborative from a neurodegenerative perspective. These measurements of two unrelated anatomical structures are easily applied, quick, non-invasive and reproducible. They can be used in outpatient clinics and also in clinical trials investigating and tracking neurodegeneration and, potentially, neuroprotection and neurorestoration in MS. Accordingly, we recommend that clinical trials investigating treatment options should use these two measures in conjunction to monitor treatment response.
Conclusions
In conclusion, our data shows that CC and RNFLT values are lower in MS patients when compared with healthy control subjects. Our findings also show a correlation between CCI and RNFLT values independent of ON history. This supports the possibility that damage to axons in the retina may be concurrent with similar changes in the brain. In the absence of clinically evident inflammation, the presence of axonal loss may play a significant role in detecting neurodegeneration.
References
Friese MA, Montalban X, Willcox N, Bell JI, Martin R, Fugger L (2006) The value of animal models for drug development in multiple sclerosis. Brain 129:1940–1952
Manrique-Hoyos N, Jürgens T, Grønborg M, Kreutzfeldt M, Schedensack M, Kuhlmann T, Schrick C, Brück W, Urlaub H, Simons M, Merkler D (2012) Late motor decline after accomplished remyelination: impact for progressive multiple sclerosis. Ann Neurol 71:227–244
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Carmen AP, James GF (1991) Optical coherence tomography. Science 254:1178–1181
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, Frohman EM, Cutter G, Calabresi PA (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69:1603–1609
Grazioli E, Zivadinov R, Weinstock-Guttman B, Lincoff N, Baier M, Wong JR, Hussein S, Cox JL, Hojnacki D, Ramanathan M (2007) Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J Neurol Sci 268:12–17
Hines M, Chiu L, McAdams LA, Bentler PM, Lİpcamon J (1992) Cognition and the corpus callosum: verbal fluency, visuospatial ability, and language lateralization related to midsagittal surface areas of callosal subregions. Behav Neurosci 106:3–14
Pelletier J, Suchet L, Witjas T, Habib M, Guttmann CR, Salamon G, Lyon-Caen O, Chérif AA (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing remitting multiple sclerosis. Arch Neurol 58:105–111
Barkhof FJ, Elton M, Lindeboom J, Tas MW, Schmidt WF, Hommes OR, Polman CH, Kok A, Valk J (1998) Functional correlates of callosal atrophy in relapsing-remitting multiple sclerosis patients. A preliminary MRI study. J Neurol 245:153–158
Figueira FF, Santos VS, Figueira GM, Silva AC (2007) Corpus callosum index: a practical method for long-term follow-up in multiple sclerosis. Arq Neuropsiquiatr 65:931–935
Yaldizli Ö, Atefy R, Gass A, Sturm D, Glassl S, Tettenborn B, Putzki NJ (2010) Corpus callosum index and long-term disability in multiple sclerosis patients. Neurol 257:1256–1264
Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ (2008) Optical coherence tomography: a window in to the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 4:664–675
Petzold A, De Boer JF, Schippling S, Vermersch P, Kardon R, Green A, Calabresi PA, Polman C (2010) Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 9:921–932
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol 58:840–846
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 33:1444–1452
Consortium of Multiple Sclerosis Centers (2003) Available at http://www.mscare.org/
Pretegiani E, Rosini F, Dotti MT, Bianchi S, Federico A, Rufa A (2013) Visual system involvement in CADASIL. J Stroke Cerebrovasc Dis 22:1377–1384
La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, Sambati L, Pan BX, Tozer KR, Barboni P, Provini F, Avanzini P, Carbonelli M, Pelosi A, Chui H, Liquori R, Baruzzi A, Koronyo M, Sadun AA, Carelli V (2016) Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol 79:90–109
Oktem EO, Derle E, Kibaroglu S, Oktem C, Akkoyun I, Can U (2015) The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci 36:1141–1146
Jimenez B, Ascaso FJ, Cristobal JA, Val JL (2014) Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov Disord 29:68–74
Scheel M, Finke C, Oberwahrenbrock T, Freing A, Pech LM, Schlichting J, Sömmer C, Wuerfel J, Paul F, Brandt AU (2014) Retinal nerve fibre layer thickness correlates with brain white matter damage in multiple sclerosis: a combined optical coherence tomography and diffusion tensor imaging study. Mult Scler 20:1904–1907
Sullivan EV, Rohlfing T, Pfefferbaum A (2010) Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking. Dev Neuropsychol 35:233–256
Granberg T, Martola J, Bergendal G, Shams S, Damangir S, Aspelin P, Fredrikson S, Kristoffersen-Wiberg M (2015) Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: results of a 17-year longitudinal study. Mult Scler 21:1151–1158
Caligiuri ME, Barone S, Cherubini A, Augimeri A, Chiriaco C, Trotta M, Granata A, Filippelli E, Perrotta P, Valentino P, Quattrone A (2014) The relationship between regional microstructural abnormalities of the corpus callosum and physical and cognitive disability in relapsing–remitting multiple sclerosis. Neuroimage Clin 7:28–33
Young KL, Brandt AU, Petzold A, Reitz LY, Lintze F, Paul F, Martin R, Schippling S (2013) Loss of retinal nerve fibre layer axons indicates white but not grey matter damage in early multiple sclerosis. Eur J Neurol 20:803–811
Zivadinov R, Bergsland N, Cappellani R, Hagemeier J, Melia R, Carl E, Dwyer MG, Lincoff N, Weinstock-Guttman B, Ramanathan M (2014) Retinal nerve fiber layer thickness and thalamus pathology in multiple sclerosis patients. Eur J Neurol 21:1137–1144
Vogt J, Paul F, Aktas O, Müller-Wielsch K, Dörr J, Dörr S, Bharathi BS, Glumm R, Schmitz C, Steinbusch H, Raine CS, Tsokos M, Nitsch R, Zipp F (2009) Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann Neurol 66:310–322
Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J, Harmel J, Ruprecht K, Schippling S, Hartung HP, Aktas O, Brandt AU, Paul F (2013) Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler 19:1887–1895
Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Brück W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365:2188–2197
Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, Crainiceanu CM, Durbin MK, Oakley JD, Meyer SA, Frohman EM, Calabresi PA (2012) Optical coherence tomographysegmentation reveals ganglion cell layer pathology after optic neuritis. Brain 135:521–533
Huang-Link YM, Al-Havasi A, Lindehammar H (2015) Acute optic neuritis: retinal ganglion cell loss precedes retinal nerve fiber thinning. Neurol Sci 36:617–620
Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R (2010) Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 133:1591–1601
Kupersmith MJ, Gravin MK, Wang JK, Durbin M, Kardon R (2016) Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler 22:641–648
Jehna M, Pirpamer L, Khalil M, Fuchs S, Ropele S, Langkammer C, Pichler A, Stulnig F, Deutschmann H, Fazekas F, Enzinger C (2015) Periventricular lesions correlate with cortical thinning in multiple sclerosis. Ann Neurol 78:530–539
Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, Miller DH (2008) An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 131:277–287
Huang-Link YM, Fredrikson M, Link H (2015) Benign multiple sclerosis is associated with reduced thinning of the retinal nerve fibre and ganglion cell layers in non-optic-neuritis eyes. J Clin Neurol 11:241–224
Klistorner A, Garrick R, Barnett MH, Graham SL, Arvind H, Sriram P, Yiannikas C (2012) Axonal loss in non-optic neuritis eyesof patients with multiple sclerosis linked to delayed visual evoked potential. Neurology 80:242–245
Sriram P, Wang C, Yiannikas C, Garrick R, Barnett M, Parratt J, Graham SL, Arvind H, Klistorner A (2014) Relationship between optical coherence tomography and electrophysiology of the visual pathway in non-optic neuritis eyes of multiple sclerosis patients. PLoS One 9(8):102546
Balk L, Tewarie P, Killestein J, Polman C, Uitdehaag B, Petzold A (2014) Disease course heterogeneity and OCT in multiple sclerosis. Mult Scler 20:1198–1206
Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ (2012) Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One 7:e36847
Costello F, Hodge W, Pan YI, Eggenberger E, Freedman MS (2010) Using retinal architecture to help characterize multiple sclerosis patients. Can J Ophthalmol 45:520–526
Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C (2009) Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci 281:74–79
Saidha S, Calabresi AP (2014) Optical coherence tomography should be part of the routine monitoring of patients with multiple sclerosis: yes. Mult Scler 20(10):1296–1298
Acknowledgments
We would like to thank our patients and healthy volunteers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Institute the study was conducted: Yuzuncu Yil University Faculty of Medicine Neurology Clinic
Rights and permissions
About this article
Cite this article
Cilingir, V., Batur, M., Bulut, M.D. et al. The association between retinal nerve fibre layer thickness and corpus callosum index in different clinical subtypes of multiple sclerosis. Neurol Sci 38, 1223–1232 (2017). https://doi.org/10.1007/s10072-017-2947-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-2947-0