Abstract
Background
Neurodegenerative processes are present since the early stages of multiple sclerosis (MS), constituting the primary substrate of disability. As part of the CNS, retinal damage could be considered a reliable prognostic biomarker of neurodegeneration in MS.
Objectives
To characterize longitudinal changes in the retinal layers’ thickness and to investigate correlations between retinal atrophy and other prognostic biomarkers, i.e., cerebrospinal fluid (CSF) β-amyloid1–42 (Aβ) levels.
Methods
Forty-two eyes without a history of optic neuritis of 23 MS patients were recruited. All patients underwent spectral-domain-OCT scans (SD-OCT), brain magnetic resonance imaging (MRI), and lumbar puncture at baseline. SD-OCT and brain MRI were repeated after 12 months. Ten controls underwent the same OCT procedure.
Results
At baseline, macular ganglion cell/inner plexiform layer (mGCIPL) thickness was reduced in patients compared to controls (p = 0.008), without retinal nerve fiber layer (RNFL) thinning, that was revealed only at follow-up (p = 0.005). Patients with lower CSF Aβ levels displayed reduced RNFL thickness values, both at baseline and follow-up.
Conclusions
At very early clinical stages, mGCIPL thickness values were reduced without a concomitant peripapillary RNFL thinning. The longitudinal assessment demonstrated a RNFL loss in patients compared to HC, together with a plateau of mGCIPL thinning. Aβlow subgroup of patients showed a reduction of retinal nerve fiber layer thickness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) represents a potentially severe cause of disability throughout adult life [1]. Alongside with chronic inflammation of the central nervous system (CNS), neurodegenerative processes may be present since the early stages of MS, constituting the primary substrate of irreversible disability [2,3,4,5,6,7,8].
As part of the CNS, the optic nerve (ON) is a major target of MS [3]. Optical coherence tomography (OCT), and particularly macular segmentation using spectral-domain-OCT (SD-OCT), represents a sensitive easy-accessible tool to investigate retinal damage in MS, and is currently considered a reliable prognostic biomarker of neurodegeneration [9,10,11,12,13]. Peripapillary and macular retinal nerve fiber layer (pRNFL and mRNFL), and macular ganglion cell/inner plexiform layer (mGCIPL) thinning results from axonal loss and neuronal damage of the inner retina. They correlate with visual function, global disability, and brain atrophy [14,15,16,17], and reflect MS-related ON neurodegeneration [11]. Conversely, inner nuclear layer (INL) thickening is linked to CNS inflammation, possibly due to the formation of microcystic macular oedema [18, 19].
The reduction of RNFL and mGCIPL results primarily from dying-back axonal loss in the ON after retrobulbar acute optic neuritis (ONe) [3], and is accelerated in MS patients exhibiting clinical-radiological disease activity [20,21,22]. Nevertheless, RNFL and mGCIPL thinning are described in MS even in the absence of clinical ONe [23, 24]. This is possibly due either to subclinical inflammation of the retina/ON, or to lesions in the visual cortex/optic radiations that lead to retinal structural changes (retrograde trans-synaptic degeneration) [9, 25]. Independently from optic neuropathy, several studies have shown that a primary process targeting retinal neurons may also act in MS, in a way analogous to the early damage of the gray matter (GM) [26, 27]. In a post-portem study, Green et al. observed retinal atrophy beyond the macular ganglion cell layer (mGCL) in MS patients and hypothesized that outer retina cell loss could be the consequence of a direct immune-mediated process [28]. Saidha et al. described a subset of MS patients with normal pRNFL but reduced macular thickness, not attributable to retrograde degeneration [29].
We recently observed in a small group of newly diagnosed MS without ONe (MSNON) patients a significant mRNFL and mGCIPL thinning compared to healthy controls (HC), without a concomitant reduction of the pRNFL [30]. Furthermore, we proposed cerebrospinal fluid (CSF) β-amyloid1–42 (Aβ) levels as a predictive biomarker of disease progression in MS. CSF Aβ levels were found to predict patients’ expanded disability status scale (EDSS) increase at 3 and 5 year of follow-up [31], and to correlate with GM atrophy and white matter (WM) damage [31, 32]. In this scenario, a possible cut-off value of CSF Aβ levels to identify patients with a worse prognosis has been proposed [32, 33].
Given these premises, the main aim of this study was to evaluate longitudinal changes in OCT measurements over a 12-month period in a cohort of newly diagnosed relapsing-remitting (RR)-MSNON patients in comparison with HC. Moreover, we evaluated whether CSF Aβ levels at diagnosis may be related to the structural retinal imaging findings, which are important biomarkers for early recognition of neurodegeneration in MS.
Materials and methods
Subjects
Table 1 summarized the main demographic and clinical characteristics of the population. Twenty-three patients with a new diagnosis of RR-MS according to the 2017 revisions of the McDonald criteria [34] were recruited and followed-up for 12 months.
All patients underwent lumbar puncture (LP) at baseline. Neurological evaluation with assessment of the EDSS, ophthalmological exam, SD-OCT, and brain magnetic resonance imaging (MRI) were performed at baseline and repeated after 12 months. Exams were performed before starting any treatment, including corticosteroids. After diagnosis the patients started a MS specific treatment, as specified in Table 1. No evidence of disease activity (NEDA) status was assessed at follow-up for each recruited subject as the absence of clinical relapses, increase in disability (as measured by EDSS), and new or enhancing lesions on their MRI scans. Ten age- and sex-matched healthy volunteers (HC) were also included and underwent ophthalmological visit and SD-OCT at baseline and follow-up.
Eyes with a history of ONe were excluded from the study, at baseline as well as the follow-up. Visuospatial abilities were carefully evaluated, with no evidence of impairment requiring further specific investigation. Regarding a potential subclinical ophthalmological involvement, subjects’ visual acuity and color vision were evaluated. All participants with a refractive error greater than 5.0 diopters (D), media opacity, systemic conditions that could affect the visual system, history of ocular trauma, or concomitant ocular diseases (including glaucoma or other known optic neuropathy) were excluded.
The current study was approved by the Institutional Review Board of the Fondazione IRCCS Ca′ Granda, Ospedale Maggiore Policlinico (Milan, Italy). All MS patients and HC gave their written informed consent for this research before entering the study.
Optical coherence tomography
OCT imaging in all subjects were performed with a SD-OCT device, using the built-in segmentation software of the Heidelberg Eye Explorer, version 1.10.2.0 (Spectralis, Heidelberg Engineering, Germany). All the sequences were acquired using the machine follow-up acquisition mode. The standard scanning protocol used at the baseline of the study included 61 high-speed B-scans and each scan was approximately 8.5 mm in length and spaced 118 μm apart. All 61 B-scans were acquired in a continuous, automated sequence, and covered a 30°× 25° area. A minimum of 20 frames were averaged automatically and used to obtain a good quality image. The central fixation target was used to center the raster scan to the fovea. Color-coded retinal thickness maps were generated automatically by the built-in software of the device by applying the ETDRS grid on the fovea and measurements of the retinal thickness were recorded. The ETDRS grid divides the macula into 3 concentric rings (center, inner, and outer), with the inner ring measuring 1 to 3 mm and the outer ring measuring 3 to 6 mm of diameter (referring to a ring with a diameter of 1-mm centered on the fovea). The grid further divides inner and outer rings into 4 quadrants (superior, inferior, temporal, and nasal) (Fig. 1). All individual retinal layers were measured with the new SD-OCT automatic segmentation explorer mapping software of the device (Heidelberg Engineering, Heidelberg, Germany). Good scan quality and automatic segmentation were assessed prior to the analysis by a trained ophthalmologist, and poor-quality images were rejected. The automated retinal segmentation software was applied to determine thicknesses of the following parameters: mRNFL, mGCL, macular inner plexiform layer (mIPL), macular inner nuclear layer (mINL), macular outer plexiform layer (mOPL), macular outer nuclear layer (mONL), and macular retinal pigment epithelium layer (mRPE). The mGCIPL was determined by combining the mGCL and mIPL parameters. For each layer, average thicknesses were calculated within ETDRS grid quadrants and were compared between the two groups. Using the follow-up acquisition mode, pRNFL thickness was measured with a 12° circular scan around the optic nerve with the activated eye tracker. The global average pRNFL thickness and the temporal sector RNFL thickness (pRNFL-T) were evaluated.
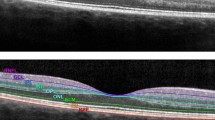
Spectral-domain optical coherence tomography (SD-OCT) follow-up analysis. Left column a: example, pRNFL thickness measurement follow-up analysis. First row: baseline exam; second row: follow-up exam.
Right column b: example, follow-up analysis of the automatic retinal segmentation follow-up analysis, specifically GCL thickness and color-coded thickness map. First row: baseline exam; second row: follow-up exam
CSF collection and Aβ determination
CSF samples were collected by LP in the L3/L4 or L4/L5 interspace. CSF samples were centrifuged at 8000 rpm for 10 min. The supernatant was aliquoted in polypropylene tubes and stored at − 80 °C until use. CSF cell counts, glucose, and proteins were determined. Albumin was measured by rate nephelometry. CSF Aβ was measured using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit (Fujirebio, Ghent, Belgium). According to our previous findings [40], we considered the threshold value of 813 pg/mL to divide patients into two groups (Aβhigh versus Aβlow).
Statistical analysis
Data were reported as mean (± standard deviation) and number (percentage) for continuous and categorical variables, respectively. Group differences between MS patients and HC were tested by Chi-square test for sex and Wilcoxon rank-sum test for age.
A generalized estimating equation (GEE) model was performed to assess differences between groups for RNFL variables (Table 3) investigated, in order to take into account the correlation between observations originated from the same patient (right and left eye). In the GEE model, the RNFL variables were used as dependent variables and an unstructured correlation matrix was used as correlation structure.
We also compared between-group changes at baseline and 12 months with a mixed model, using time and group (MS patients and HC) as fixed factors, interaction between time and group, patient as variable with a random effect, and an unstructured covariance matrix.
A p value lower than 0.05 was considered statistically significant. SAS 9.3 (Institute Inc., Cary, NC, USA) and R software (version 3.5.0) were used for all the analysis.
Results
Forty-two eyes of 23 RR-MS patients, without impairment of vision, color vision, and visuospatial abilities, were evaluated at baseline and follow-up, together with 20 eyes of 10 age- and sex-matched HC. Four eyes of 4 patients were excluded from the analysis due to previous history of ONe. At follow-up, 14 patients (27 eyes) were classified as NEDA, whereas 9 patients (15 eyes) do not reach all NEDA criteria. No lesions in the anterior visual pathway were observed at brain MRI at both time points of the study. A detailed list of all OCT measurements at the two time points of the study is listed in Table 3.
At baseline, mRNFL, mGCL, mIPL, and mGCIPL thickness values were significantly reduced in MS patients compared to HC (p = 0.03, p = 0.004, p = 0.02, and p = 0.008, respectively, Fig. 2), whereas no differences were found in pRNFL and pRNFL-T thickness. The longitudinal evaluation after 12 months confirmed that mRNFL, mGCL, mIPL, and mGCIPL thickness are reduced in MS patients compared to HC (p = 0.05, p = 0.008, p = 0.01, and p = 0.01, respectively, Fig. 3). At follow-up, mIPL was found significantly thinned compared to baseline in MS patients only (p = 0.02), and there was a trend towards mGCIPL and mINL thinning (p = 0.067 and p = 0.061, respectively). Follow-up revealed pRNFL and pRNFL-T loss in MS patients (both p = 0.005) and not in HC, but their thickness values still did not result significantly different in the two groups, except for a trend towards pRNFL-T thinning (p = 0.09). At both baseline and follow-up, mINL, mOPL, mONL, and mRPE did not differ between MS patients and HC, and mRNFL, mGCL, mOPL, mONL, and mRPE revealed no longitudinal changes in the two groups.
Baseline differences in mRNFL (a), mGCL (b), mIPL (c), and mGCIPL (d) thickness (in μm) between patients and controls. mRNFL, macular retinal nerve fiber layer; mGCL, macular ganglion cell layer; mIPL, macular inner plexiform layer; mGCIPL, ganglion cell layer-inner plexiform layer, HC, healthy control, MS, multiple sclerosis
Differences in mRNFL (a), mGCL (b), mIPL (c), and mGCIPL (d) thickness (in μm) betwenn patients and controls at follow-up (12 months after baseline). mRNFL, macular retinal nerve fiber layer; mGCL, macular ganglion cell layer; mIPL, macular inner plexiform layer; mGCIPL, ganglion cell layer-inner plexiform layer; HC, healthy control; MS, multiple sclerosis
No difference in all OCT measurements was found at baseline between active and non-active MS patients. Nevertheless, patients who do not reach all NEDA criteria at follow-up had a significant pRNFL, pRNFL-T, mGCL, mIPL, and mGCIPL loss (p = 0.026, p = 0.006, p = 0.017, p = 0.006, 0 = 0.014, respectively) that was not observed in patients classified as NEDA. EDSS score at follow-up was found to be associated with the thinning of mGCL (β = 0.029, p = 0.017) and mGCIPL (β = 0.47, p = 0.03) in MS patients.
We split our cohort of patients into two groups based on CSF Aβ levels (Aβlow under 813 pg/ml and Aβhigh over 813 pg/ml) [39]. According to their CSF Aβ levels, 9 MS patients were Aβlow (18 eyes) and 14 Aβhigh (24 eyes). No significant differences were found between Aβlow and Aβhigh subgroup of patients at baseline, i.e., age, gender, disease duration, and EDSS score (Table 2). Aβlow MS patients displayed reduced pRNFL-T and mRNFL thickness values as compared with Aβhigh already at baseline (p = 0.003 and p = 0.03, respectively), and the same result was observed longitudinally at follow-up for pRNFL-T (p = 0.003; Fig. 4), but not for mRNFL. However, the longitudinal evaluation revealed a significant mRNFL loss in the Aβlow group (p = 0.05) that was not observed in the Aβhigh group.
Discussion
In this study, we first confirmed that, at very early clinical stages, mRNFL, mGCL, mIPL, and mGCIPL thickness values were significantly reduced in MSNON patients compared to controls, whereas there were no differences in pRNFL thickness. Secondly, the longitudinal assessment after 12 months demonstrated pRNFL and pRNFL-T loss in MS patients and not in HC, without a significant and concomitant progressive thinning of mGCIPL.
Different studies have described longitudinal data regarding OCT finding in MS patients, showing both pRNFL and inner retinal thinning. According to a recently published systematic review and meta-analysis [12], the reported annual pRNFL atrophy rate was higher in MSON eyes (− 0.91 μm/year) compared with MSNON eyes (− 0.53 μm/year) [34], while the highest annual atrophy rate in MSNON patients was found in those who had a shorter disease duration [8]. A plateau effect was observed in patients with a longer disease duration (> 20 years).
Consistent with the data regarding the RNFL [12], atrophy of mGCIPL was more severe in MSON eyes than in MSNON eyes. Inner retinal layers thinning preceded pRNFL loss: most of the studies showed significant thinning of the GCL within the first month from symptoms onset [35], while RNFL atrophy became detectable only after at least 3 months.
Likewise, in our longitudinal study, we found a significant thinning over the time of both pRNFL and pRNFL-T in MS patients in comparison with HC, with an atrophy rate of − 2.5 μm/year, higher than the previous studies. Regarding the inner retinal layers longitudinal data, we found a decrease of their thickness (mRNFL, GCL, IPL, and GCIPL), although it was statistically significant only for IPL (atrophy rate 0.5 μm/year). According to our results, in MSNON patients pRNFL loss was more evident than inner retinal layers throughout the course of 12 months of the follow-up time.
We speculate that these data can reflect (1) the progressive, incipient axonal atrophy, that is known to happen later in time; (2) the plateau of the GCIPL atrophy, which was already detectable at the very beginning of the disease (the baseline of the study). The difference in annual atrophy rates of this study compared with previous ones (− 2.5 μm/year vs. − 0.53 μm/year) could be explained by differences in the demographic data, since the disease duration of our population was only 18 months, significantly shorter than the disease duration of the other cohorts.
Since a significant difference of pRNFL thinning between patients and HC was not found, we hypothesize that the amount of atrophy that occurred over a period of 12 months was not enough to reach the statistical significance. A longer follow-up is needed to support these hypotheses. Furthermore, considering that some studies conducted over a longer period of time described the progressive thinning of both pRNFL and inner retinal layer, we speculate that the plateau of the inner retinal atrophy might be transient in time, possibly mirroring the relapsing-remitting nature of the disease. An alternative hypothesis, or at least additional, that could explain our findings is based on the assumption that the ganglion cells’ damage could potentially precede peripapillary axons abnormalities in MS patients. In other words, the earliness of ganglion cell thinning may reflect a primary process targeting retinal neurons’ bodies before affecting the peripapillary axons. As already proposed [30], it is likely that previous studies reported a concomitant thinning of GCIPL and pRNFL because their results were not obtained during the very early stages of the disease. In line with this framework, we speculated that the retina itself may be a primary target of degenerative processes in MS, possibly in combination with inflammatory mechanisms, and as a finding of this study, we supported the previous hypothesis that retinal damage could begin from the macular ganglion cells and might not be only a consequence of inflammatory attacks to the anterior optic pathway. Supporting this theory, the thinning of the IPL observed in this study could reflect a retrograde atrophy secondary the ganglion cell loss.
In our view, a pRNFL-T significant loss with a concomitant plateau of mGCIPL thinning, already in the very early stages of the disease, is the most significant finding that seems to emerge from our study, as if to suggest a subsequent involvement of the peripapillary axons shortly after affecting the retinal neurons’ bodies.
In agreement with the previous findings in the literature [8, 9, 29], we did not find a significant progressive INL thinning, although a trend was observed. However, controversial results have been reported. Indeed, a thickening of this layer has been previously described and some authors tried to explain these differences by the occasional presence of macular oedema. Furthermore, although the INL has been described as a physiological barrier to retrograde trans-synaptic axonal degeneration [7], in human retinal post-mortem samples, extensive loss of neurons in the INL has been demonstrated after about 20 years of disease duration. Most studies suggested that the outer retinal layers do not differ in either MSON eyes or MSNON eyes compared with control eyes and that they do not change over a period of time. Likewise, we could not find any statistical difference or any progressive thinning of these layers over 12 months.
Interestingly, EDSS score was found to be associated with the thinning of mGCL and mGCIPL at follow-up. Several studies have examined the relationship between OCT measures and EDSS scores and inconsistent results have been reported [8, 22, 26]. Nevertheless, in a study performed in 132 patients, Saidha et al. [29] demonstrated that the GCIP thickness in MS patients correlated better than pRNFL thickness with EDSS score. Similarly, in our study, patients without all NEDA criteria at follow-up had a significant inner retinal layers loss that was not observed in patients classified as NEDA. Further follow-up time is needed to better elucidate these correlations over the time.
Moreover, we recently described a relationship between low CSF Aβ levels and worse prognosis in MS [31,32,33, 36, 37]. In line with these findings, Aβlow MS patients interestingly displayed a reduced internal retinal layers thickness in its entirety, compared to Aβhigh, although without reaching statistical significance for each layer. More in details, the most significant data concerned the reduced pRNFL-T thickness values in the Aβlow subgroup compared to Aβhigh, both at baseline and at follow-up. mGCIPL thickness values appeared reduced already at the baseline in MS patients compared to HC, although without significant differences between the two patient subgroups. We speculate that since mGCIPL are reduced at very early clinical stages, no difference could be detected among affected subjects. Taken together, this data seems to confirm a worse prognosis in Aβlow patients compared to Aβhigh, due to an early retinal involvement. In line with previous findings and speculations [31,32,33, 36, 37], the link we describe between CSF Aβ concentration and early retinal volume loss suggests the retinal layers may represent a site particularly susceptible both to inflammation and neurodegeneration.
There are some limitations when considering our study. First, we acknowledge that this represents an exploratory study and that a larger cohort of patients will be needed to confirm our findings. Second, it would be necessary to prolong the follow-up time to clarify the longitudinal changes of retinal layers in MS. Third, data from visual evoked potentials were not available. Further studies combining neurophysiological, neuroradiological, and OCT data are needed.
In conclusion, in this study we documented that, at very early clinical stages, mRNFL, mGCL, mIPL, and mGCIPL thickness values were significantly reduced in MSNON patients without a concomitant pRNFL thinning. The longitudinal assessment after 12 months demonstrated a pRNFL-T significant loss in MSNON patients compared to HC, together instead with a sort of plateau of mGCIPL thinning. Lastly, Aβlow subgroup of MSNON patients showed a significant reduction of retinal nerve fiber layer thickness compared to Aβhigh subgroup, already at the baseline.
References
Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, Khalil IA, Krohn KJ, Liang X, Naghavi M, Mokdad AH, Nixon MR, Reiner RC, Sartorius B, Smith M, Topor-Madry R, Werdecker A, Vos T, Feigin VL, Murray CJL (2019) Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study. Lancet Neurol
Friese MA, Schattling B, Fugger L (2014) Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol 10(4):225–238
Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378(2):169–180
Lassmann H (2018) Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol 9:3116
Bjartmar C, Trapp BD (2001) Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr Opin Neurol 14(3):271–278
Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132(5):1175–1189
Petzold A. (2018) Neurodegeneration and multiple sclerosis. In: NeurodegeneratIve Diseases: Clinical Aspects, Molecular Genetics and Biomarkers.; 379–400
Balk LJ, Cruz-Herranz A, Albrecht P, Arnow S, Gelfand JM, Tewarie P, Killestein J, Uitdehaag BMJ, Petzold A, Green AJ (2016) Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol 263(7):1323–1331
Costello F, Burton J (2018) Retinal imaging with optical coherence tomography: a biomarker in multiple sclerosis? Eye Brain 10:47–63
Costello F (2013) The afferent visual pathway: designing a structural-functional paradigm of multiple sclerosis. ISRN Neurol 2013:1–17
Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ (2008) Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 4(12):664–675
Petzold A, Balcer L, Calabresi PA et al (2017) Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 16(10):797–812
Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, Calabresi PA, Polman C (2010) Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 9(9):921–932
Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ (2006) Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 113(2):324–332
Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, Brandt AU, Pablo LE, Guerrieri S, Gonzalez I, Outteryck O, Mueller AK, Albrecht P, Chan W, Lukas S, Balk LJ, Fraser C, Frederiksen JL, Resto J, Frohman T, Cordano C, Zubizarreta I, Andorra M, Sanchez-Dalmau B, Saiz A, Bermel R, Klistorner A, Petzold A, Schippling S, Costello F, Aktas O, Vermersch P, Oreja-Guevara C, Comi G, Leocani L, Garcia-Martin E, Paul F, Havrdova E, Frohman E, Balcer LJ, Green AJ, Calabresi PA, Villoslada P, IMSVISUAL consortium (2016) Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol 15(6):574–584
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, Frohman EM, Cutter G, Calabresi PA (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 69(16):1603–1609
Saidha S, Al-Louzi O, Ratchford JN et al (2015) Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol 78(5):801–813
Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, Ratchford JN, Oh J, Seigo MA, Newsome SD, Balcer LJ, Frohman EM, Green AJ, Nguyen QD, Calabresi PA (2012) Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 11(11):963–972
Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ (2012) Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 135(6):1786–1793
Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P (2007) Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 68(18):1488–1494
Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C, Durbin MK, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome SD, Balcer LJ, Frohman EM, Calabresi PA (2013) Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 80(1):47–54
Pisa M, Guerrieri S, Di Maggio G et al (2017) No evidence of disease activity is associated with reduced rate of axonal retinal atrophy in MS. Neurology. 89(24):2469–2475
Talman LS, Bisker ER, Sackel DJ, Long da Jr, Galetta KM, Ratchford JN, Lile DJ, Farrell SK, Loguidice MJ, Remington G, Conger A, Frohman TC, Jacobs DA, Markowitz CE, Cutter GR, Ying GS, Dai Y, Maguire MG, Galetta SL, Frohman EM, Calabresi PA, Balcer LJ (2010) Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 67(6):749–760
Garcia-Martin E, Pueyo V, Almarcegui C, Martin J, Ara JR, Sancho E, Pablo LE, Dolz I, Fernandez J (2011) Risk factors for progressive axonal degeneration of the retinal nerve fibre layer in multiple sclerosis patients. Br J Ophthalmol 95(11):1577–1582
Gabilondo I, Sanchez-Dalmau B, Berenguer J et al (2013) Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol 75(1):98–107
Geurts JJG, Bö L, Pouwels PJW et al (2005) Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. Am J Neuroradiol 26(3):572–577
Calabrese M, Atzori M, Bernardi V, Morra A, Romualdi C, Rinaldi L, McAuliffe MJM, Barachino L, Perini P, Fischl B, Battistin L, Gallo P (2007) Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol 254(9):1212–1220
Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R (2010) Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 133(6):1591–1601
Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome S, Ratchford JN, Frohman EM, Calabresi PA (2011) Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler J 17(12):1449–1463
Pietroboni AM, Dell’Arti L, Caprioli M, Scarioni M, Carandini T, Arighi A, Ghezzi L, Fumagalli GG, de Riz MA, Basilico P, Colombi A, Benatti E, Triulzi F, Scarpini E, Viola F, Galimberti D (2019) The loss of macular ganglion cells begins from the early stages of disease and correlates with brain atrophy in multiple sclerosis patients. Mult Scler J 25(1):31–38
Pietroboni AM, Schiano Di Cola F, Scarioni M et al (2017) CSF β-amyloid as a putative biomarker of disease progression in multiple sclerosis. Mult Scler 23(8):1085–1091
Pietroboni AM, Caprioli M, Carandini T, et al. (2018) CSF β-amyloid predicts prognosis in patients with multiple sclerosis. Mult Scler J 135245851879170
Pietroboni AM, Carandini T, Colombi A, Mercurio M, Ghezzi L, Giulietti G, Scarioni M, Arighi A, Fenoglio C, de Riz MA, Fumagalli GG, Basilico P, Serpente M, Bozzali M, Scarpini E, Galimberti D, Marotta G (2019) Amyloid PET as a marker of normal-appearing white matter early damage in multiple sclerosis: correlation with CSF β-amyloid levels and brain volumes. Eur J Nucl Med Mol Imaging 46(2):280–287
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol
Graham EC, You Y, Yiannikas C, Garrick R, Parratt J, Barnett MH, Klistorner A (2016) Progressive loss of retinal ganglion cells and axons in nonoptic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Invest Ophthalmol Vis Sci 57(4):2311–2317
Pietroboni AM, Schiano di Cola F, Colombi A et al (2020) CSF β-amyloid predicts early cerebellar atrophy and is associated with a poor prognosis in multiple sclerosis. Mult Scler Relat Disord 37:101462. https://doi.org/10.1016/j.msard.2019.101462
Pietroboni AM, Colombi A, Carandini T et al (2020) Low CSF β-amyloid levels predict early regional grey matter atrophy in multiple sclerosis. Mult Scler Relat Disord 39:101899. https://doi.org/10.1016/j.msard.2019.101899
Acknowledgments
The authors thank Beatrice Clementi (University of Milan, Italy) for helping in collecting the data of the study.
Funding
This research was supported by the Italian Ministry of Health (Ricerca Corrente to ES, FV, and FT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The research documented in this manuscript has been carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pietroboni, A.M., Carandini, T., Dell’Arti, L. et al. Evidence of retinal anterograde neurodegeneration in the very early stages of multiple sclerosis: a longitudinal OCT study. Neurol Sci 41, 3175–3183 (2020). https://doi.org/10.1007/s10072-020-04431-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04431-4