Abstract
Objective
To evaluate volumetric changes and discriminative power of intra-retinal layers in early-stage multiple sclerosis (MS) using a 3D optical coherence tomography (OCT) imaging method based on an in-house segmentation algorithm.
Methods
3D analysis of intra-retinal layers was performed in 71 patients with early-stage MS (mean disease duration 2.2 ± 3.5 years) at baseline and 40 healthy controls (HCs). All patients underwent a follow-up OCT scan within 23 ± 9 months. Patients with a clinical episode of optic neuritis (ON) more than 6 months prior to study entrance were compared with patients who never experienced clinical symptoms of an ON episode (NON).
Results
Significantly decreased total retinal volume (TRV), macular retinal nerve fiber layer (mRNFL) and ganglion cell—inner plexiform layer (GCIPL) volumes were detected in ON patients compared to NON patients (all p values < 0.05) at baseline. Each parameter on its own allowed identification of prior clinical ON based on a discriminative model (ROC analysis). Over time, TRV decreased in both ON (p = 0.013) and NON patients (p = 0.002), whereas mRNFL volume (p = 0.028) decreased only in ON and GCIPL volume (p = 0.003) decreased only in NON patients.
Conclusion
Our 3D-OCT data demonstrated that TRV, mRNFL and GCIPL allow discrimination between ON and NON patients in a cross-sectional analysis. However, the subsequent retinal atrophy pattern diverges in the initial phase of MS: Prior ON promotes sustained axonal thinning over time indicated by mRNFL loss, whereas longitudinal measurement of GCIPL volume better depicts continuous retrograde neurodegeneration in NON patients in early-stage MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple Sclerosis (MS) is a chronic neuroinflammatory disease leading to progressive disability with varying degrees of rapidity and severity among patients [1]. Therefore, the development of a sensitive and non-invasive imaging method which can mirror disease progression or allow prediction of the clinical outcome is essential for an optimized clinical management of the disease.
Recently, optical coherence tomography (OCT), using reflection patterns of spectral light from the retinal tissue to generate high-resolution images, has emerged as an encouraging paraclinical tool for quantifying the thickness of the retina, thus enabling the monitoring of retinal neurodegeneration [2]. A precise and detailed evaluation of retinal atrophy is feasible via segmentation of intra-retinal layers from OCT datasets.
The optic nerve is known to be a predilection site for inflammatory activity in MS, manifesting in retinal atrophy and visual deficits in these patients [3]. Between 25 and 75% of patients who experienced episodes of optic neuritis (ON) develop MS within 15 years depending on the presence or absence of white matter (WM) lesions [4].
In ON eyes, both initial swelling and eventual atrophy of the optic nerve are results of inflammation. Over time, these processes lead to thinning of the peripapillary retinal nerve fiber layer (pRNFL) and macular retinal nerve fiber layer (mRNFL) as well as the ganglion cell layer-inner plexiform layer (GCIPL) [5,6,7,8,9,10,11,12]. The relationship between the thickness of these intra-retinal layers and clinical and paraclinical progression markers has been investigated over the last years [7, 13].
Furthermore, widespread retinal damage was found to be more pronounced in ON eyes compared to non-affected (NON) eyes of MS patients, and retinal atrophy was shown to correlate with clinical disability [12, 14,15,16,17]. Moreover, the observed decrease in intra-retinal layer thickness was found to be related to “non-ocular” disease burden measurements depicted by magnetic resonance imaging (MRI) such as T2-hyperintense lesion load, WM and deep grey matter tissue volumes [10, 18, 19].
Two recent longitudinal studies have demonstrated that, in particular, degeneration of the pRNFL and GCIPL occurs most rapidly in the early stages of the disease and reaches a plateau in advanced stages [20, 21]. However, whether mRNFL and GCIPL atrophy, which are measurements of different compartments of the same cellular structures within the macula, run completely in parallel during the disease course or diverge with regard to prior ON is largely unknown.
The aim of the current study was to assess the discriminative power of intra-retinal layer volumes in the initial phase of the disease to distinguish between prior history of ON and NON in a cross-sectional analysis and to longitudinally evaluate the volume changes of the intra-retinal layers in patients with a history of ON in comparison to NON patients in an early-stage MS cohort using an extension of a published software for automated segmentation of OCT datasets for 3D data.
Methods
Participants
71 patients [51 relapsing–remitting (RR)MS, 20 clinically isolated syndrome (CIS)] and 40 healthy controls (HCs) were recruited at the outpatient clinics of the Department of Neurology, at the University Medical Centers in Mainz (Germany) and Münster (Germany).
The RRMS patients were diagnosed according to the 2010 revised McDonald diagnostic criteria [22]. CIS was defined as patients who had a first clinical episode with features suggestive of MS, but had not demonstrated dissemination in time required for the diagnosis of RRMS. Due to the joint group of CIS and early RRMS patients we refer to our cohort as “early-stage MS” patients comprising CIS and early RRMS patients with a mean disease duration of 2.2 ± 3.5 years.
Each patient was clinically assessed by an experienced neurologist and their Expanded Disability Status Scale (EDSS) score and corrected visual acuity (using Snellen’s chart test) were determined at study entrance.
Study protocol
The Advised Protocol for OCT Study Terminology and Elements (APOSTEL) recommendations were followed [23]. For this study, patients with a clinical ON episode more than 6 months prior to study entrance were compared with patients who had never experienced clinical symptoms of an ON episode (NON). From the enrolled patients, 14 presented with a previous, unilateral ON episode and three with a previous, bilateral ON episode. Patients with a new ON during the follow-up period (regardless of whether they were classified as an ON or NON patient at baseline) were excluded from the longitudinal analysis.
Other diseases affecting the optic nerve such as neuromyelitis optica spectrum disorder or chronic relapsing inflammatory optic neuropathy were excluded. In all patients, no history of glaucoma, high myopia (≥ − 6 dpt), retinopathy or other neurological disorders (besides CIS or RRMS) was recorded.
OCT image acquisition and scanning protocol
In both participating centers, OCT image acquisition was performed by an experienced operator following a unified standard acquisition protocol using a spectral-domain (SD)-OCT (Heidelberg Spectralis, Heidelberg Engineering, Germany) with Heidelberg Eye Explorer software (HEYEX). Scans were acquired without pupil dilatation in a shaded room with natural light. Macular volume was measured by a standardized scan compromising 61 vertical or horizontal B-scans while focusing on the fovea at a scanning angle of 30° × 25° and a resolution of 768 × 496 pixels, automatic real time (ART) = 9. HCs underwent one OCT scan, whereas early-stage MS patients had a follow-up OCT scan 23 ± 9 months later. All datasets were anonymized prior to exporting and further analysis. No further fundoscopic imaging was done.
Postacquisition data selection and analysis
For quality control of the OCT scans, all images were carefully reviewed to ensure that they fulfilled OSCAR-IB criteria [24]. To account for inter-eye within-patient dependencies, we calculated the mean of NON eyes in NON patients and the mean of ON eyes in ON patients with bilateral ON. In ON patients with a unilateral ON we only used the OCT scan of the affected eye. Hence, the main statistical analysis was performed on a per-patient level and only the visual acuity subanalysis was performed on a per-eye level. Detailed statistical approaches are described below.
OCT image segmentation analysis
For segmentation, the obtained scans were converted from E2E to non-compressed JPEG format, taking into account the relationship of 1 pixel: 3.9 µm. Our analysis used a modified version of a previously validated automatic segmentation technique: a MATLAB-based 2D segmentation algorithm [25]. This analysis approach was typically used for the center scan of the macula. Now, this automated segmentation was applied over a series of B-scans to calculate three dimensional volumes of the intra-retinal layers (Fig. 1). A detailed description of the segmentation analysis and its validation is given in the supplement (Supplementary Table S1 & S2 and Fig. S1). The total retinal volume (TRV) as well as the volumes of mRNFL, GCIPL, the inner nuclear layer (INL), the outer plexiform layer (OPL), the outer nuclear layer-inner segment (ONL-IS) and the outer segment-retinal pigment epithelium layers (OS-RPE) were measured.
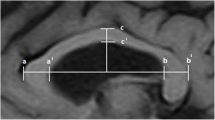
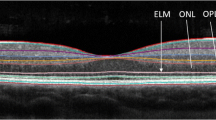
Flow scheme of automated 3D-OCT segmentation procedure. a Original volumetric OCT scan. b Retinal slice at macular level depicting the intra-retinal layers. c Border detection of the intra-retinal layers. d Envelope reconstruction in 3D for the segmented intra-retinal layers. mRNFL macular retinal nerve fiber layer, GCIPL ganglion cell-inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer, ONL-IS outer nuclear layer-inner segment, OS-RPE outer segment-retinal pigment epithelium layers
Statistical analysis
Statistical analysis was performed using SPSS 23 software (SPSS, Chicago, IL, USA) and was based on established statistical approaches in ophthalmology [26]. First, the Shapiro–Wilk test was performed on each dataset to check for normal distribution. The test showed non-normality for the clinical and demographic variables. Therefore, continuous (age and disease duration) and ordinal (EDSS) variables were compared using a Mann–Whitney U test. Categorical variables (sex) were compared using a Pearson’s Chi-square test.
With regard to the intra-retinal layer volumes, the Shapiro–Wilk test indicated that the assumption of normality had not been violated. Hence, we opted to calculate statistical significance for cross-sectional analyses using a multiple linear regression model for the different intra-retinal layers adjusting for age and sex (and additionally for EDSS and disease duration in patients) to compare differences in intra-retinal volumes at baseline between HCs and NON or ON patients, separately. The annual rate of volumetric change was calculated for each layer. Moreover, a paired t test was performed comparing volumetric changes over the study period in each group (NON and ON patients) in the investigated MS patients.
Furthermore, predictive discriminating values were calculated by a receiver operating characteristic (ROC) analysis using MedCalc© software (Version 17.2). This statistical method is preferentially used to make a series of discriminations into two different states (e.g., prior history of ON vs. NON) on the basis of a specific diagnostic variable (e.g., the individual intra-retinal layer volumes). Every value of that discriminating variable is used as a cut-off with calculation of the corresponding sensitivity and specificity. The maximum value of the Youden’s index, which assesses a classifying test’s performance, was calculated and used to select the optimal cut-off value for each ROC curve [27]. Finally, we compared ROC curves to test for statistical significance of the difference between the areas under the curves (AUCs) with the method of DeLong et al. [28] using MedCalc© software. p values less than 0.05 were considered statistically significant.
In a per-eye subanalysis, intra-retinal layer volumes were compared between ON eyes with impaired vision (corrected visual acuity < 1.0) and ON eyes with normal vision (corrected visual acuity ≥ 1.0) at baseline to determine if the volumes differed between these two groups at study entrance.
Results
Demographics
An overview of the demographic and clinical data of the investigated cohort is shown in Tables 1 and 2. The mean (± SD) age of the 71 patients was 32.0 ± 8.5 years. The mean disease duration was 2.2 ± 3.5 years and the median disability (quantified with the EDSS [29] score) was 1.5 [range 0–4.5]. The mean age of the 40 HCs was 35.9 ± 11.9 years.
Visual acuity
Corrected visual acuity was impaired in two NON patients (two NON eyes). Within the ON patients (including 20 ON eyes), eight ON eyes had an impaired vision, whereas the remaining 12 ON eyes demonstrated full visual recovery at study entrance. There was no significant difference between ON eyes with impaired vision and ON eyes with normal vision in all intra-retinal layer volumes (Supplementary Table S3).
Baseline 3D OCT data comparisons
At baseline, the segmented intra-retinal layer volumes for HCs, NON and ON patients were compared (Table 3). Volume loss of the TRV (p = 0.009), mRNFL (p < 0.001) and GCIPL (p < 0.001) was detected in ON patients in comparison to HCs. Accordingly, volume reductions in these layers were also seen in the comparison between ON and NON patients (p = 0.018 for TRV, p = 0.020 for mRNFL and p = 0.002 for GCIPL). Interestingly, no significant volume loss was seen in TRV, mRNFL and GCIPL comparing HCs and NON patients at study entrance (all p values > 0.05).
At baseline, between-group analyses based on the intra-retinal layer volumes obtained via automated 3D segmentation revealed a volume increase in INL (p < 0.001 for HCs vs. NON patients and p = 0.002 for HCs vs. ON patients) and a volume increase in OPL (p < 0.001 for HCs vs. NON patients and p = 0.006 for HCs vs. ON patients) in both NON and ON patients compared with the HC group (Table 3). The ONL-IS and OS-RPE volumes showed no significant difference between the groups (all p values > 0.05).
ROC analysis of baseline 3D OCT data
An overall ROC analysis (Fig. 2) was performed to determine the predictive discriminating value of the volumes of those retinal layers that were significantly different between NON and ON patients at baseline (namely TRV, mRNFL and GCIPL volumes). For all three ROC curves, the p-value for testing AUC = 0.5 vs. AUC ≠ 0.5 was less than 0.001 (Table 4). GCIPL volume below or above 2.06 mm3 predicted prior history of ON with a sensitivity of 70.6% and specificity of 79.6% (AUC = 0.794). TRV below or above 10.23 mm3 predicted prior history of ON with 70.6% sensitivity and 68.5% specificity (AUC = 0.751). Finally, mRNFL volume below or above 1.31 mm3 predicted prior history of ON with 58.8% sensitivity and 85.2% specificity (AUC = 0.733). The AUCs of the three ROC curves were not significantly different from each other (all p values > 0.05) indicating no superiority of one volumetric measurement over the other.
Receiver operating characteristic (ROC) curve classifying the groups (ON vs. NON patients) at study entrance. Predictive discrimination of total retinal volume (TRV), macular retinal nerve fiber layer (mRNFL) or ganglion cell-inner plexiform layer (GCIPL) volumes at baseline in ON vs. NON patients. The ROC curves of TRV (blue), mRNFL volume (green) and GCIPL volume (orange) are shown; the optimal cut-off value for each curve with the corresponding sensitivity and specificity based on the Youden’s index are indicated by dots
Longitudinal changes of the intra-retinal layers in NON and ON patients
During the follow-up period, five patients (3 in the ON group and 2 in the NON group) experienced an ON episode and were consequently excluded from the longitudinal analysis. Table 5 demonstrates the patterns of change in intra-retinal layer volumes within each group over time. In NON patients, significant decreases in TRV (p = 0.002), GCIPL (p = 0.003) and INL (p = 0.009) volumes were observed over the follow-up period. Patients with a previous ON episode (and without another ON episode during follow-up) showed a significant decrease in TRV (p = 0.013), mRNFL (p = 0.028) and INL (p = 0.042) over the follow-up time period (Table 5). In summary, TRV and INL decreased in both ON and NON patients, whereas mRNFL volume decreased only in ON and GCIPL volume only in NON patients.
Volumes of OPL, ONL-IS and OS-RPE did not significantly change over the study period in either NON or ON patients (all p values > 0.05).
The annual rate of change over time for each layer volume is given in Supplementary Table S4.
ROC analysis of longitudinal 3D OCT data
Finally, an overall ROC analysis was also performed to determine the discriminating power of the intra-retinal atrophy rates (longitudinal atrophy rates) and showed no significant results (data not shown).
Discussion
Marked thinning of the neuronal and axonal components of the retina was demonstrated early in the course of MS [12, 30]. Studies focusing on the discrimination between healthy controls and MS patients have pointed towards a better sensitivity of the GCIPL compared to pRNFL [31]. However, apart from the presence or absence of prior ON, it is important to capture the atrophy over time, as retinal atrophy during the initial stages of the disease mirrors disease progression [13]. Thus, the aim of our study was to evaluate volumetric changes of intra-retinal layers in early-stage MS patients using a 3D OCT imaging method based on an in-house segmentation algorithm and to determine the potential to infer a prior ON episode based on these changes.
The cross-sectional analysis comparing NON and ON patients demonstrated that ON patients had markedly reduced TRV, mRNFL and GCIPL volumes at least 6 months after ON, whereas no significant changes were seen in the remaining layers. Probably due to the short disease duration, only INL and OPL in NON patients revealed a significant difference compared to healthy subjects at baseline.
In the discrimination model, the decrease in mRNFL or GCIPL was found to predict a prior clinical episode of ON with a moderate sensitivity and high-specificity without demonstrating superiority of any one measure. Overall, the discriminative ability of TRV, mRNFL and GCIPL in regard to prior ON was not significantly different. The analysis additionally provided volumetric cut-off values for these layers to distinguish ON from NON patients.
Finally, a substantial loss of TRV could be shown in both patients with and without ON during the almost 2-year observation period. However, in this early-stage MS cohort and with a period of at least 6 months since suffering from an acute ON in the ON group, during the follow-up period mRNFL volume decreased only in ON, and GCIPL volume decreased only in NON patients, despite both volumes being lower in ON patients at baseline. This suggests that documented acute inflammatory episodes have minor impact on the atrophy of the GCIPL 6 months beyond the event.
Our cross-sectional results demonstrate that a focal inflammatory attack of the optic nerve’s retrobulbar axons leads to a thinning of the inner-retinal axons and ganglion cell degeneration as seen by a reduction in GCIPL and mRNFL volumes in comparison to NON patients at baseline. In addition, our study provides evidence for a divergent pattern of those two layers in the initial phase of the disease (and 6 months beyond the event in the ON group). Over the observation period of an average of almost 24 months we were able to demonstrate a significant decrease in TRV for ON as well as for NON patients. Interestingly, for NON patients this seems to be mainly due to a loss in GCIPL whereas for ON patients the loss in TRV was mostly due to a loss in mRNFL.
The sustained mRNFL volume loss in ON patients and the absence of significant GCIPL loss in those patients supports the idea that early functional changes of the axons in the optic nerve may contribute to cell shrinkage or loss (immediate GCIPL loss) before structural damage of axons occurs (later mRNFL thinning).
This divergent atrophy pattern over time is partly in line with previous observations that provided evidence that the thickness of the GCIPL predominantly reduces within the first weeks of an acute ON onset indicating that GCIPL thinning takes place quite early after an ON [32], but might reach a plateau afterwards as indicated by our findings. Hence, the results of the cross-sectional and longitudinal analysis suggest that demyelination of optic nerve axons after an acute ON leads to sustained thinning of the unmyelinated axons over time (mRNFL volume reduction in ON patients), whereas longitudinal GCIPL volume loss likely provides a better estimate in NON patients in terms of continuous neurodegeneration independent of clinical ON involvement.
Our observations extend previous reports based on 2D segmentation of OCT data [6, 7, 16, 21, 33] as well as studies using other segmentation tools indicating that atrophy of mRNFL and GCIPL is a direct result of the demyelination of the optic nerve [8, 11, 12]. A loss in visual acuity as well as a loss in color vision has been linked to the thickness of GCIPL and mRNFL [34, 35], providing potential OCT-based predictors of visual impairment following ON [36]. In our cohort, the lack of difference in intra-retinal layer volumes between ON patients with and without persistent visual impairment may relate to the small amount of ON patients or the imprecision of visual acuity testing with Snellen’s charts. However, reported correlations between visual acuity and intra-retinal layer thickness were similar for ON and NON eyes [37] and relatively weak [38], presumably due to the fact that MS-related visual impairment can be additionally affected by posterior visual pathway pathology or impaired ocular motility, which are not captured with OCT. Moreover, thinning in GCIPL has been correlated with reduced quality of life in MS patients with or without ON [20]. In addition, both pRNFL and mRNFL thinning, which is primarily an estimate for neuroaxonal damage of the retina, has been associated with brain volume loss and also with disability worsening [15, 39, 40].
In summary, our findings demonstrate that mRNFL and GCIPL seem to reveal a different timing of retinal neuronal and axonal loss in early-stage MS patients. We can argue that GCIPL thinning in ON patients seems to precede mRNFL thinning, which has been shown for pRNFL in a longitudinal pilot study with unilateral ON [41] and is also in line with previous findings demonstrating that mRNFL atrophy develops more slowly than GCIPL atrophy in ON patients [32, 42].
However, some caveats need to be considered when interpreting these divergent patterns. First, there is certainly a non-significant mRNFL loss over time also for NON patients, prompting us to interpret the mRNFL difference between ON an NON patients with more caution. On the other hand, the longitudinal difference in GCIPL between NON and ON patients seems to be marked. Second, the rationale for diverging mRNFL and GCIPL measures is inherently difficult (when investigating mRNFL and GCIPL separately), as these two measures reflect biological extensions of one another, rather than being remotely connected components of the CNS. Third, when comparing the atrophy rates over the observation period we found no significant discriminative power in the ROC analysis (in any intra-retinal layer), suggesting that both the rate and strength of atrophy among patients, in particular within the ON group, are too heterogeneous to make a precise discrimination between ON and NON patients based on our longitudinal data. In contrast, Graham and colleagues demonstrated in a ROC analysis that pRNFL provided better sensitivity/specificity for detecting change over time than GCIPL [21].
Taken together, available longitudinal studies consistently demonstrate progressive loss of mRNFL and pRNFL as well as GCIPL in patients with MS and that the decline does not follow a linear process, but is rather the greatest in early phases of the disease [20]. The present data expand these observations by further providing a distinct atrophy pattern for those layers in early-stage MS patients, but without sufficient discriminative power in the longitudinal ROC analysis.
Besides the main findings, we could further demonstrate higher volumes of INL and ONL at baseline for patients with or without ON in comparison to HCs. Over the observation period, a subtle (yet significant) thinning of INL over time was documented in both ON and NON patients. The aetiology behind these changes in INL volumes is currently under debate [8]. Most likely the initial thickening in INL and ONL is due to an inflammatory process with gliosis [43]. In a recent study investigating the link between retinal atrophy and MRI activity, INL thickness was found to positively correlate with MRI T2-lesion load in patients with radiologically isolated syndrome, and in CIS patients INL thickness was associated with the progression to clinically definite MS [44]. The initial thickening of INL reflects chronic inflammatory processes probably linked to glial activation in the retina as well as continuous inflammatory activity in the CNS [30]. Our data further suggest that this initial INL thickening seems to fade out over time, pointing towards a primordial response of the layer after disease manifestation.
Our study is not without limitations. On the one hand, the group of ON patients was small as we retrospectively divided the patients into ON and NON patients, and hence the statistical power could be increased with a larger and equivalent number of patients. On the other hand, we specifically concentrated on patients in the initial phase of the disease, but did not further distinguish between CIS and RRMS, which might also display differences in OCT measurements and should be further investigated in a comparative study.
Ultimately, our data emphasize the effects of previous episodes of ON on mRNFL and GCIPL in the initial stage of the disease. The 3D-OCT data demonstrated that TRV, mRNFL and GCIPL allow discrimination between ON and NON patients in a cross-sectional analysis without demonstrating superiority of any one measure. Our almost 2-year follow-up findings, however, provide evidence that the subsequent retinal atrophy pattern diverges in the initial phase of MS: Prior ON promotes sustained axonal thinning over time indicated by mRNFL loss, whereas longitudinal measurement of GCIPL volume better depicts continuous retrograde neurodegeneration in NON patients in early-stage MS.
References
Zipp F, Gold R, Wiendl H (2013) Identification of inflammatory neuronal injury and prevention of neuronal damage in multiple sclerosis: hope for novel therapies? JAMA Neurol 70(12):1569–1574
Balk LJ, Twisk JW, Steenwijk MD, Daams M, Tewarie P, Killestein J et al (2014) A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry 85(7):782–789
Barkhof F, Calabresi PA, Miller DH, Reingold SC (2009) Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nature Rev Neurol 5(5):256–266
Shams PN, Plant GT (2009) Optic neuritis: a review. Int MS J 16(3):82–89
Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A et al (2010) Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 9(9):921–932
Balk LJ, Petzold A (2014) Current and future potential of retinal optical coherence tomography in multiple sclerosis with and without optic neuritis. Neurodegener Dis Manag 4(2):165–176
Garcia-Martin E, Ara JR, Martin J, Almarcegui C, Dolz I, Vilades E et al (2017) Retinal and optic nerve degeneration in patients with multiple sclerosis followed up for 5 years. Ophthalmology 124:688–696
Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R (2010) Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 133(Pt 6):1591–1601
Sriram P, Graham SL, Wang C, Yiannikas C, Garrick R, Klistorner A (2012) Transsynaptic retinal degeneration in optic neuropathies: optical coherence tomography study. Investig Ophthalmol Vis Sci 53(3):1271–1275
Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C et al (2013) Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 80(1):47–54
Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E et al (2012) Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 135(Pt 2):521–533
Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J et al (2013) Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler 19(14):1887–1895
Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T et al (2016) Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol 15(6):574–584
Albrecht P, Frohlich R, Hartung HP, Kieseier BC, Methner A (2007) Optical coherence tomography measures axonal loss in multiple sclerosis independently of optic neuritis. J Neurol 254(11):1595–1596
Dorr J, Wernecke KD, Bock M, Gaede G, Wuerfel JT, Pfueller CF et al (2011) Association of retinal and macular damage with brain atrophy in multiple sclerosis. PloS One 6(4):e18132
Gracien RM, Jurcoane A, Wagner M, Reitz SC, Mayer C, Volz S et al (2016) Multimodal quantitative MRI assessment of cortical damage in relapsing-remitting multiple sclerosis. J Magn Resonance Imaging JMRI 44(6):1600–1607
Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, Keser N et al (2012) Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int 2012:530305
Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD et al (2015) Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 78(5):801–813
Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, Shiee N et al (2013) Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol 70(1):34–43
Balk LJ, Cruz-Herranz A, Albrecht P, Arnow S, Gelfand JM, Tewarie P et al (2016) Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol 263(7):1323–1331
Graham EC, You Y, Yiannikas C, Garrick R, Parratt J, Barnett MH et al (2016) Progressive loss of retinal ganglion cells and axons in nonoptic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Investig Ophthalmol Vis Sci 57(4):2311–2317
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA et al (2016) The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 86(24):2303–2309
Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S et al (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PloS One 7(4):e34823
Droby A, Panagoulias M, Albrecht P, Reuter E, Duning T, Hildebrandt A et al (2016) A novel automated segmentation method for retinal layers in OCT images proves retinal degeneration after optic neuritis. Br J Ophthalmol 100(4):484–490
Fan Q, Teo YY, Saw SM (2011) Application of advanced statistics in ophthalmology. Investig Ophthalmol Vis Sci 52(9):6059–6065
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3(1):32–35
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
International Multiple Sclerosis Genetics Consortium, Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A et al (2013) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nature Genet 45(11):1353–1360
Knier B, Berthele A, Buck D, Schmidt P, Zimmer C, Muhlau M et al (2016) Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler 22(7):893–900
Narayanan D, Cheng H, Bonem KN, Saenz R, Tang RA, Frishman LJ (2014) Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler 20(10):1331–1341
Gabilondo I, Martinez-Lapiscina EH, Fraga-Pumar E, Ortiz-Perez S, Torres-Torres R, Andorra M et al (2015) Dynamics of retinal injury after acute optic neuritis. Ann Neurol 77(3):517–528
Cerovski B, Kutija MB, Vidovic T, Popovic-Suic S, Jandrokovic S, Kordic R et al (2013) The role of optical coherence tomography (OCT) in optic neuritis (ON). Coll Antropol 37(Suppl 1):121–125
Lampert EJ, Andorra M, Torres-Torres R, Ortiz-Perez S, Llufriu S, Sepulveda M et al (2015) Color vision impairment in multiple sclerosis points to retinal ganglion cell damage. J Neurol 262(11):2491–2497
Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB et al (2012) Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 119(6):1250–1257
Sanchez-Dalmau B, Martinez-Lapiscina EH, Torres-Torres R, Ortiz-Perez S, Zubizarreta I, Pulido-Valdeolivas IV et al (2018) Early retinal atrophy predicts long-term visual impairment after acute optic neuritis. Mult Scler 24(9):1196–1204
Balk LJ, Coric D, Nij Bijvank JA, Killestein J, Uitdehaag BM, Petzold A (2018) Retinal atrophy in relation to visual functioning and vision-related quality of life in patients with multiple sclerosis. Mult Scler 24(6):767–776
Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA (2007) Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 69(22):2085–2092
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ et al (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69(16):1603–1609
Pietroboni AM, Dell’Arti L, Caprioli M, Scarioni M, Carandini T, Arighi A et al (2017) The loss of macular ganglion cells begins from the early stages of disease and correlates with brain atrophy in multiple sclerosis patients. Mult Scler. https://doi.org/10.1177/1352458517740214
Huang-Link YM, Al-Hawasi A, Lindehammar H (2015) Acute optic neuritis: retinal ganglion cell loss precedes retinal nerve fiber thinning. Neurol Sci 36(4):617–620
Gabilondo I, Sepulveda M, Ortiz-Perez S, Fraga-Pumar E, Martinez-Lapiscina EH, Llufriu S et al (2013) Retrograde retinal damage after acute optic tract lesion in MS. J Neurol Neurosurg Psychiatry 84(7):824–826
Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM et al (2017) Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 16(10):797–812
Knier B, Schmidt P, Aly L, Buck D, Berthele A, Muhlau M et al (2016) Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 139:2855–2863
Acknowledgements
This study was funded by the German Ministry for Education and Research (BMBF) German Competence Network Multiple Sclerosis (KKNMS) to FZ, SG, SGM and HW. We would like to thank Dr. Cheryl Ernest for proofreading the manuscript. The results presented are part of the doctoral thesis of LZ.
Author information
Authors and Affiliations
Contributions
CBS and AD analyzed and interpreted the data and wrote the manuscript. LZ collected and analyzed the data. MP developed the 3D expansion of the 2D automatic segmentation algorithm. LK, HW, JK and SGM organized patient recruitment and logistics and provided clinical information. SG helped to conceptualize the study, analyzed and interpreted the data. FZ and VF designed the project, were responsible for the concept, generated funding, organized patient recruitment, provided clinical information and wrote the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study was approved by the local ethics committees at the University Medical Centers in Mainz (Germany) and Münster (Germany) and performed in accordance with the Declaration of Helsinki. All participants gave their informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seitz, C.B., Droby, A., Zaubitzer, L. et al. Discriminative power of intra-retinal layers in early multiple sclerosis using 3D OCT imaging. J Neurol 265, 2284–2294 (2018). https://doi.org/10.1007/s00415-018-8988-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8988-3