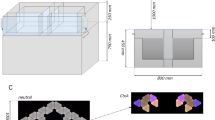
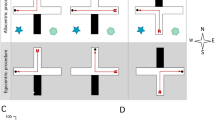
Abstract
The nature of the representation guiding spatial navigation has been investigated extensively; however, most of this work has used behavioral tasks that involved learning the location of food reward or an escape platform. In contrast, relatively few studies have focused on the spatial representation of a home base, a ubiquitous feature of open-field behavior, and its ability to be encoded relative to environmental cues. The current set of experiments investigated acquisition and retention of the location of home base establishment. In general, proximal cues anchored the position of the home base during acquisition sessions across all four experiments. Although mice established a home base during retention sessions, previous experience did not influence its position during retention sessions. These observations demonstrate that stimulus control of home base position depends on access to proximal cues. Further work is needed to determine the extent that home base establishment may provide a framework to encode goal-directed spatial behaviors.













Similar content being viewed by others
References
Akers KG, Candelaria FT, Hamilton DA (2007) Preweanling rats solve the Morris water task via directional navigation. Behav Neurosci 121:1426
Altmann J, Samuels A (1992) Costs of maternal care: infant-carrying in baboons. Behav Ecol Sociobiol 29:391–398
Alyan S, Jander R (1994) Short-range homing in the house mouse, Mus musculus: stages in the learning of directions. Anim Behav 48:285–298
Alyan S, Jander R (1997a) Exploration is sufficient but not necessary for navigation with landmarks in the house mouse (Mus musculus). Learn Motiv 28:558–576
Alyan SH, Jander R (1997b) Interplay of directional navigation mechanisms as a function of near-goal distance: experiments with the house mouse. Behav Process 41:245–255
Banovetz MT, Lake RI, Blackwell AA et al (2021) Effects of acquired vestibular pathology on the organization of mouse exploratory behavior. Exp Brain Res 239:1125–1139
Batschelet E (1981) Circular statistics in biology. Acad Press, NY
Blankenship PA, Cherep LA, Donaldson TN et al (2017) Otolith dysfunction alters exploratory movement in mice. Behav Brain Res 325:1–11
Blodgett HC, McCutchan K, Mathews R (1949) Spatial learning in the T-maze: the influence of direction, turn, and food location. J Exp Psychol 39:800
Burke CJ, Whishaw IQ (2020) Sniff, look and loop excursions as the unit of “exploration” in the horse (Equus ferus caballis) when free or under saddle in an equestrian arena. Behav Process 173:104065
Cheng K (1986) A purely geometric module in the rat’s spatial representation. Cognition 23:149–178
Clark BJ, Hines DJ, Hamilton DA, Whishaw IQ (2005) Movements of exploration intact in rats with hippocampal lesions. Behav Brain Res 163:91–99
Clark BJ, Hamilton DA, Whishaw IQ (2006) Motor activity (exploration) and formation of home bases in mice (C57BL/6) influenced by visual and tactile cues: modification of movement distribution, distance, location, and speed. Physiol Behav 87:805–816
Devan BD, Petri HL, Mishkin M et al (2002) A room with a view and a polarizing cue: Individual differences in the stimulus control of place navigation and passive latent learning in the water maze. Neurobiol Learn Mem 78:79–99
Donaldson TN, Jennings KT, Cherep LA et al (2018) Antisense oligonucleotide therapy rescues disruptions in organization of exploratory movements associated with Usher syndrome type 1C in mice. Behav Brain Res 338:76–87
Donaldson TN, Jennings KT, Cherep LA et al (2019) Progression and stop organization reveals conservation of movement organization during dark exploration across rats and mice. Behav Process 162:29–38
Eilam D, Golani I (1989) Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res 34:199–211
Fonio E, Benjamini Y, Golani I (2009) Freedom of movement and the stability of its unfolding in free exploration of mice. Proc Natl Acad Sci 106:21335–21340
Frostig T, Alonim H, Scheingesicht G et al (2020) Exploration in the presence of mother in typically and non-typically developing pre-walking human infants. Front Behav Neurosci 14:205
Golani I, Benjamini Y, Eilam D (1993) Stopping behavior: constraints on exploration in rats (Rattus norvegicus). Behav Brain Res 53:21–33
Hamilton DA, Sutherland RJ (1999) Blocking in human place learning: evidence from virtual navigation. Psychobiology 27:453–461
Hamilton DA, Driscoll I, Sutherland RJ (2002) Human place learning in a virtual Morris water task: some important constraints on the flexibility of place navigation. Behav Brain Res 129:159–170
Hamilton DA, Akers KG, Weisend MP, Sutherland RJ (2007) How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. J Exp Psychol Anim Behav Process 33:100
Hamilton DA, Akers KG, Johnson TE et al (2009) Evidence for a shift from place navigation to directional responding in one variant of the Morris water task. J Exp Psychol Anim Behav Process 35:271
Hines DJ, Whishaw IQ (2005) Home bases formed to visual cues but not to self-movement (dead reckoning) cues in exploring hippocampectomized rats. Eur J Neurosci 22:2363–2375
Köppen JR, Winter SS, Loda EL et al (2013) Analysis of movement kinematics on analogous spatial learning tasks demonstrates conservation of direction and distance estimation across humans (Homo sapiens) and rats (Rattus norvegicus). J Comp Psychol 127:179
Lehmann H, Clark BJ, Whishaw IQ (2007) Similar development of cued and learned home bases in control and hippocampal-damaged rats in an open field exploratory task. Hippocampus 17:370–380
Nemati F, Whishaw IQ (2007) The point of entry contributes to the organization of exploratory behavior of rats on an open field: an example of spontaneous episodic memory. Behav Brain Res 182:119–128
Osterlund Oltmanns JR, Lipton MH, Adamczyk N et al (2021) Organization of exploratory behavior under dark conditions in female and male rats. Behav Process 189:104437
Osterlund Oltmanns JR, Schaeffer EA, Blackwell AA et al (2022a) Age-related changes in the organization of spontaneously occurring behaviors. Behav Process 201:104713
Osterlund Oltmanns JR, Schaeffer EA, Donaldson TN et al (2022b) Sexually dimorphic organization of open field behavior following moderate prenatal alcohol exposure. Alcohol Clin Exp Res 46:861–875
Patil SS, Sunyer B, Höger H, Lubec G (2009) Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res 198:58–68
Schaeffer EA, Blackwell AA, Oltmanns JO et al (2022) Differential organization of open field behavior in mice following acute or chronic simulated GCR exposure. Behav Brain Res 416:113577
Skinner DM, Etchegary CM, Ekert-Maret EC et al (2003) An analysis of response, direction and place learning in an open field and T maze. J Exp Psychol Anim Behav Process 29:3
Stewart A, Cachat J, Wong K et al (2010) Homebase behavior of zebrafish in novelty-based paradigms. Behav Process 85:198–203
Tolman EC, Ritchie BF, Kalish D (1946) Studies in spatial learning. II. Place learning versus response learning. J Exp Psychol 36:221
Tchernichovski O, Golani I (1995) A phase plane representation of rat exploratory behavior. J Neurosci Methods 62:21–27
Von Uexküll J, Von Uexküll J, Winthrop-Young G (2010) A foray into the worlds of animals and humans: With a theory of meaning. SciELO Brasil
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Wallace DG, Hamilton DA, Whishaw IQ (2006) Movement characteristics support a role for dead reckoning in organizing exploratory behavior. Anim Cogn 9:219–228
Whishaw IQ, Gharbawie OA, Clark BJ, Lehmann H (2006) The exploratory behavior of rats in an open environment optimizes security. Behav Brain Res 171:230–239
Woodgate JL, Makinson JC, Lim KS et al (2016) Life-long radar tracking of bumblebees. PLoS ONE 11:e0160333
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
All protocols and procedures were approved by the Northern Illinois University (NIU) Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was in partial fulfillment of a masters thesis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schaeffer, E.A., Campbell, N., Sampson, H. et al. Cue polarization and representation in mouse home base behaviors. Anim Cogn 26, 861–883 (2023). https://doi.org/10.1007/s10071-022-01729-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-022-01729-y