Abstract
Rodent open field behavior is highly organized and occurs spontaneously in novel environments. This organization is disrupted in mice with vestibular pathology, suggesting vestibular signals provide important contributions to this behavior. A caveat to this interpretation is that previous studies have investigated open field behavior in adult mice with congenital vestibular dysfunction, and the observed deficits may have resulted from developmental changes instead of the lack of vestibular signals. To determine which aspects of open field behavior depend specifically on vestibular signals, mouse movement organization was examined under dark and light conditions at two time points, 1 and 2 months, after bilateral chemical labyrinthectomy. Our results show that acquired vestibular damage selectively disrupted the organization of open field behavior. Access to visual environmental cues attenuated, but did not eliminate, these significant group differences. Improvement in movement organization from the first to the second testing session was limited to progression path circuity. These observations provide evidence for the role of the vestibular system in maintaining spatial orientation and establishes a foundation to investigate neuroplasticity in brain systems that process self-movement information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sequential organization of movement around a home base is a ubiquitous characteristic of rodent open field behavior. For example, rats and mice exposed to a new environment quickly establish a home base that is a position marked by frequent grooming, rearing, and stopping behavior (Eilam and Golani 1989; Golani, Benjamini et al. 1993; Donaldson, Jennings et al. 2019). In addition, movements directed toward the home base are faster, relative to movements away from the home base (Tchernichovski and Golani 1995). Several factors influence the home base position. First, visual and tactile cues have been observed to polarize home base location (Hines and Whishaw 2005; Clark, Hamilton et al. 2006; Thompson, Berkowitz et al. 2018). Next, the point of entry has been observed to anchor the position of the home base independent of access to visual environmental cues (Nemati and Whishaw 2007). However, home base establishment and movement organization do not depend on olfactory cues (Hines and Whishaw 2005). Overall, these observations add to a growing literature demonstrating that rodents use multiple sources of information to organize their open field behavior.
Recent work has shown that the vestibular system provides a crucial source of self-movement cue information to organize open field behavior. In the absence of visual environmental cues, rodents establish a focal home base and organize their movements around this location (Avni, Zadicario et al. 2006; Donaldson, Jennings et al. 2019). Genetic mouse models of vestibular pathology have shown disrupted home base establishment and movement organization specific to dark environmental conditions, where navigation putatively relies heavily on self-movement cues (Avni, Elkan et al. 2009; Blankenship, Cherep et al. 2017). Importantly, these genetic models are associated with an early onset of vestibular pathology, suggesting that learning processes are either directly impaired or are otherwise unable to compensate for the lack of vestibular signals. Indeed, the loss of vestibular function has been observed to impact the structure (Brandt, Schautzer et al. 2005) and neuronal function (Stackman, Clark et al. 2002; Russell, Horii et al. 2006; Harvey, Rutan et al. 2018) of the hippocampus, but at this time we do not have sufficient evidence to determine whether the disruption in behavioral organization reflects developmental changes in the hippocampus or merely the loss of crucial sensory information. Regardless, vestibular pathology sustained as an adult has been shown to have the greatest impact on homing performance in conditions in which visual environmental cues are absent or conflict with previous experience (Wallace, Hines et al. 2002; Zheng, Goddard et al. 2009). Thus, rodents appear to establish a representation of the environment during movement around a home base, and vestibular dysfunction may impair spatial functions by disrupting the learning that occurs during this behavior. However, no studies have examined the effects of acquired vestibular pathology on home base establishment and movement organization in a novel environment.
The current study investigated the effects of acquired vestibular pathology on the organization of open field behavior across two time points. Non-operated control mice and mice that received bilateral chemical labyrinthectomies were exposed to a circular table without walls under dark and light conditions. The first dark session occurred 3 weeks after surgery. Mice were exposed to the same table under light conditions 1 week later. The second dark and light sessions occurred 7 and 8 weeks after surgery, respectively. General measures of locomotion, circular statistical analysis of stop clustering, and stratified analysis of varied progression lengths were used to assess the effects of acquired vestibular pathology on the organization of open field behavior. The results add to a growing literature suggesting an important role for vestibular signals in maintaining spatial orientation.
Materials and methods
Animals
Young adult female C57BL/6 J mice (n = 16) bred at Jackson Laboratory (Bar Harbor, ME) were transported to Northern Illinois University (NIU). The current study used female mice to more closely follow previous work with genetic models of vestibular pathology (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018) that used female mice. Upon arrival, mice were housed in cohorts of four within clear plexiglass containers with metal cage tops. Food and water were provided ad libitum. The colony room was temperature- and humidity-controlled with a 12:12 light–dark cycle. Mice were adults (approximately 90 and 110 days of age) at the start of the experiment. All experimental procedures were carried out in accordance with the Institutional Animal Care and Use Committees at NIU following NIH guidelines for The Care and Use of The Laboratory Animals.
Surgery
The vestibular group experienced similar surgical procedures as previous studies producing bilateral chemical labyrinthectomies (Chen, Pellis et al. 1986; Ossenkopp and Hargreaves 1993; Stackman and Taube 1997; Wallace, Hines et al. 2002). Mice receiving bilateral chemical labyrinthectomies (n = 8) were anesthetized using an isoflurane-oxygen gaseous mixture. Hemostats and forceps were used to retract the outer ear so that a clear view of the surgical field within the external auditory meatus could be obtained. Bilateral intratympanic injections of sodium arsanilate solution (50 μl of 100 mg/ml solution) were performed using a syringe with a 26-gauge needle to perforate the tympanic membrane. Immediately following injection, the external auditory meatuses were packed with saline saturated Gelfoam. Mice were then removed from anesthesia and supervised upon recovery, after which they were returned to their home cages. Mice were monitored for signs of impaired sensorimotor or motivational function (i.e., barrel rolling or progressive weight loss) over the course of 2 weeks following surgery. During this time, visual inspection of ambulation and body weight were assessed daily. Mice did not exhibit barrel rolling behavior upon recovery from surgery. Subcutaneous injections of approximately 10 ml of saline solution was administered as needed for hydration. One mouse that received surgery exhibited progressive weight loss, decreased responsiveness, and was subsequently euthanized. Due to both the limited evidence that rodents use auditory cues to guide navigation (see discussion) and concern that the sham procedure may potentially impact inner ear function, performance of vestibular mice was compared to unoperated control mice (n = 8).
Assessment of vestibular function
One day after surgery, vestibular mice were swim tested to assess vestibular pathology as reported previously (Yoder and Taube 2009; Yoder and Kirby 2014). The swim test apparatus was a transparent rectangular cage (46 cm × 26 cm × 26 cm) filled with 13 cm of water. Mice were individually dropped from a height of 20 cm into the water, after which they were permitted to swim for 10 s or were immediately retrieved with a small nylon net if they exhibited barrel rolling or sank below the surface of the water.
Apparatus
The arena was a circular (122 cm diameter and 34.5 cm high) wooden table without walls that was painted white and located inside a rectangular light-proof room. Previous work investigating the organization of exploratory behavior has not attached walls to the edge of the table to minimize thigmotaxic behavior (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018, 2019). Each session was recorded by a night-vision bullet camera attached to the ceiling. The camera feed was connected to a DVD recorder in a separate room that the experimenter used to monitor the activity of the mouse during the session. All video recordings were saved on DVDs for later offline analysis. During dark sessions, infrared emitters were used to illuminate the room for video recording. In addition, night-vision binoculars were used to place the mouse on the center of the table, replace the mouse after falls, and remove the mouse at the conclusion of the session. During light sessions, fluorescent lights located on the ceiling of the room provided illumination. The room had several environmental cues, such as a poster, table, and cabinet.
Procedure
Each session occurred during the light portion of the light–dark cycle. During a session, mice were individually placed into transport cages covered with a towel, and then transported to the experimental room via a circuitous path. This procedure limited mice from learning the spatial relationship between the animal colony and testing rooms; thereby increasing the novelty of the testing room during each testing session. Upon entering the testing room, each mouse was placed on the center of the table by the researcher. Each mouse was left to move in the arena for 40 min. If a mouse fell off the table, it was retrieved from the floor and returned to the center of the table. At the end of the session, the mouse was placed back in the transport cage, covered with a towel, and then returned to the colony room following a different circuitous path. Prior to running the next animal, the arena was cleaned with ammonia-based cleaner and dried. The dark sessions (indicated in graphs with grey background) occurred 3 and 7 weeks after surgery, whereas the light sessions (indicated in graphs with white background) occurred 4 and 8 weeks after surgery. The sequence of presenting dark sessions prior to light sessions has been used in previous studies (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018) and was intended to limit the mnemonic influence of visual cues on guiding movement during dark sessions. However, testing room and arena were no longer novel during the second testing session under dark and light conditions.
Behavioral analysis
During each session, four consecutive 4-min samples of behavior were taken, starting two minutes after a mouse was placed on the center of the table. The two-minute delay was selected based on previous work showing that mice exhibit markers of home base establishment (grooming, rearing, circling) during this time frame (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018, 2019). Four-minute samples allowed sufficient time to calculate averages for each behavior, and four sequential samples allowed us to determine whether the behaviors remained stable throughout the testing session. If a mouse fell off the table, it was placed on the center of the table. Two-minutes were permitted to elapse prior to capturing a four-minute sample of behavior, providing time to reacclimate to the table. Ethovision XT 13 (Noldus) was used to digitize (five frames per second) mouse movement during each sample. The digitized x- and y-coordinates and moment-to-moment speeds were the only data points obtained from Ethovision. Custom macros in Microsoft Excel used the resulting speeds to segment the movement into progressions and stops. Variability in average speeds observed (min: 2 cm/s; max 20 cm/s; mean: 7 cm/s) prompted the use of an individual threshold for segmenting movement into progressions and stops. The threshold for each mouse was the average speed obtained from the session, such that progressions were classified as periods (at least two frames) of movement greater than or equal to the average speed and stops were periods (at least two frames) of movement less than the average speed.
General measures
Several measures were used to quantify general characteristics of behavior.
Total falls: The total number of falls were recorded during each session.
Total travel distance: Total distance travelled during progressions was calculated for each session.
Average speed: The average speed was calculated from all moment-to-moment speeds of all movement during the four four-minute samples and used as the threshold to segment movement into stops and progressions.
Average progression peak speed: The peak speeds from all progressions were averaged for each session.
Total stop time: The total time spent stopping was calculated for each session.
These measures have been used previously as an index of motor function and activity level that have not been observed to be influenced by vestibular pathology (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018).
Stopping behavior
Rodents quickly establish a home base indicated by making frequent stops at this location that are typically longer in duration relative to stops in other locations in the environment (Eilam and Golani 1989; Golani, Benjamini et al. 1993; Avni, Zadicario et al. 2006; Clark, Hamilton et al. 2006; Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018). Home bases are typically established at the edge of the table and circular statistics (Batschelet 1981) have proven an effective tool to characterize location and stability. Each second of a stop was equated to a single observation and converted from cartesian coordinates (x,y) to polar (theta, r) coordinates.
Two descriptive circular statistics (Batschelet 1981) were used to quantify the strength of stop clustering relative to the table edge:
Within-sample stop concentration (r): The density in which stops cluster within a single direction during a sample can be calculated using circular statistics. Specifically, the parameter of concentration is calculated from all stop theta values within a sample. Stops clustering in one direction will yield values close to 1.0 and stops uniformly distributed around the edge of the table will result in values that approach 0.0. The tight circling behavior associated with Usher mice, a genetic harmonin deficient model, have been observed to produce higher within-sample stop concentration, relative to control mice (Donaldson, Jennings et al. 2018). The within-sample stop concentration for a mouse reflects the average parameter of concentration value obtained from the four samples within a session.
Between-sample stop concentration (r): Stability of stop clustering between-samples can be measured by calculating the parameter of concentration for the average heading (theta) from the four samples. As concentration values approach 1.0, stops are clustered in a similar direction across samples, and a stable home base has been established. The tilted mouse, a genetic otoconia deficient model, has been observed to exhibit a home base that drifts across samples under dark conditions (Blankenship, Cherep et al. 2017). The between-sample stop concentration for a mouse reflects the parameter of concentration calculated from the average heading from the four samples in a session.
Most of the change in heading along a path occurs during stops and vestibular pathology has been observed to influence this measure (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018). One measure was used to quantify the change in heading between progressions:
Change in heading: between progression change in heading was calculated as the supplementary angle to the angle subtended by the following points: (1) the preceding progression peak speed location; (2) the average stop location; (3) the subsequent progression peak speed location. Change in heading ranges from 0 degrees (continuing straight) to 180 degrees (complete reversal of path). Change in heading for a mouse was the average of all the heading changes across the four-minute samples.
Progression behavior
Previous work has demonstrated that progression complexity was disrupted in genetic mouse models of specific vestibular pathology (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018). The large range of progression lengths observed in the current study and previous work that demonstrates rodents organize their movement into three modes of motion or “gears” (Drai, Benjamini et al. 2000; Drai and Golani 2001) prompted an analysis of three classes of progressions. First, progressions less than 20 cm (approximately 2 body lengths or “lingering gear”) were excluded from this analysis to minimize small movements related to grooming or turning behavior. Next, all progressions from a mouse were sorted from longest to shortest based on total progression length. Finally, each mouse’s ordered progressions were divided into thirds based on length. The progressions classified as long were those that were in the top third for length. The progressions classified as medium were those that were in second third for length. The remaining progressions were classified as short. Two measures were used to quantify movement topography for each class of progression.
Progression class distance: The distance travelled was calculated for each progression and averaged for each class.
Progression path circuity: Rodents tend to follow non-circuitous progressions through an environment. Path circuity of a progression was calculated as the ratio of the Euclidian distance relative to the travelled distance. Path circuity values range from 1.0 (completely straight) to values that approach 0.0 (circuitous). Genetic models of developmental vestibular pathology have been observed to exhibit significantly lower progression path circuity values, relative to controls (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018). The path circuity was calculated for each progression and averaged for each class.
Statistical analysis
Repeated measures ANOVAs were used to evaluate the main effects of acquired vestibular pathology, time or progression class, and interactions, with an alpha set at 0.05. Partial eta squared (η2p) values were reported for each main effect and interaction effect as a measure of effect size. Post hoc analyses used the Tukey HSD test with Cohen’s D as a measure of effect size. JASP 0.12.2 (University of Amsterdam) was used to calculate statistical results.
Results
Swim testing
None of the mice that received bilateral chemical labyrinthectomies passed the swim test, suggesting impaired vestibular function. Unlike control mice that swim above the surface of the water, all vestibular mice had to be quickly retrieved from the water, because they immediately spiraled below the surface of the water and failed to maintain an upright posture.
General behavior
Control mice did not fall off the table under dark conditions at either time point, while mice in the vestibular group fell off the table on average two (min = 0; max = 6) times during the first dark session and less than one (min = 0; max = 2) time during the second dark session. One mouse from the vestibular group was excluded from the study, because it did not stay on the table long enough to obtain four four-minute samples. During the first session under light conditions, the control group fell off the table less than one (min = 0; max = 1) time on average, while the vestibular group averaged one fall (min = 0; max = 3) per session. During the second light session, both control (min = 0; max = 1) and vestibular (min = 0; max = 1) mice averaged less than one fall.
Kinematic and topographic profiles are plotted for representative control (Fig. 1 panels A, C, E, G) and vestibular (Fig. 1 panels B, D, F, H) mice in the first sample of dark and light sessions. Profiles are segmented into stops and progressions. Groups were similar on measures of general behavior (Table 1). First, the total distance travelled was used to determine whether acquired vestibular dysfunction influenced activity level. Groups did not differ in total distance travelled during dark testing session, and distance travelled did not differ between sessions under dark conditions (Table 2). Similar results were observed under light conditions. Groups did not differ in total distance travelled during light testing sessions, and distance travelled did not differ between light testing sessions. Similar levels of activity were observed from both groups.
Kinematic (a and b) and topographic (c and d) characteristics of behavioral organization are plotted for a representative control and vestibular mouse for the first four-minute sample under dark conditions. Kinematic (e and f) and topographic (g and f) characteristics of behavioral organization are plotted for a representative control and vestibular mouse for the first four-minute sample under light conditions. Kinematic and topographic aspects of movement have been segmented into progressions (black lines) and stops (red lines)
Next, average speed provided a measure of general motor function. Under dark conditions, groups did not differ in average speed during testing session, and average speed did not differ between testing sessions (Table 2). Similar results were observed under light conditions. Groups did not differ in average speed during testing sessions under light conditions, and average speed did not differ between testing sessions. Average progression peak speed provided an additional assessment of motor function. Under dark conditions, groups did not differ in average progression peak speed during testing sessions, and average progression peak speed did not differ between testing sessions (Table 2). Similar results were observed under light conditions. Groups did not differ in average progression peak speed during testing sessions under light conditions, and average progression peak speed did not differ between testing sessions. The results from both measures are consistent with similar levels of general motor function observed from both groups.
Finally, total stop time was used to determine whether acquired vestibular dysfunction caused mice to stop for shorter periods of time, as has been observed in animals with experimental brain damage (Whishaw, Cassel et al. 1994). Under dark conditions, groups did not differ in total stop time during testing sessions, and total stop time did not differ between testing sessions (Table 2). Similar results were observed under light conditions. Groups did not differ in total stop time during testing sessions under light conditions, and total stop time did not differ between testing sessions. Overall, groups exhibited similar travel distances, average speeds, average progression peak speeds, and total stop times under both dark and light conditions.
Organization of stopping behavior
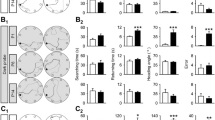
Group differences were observed in the organization of stopping behavior under dark and light conditions. First, stop clustering is plotted for one four-minute sample from representative control and vestibular mice under both conditions (Fig. 2). Within a sample, stops were observed to be more concentrated in vestibular mice relative to control mice under dark conditions (Fig. 3 panel A). Groups significantly differed at both testing sessions; however, testing sessions did not significantly differ (Table 3).
Representative stop clustering profiles are plotted for a control (a) and vestibular (b) mouse during a four-minute sample under dark conditions. Representative stop clustering profiles are plotted for a control (c) and vestibular (d) mouse during a four-minute sample under light conditions. In each cluster plot, the length and direction of the line represent the parameter of concentration and average heading, respectively
Under light conditions, stops were observed to be more concentrated in vestibular mice, relative to control mice (Fig. 3 panel B). Groups significantly differed at both testing sessions; however, testing sessions did not significantly differ (Table 3). The vestibular group exhibited significantly stronger clustering of stops within-samples at both time points under dark and light conditions; however, the effect size was smaller under light conditions.
Next, between-sample stop clustering was used to characterize the stability of the home base across the four samples. No group differences in between-sample stop clustering was observed at either testing session under dark conditions (Fig. 3 panel C). Groups did not significantly differ at both testing sessions, and testing sessions did not significantly differ (Table 3). Under light conditions, no group differences in between-sample stop concentration were observed at either testing session under light conditions (Fig. 3 panel D). Groups did not significantly differ at both testing sessions, and testing sessions did not significantly differ (Table 3). Both groups exhibited similar levels of home base stability at both time points under dark and light conditions.
Finally, during movement most of the change in heading occurs during stops (see Fig. 4 panel A and B). Vestibular mice exhibited significantly larger changes in heading between progressions relative to control mice at both time points under dark conditions (Fig. 4 panel C). Groups significantly differed at both testing sessions; however, testing sessions did not significantly differ (Table 3). Under light conditions, larger changes in heading between progressions were observed in vestibular mice, relative to control mice (see Fig. 4 panel D). Groups significantly differed at both testing sessions; however, testing sessions did not significantly differ (Table 3). The vestibular group exhibited significantly larger changes in heading between progressions at both time points under dark and light conditions.
Start position (open circle), progression (black line), peak speed (filled circle), and stop (red line and circle) are plotted for a representative movement sequence under dark (a) and light (b) conditions. The magnitude of each change in heading along the path is indicated numerically. Average change in heading is plotted for both groups under dark (c) and light (d) conditions. [Note: Error bars represent SEM; * < 0.05]
Organization of progression behavior
Progressions varied in length and complexity. To further examine these topographic characteristics of movement, all progressions from each mouse were first sorted from longest to shortest and then evenly divided into three progression classes: long, medium, and short. The average distance travelled for long, medium, and short progressions did not vary between groups at 3- or 7-week testing sessions under dark or light conditions (Table 4).
In general, group differences were observed in progression path circuity at both time points under dark and light conditions (Fig. 5). At the early session under dark conditions, vestibular mice exhibited more circuitous long, medium, and short progressions relative to control mice (Fig. 6 panel A). This was supported by observing a significant main effect of group and progression class (Table 4). At the early session under light conditions, vestibular mice only exhibited more circuitous long progressions relative to control mice (Fig. 6 panel B). This was supported by a significant effect of group, progression class, and Group by Progression class interaction (Table 4).
Representative long (blue line), medium (red line), and short (yellow line) progression classes are plotted for a control and vestibular mouse under dark (a and b) and light (c and d) conditions. Both groups of mice exhibited similar lengths for each progression class, but the progressions were significantly more circuitous for the vestibular group. This difference in progression path circuity is more pronounced in medium and long progression classes
At the late session under dark conditions, vestibular mice exhibited more circuitous long and medium progressions relative to control mice (Fig. 6 panel C). This was supported by observing a significant effect of group, progression class, and Group by Progression class interaction (Table 4). At the late session under light conditions, vestibular mice only exhibited more circuitous long progressions relative to control mice (Fig. 6 panel D). This was supported by a significant effect of group, progression class, and Group by Progression class interaction (Table 4). Access to visual cues and longer time since surgery attenuated the group differences in progression path circuity.
Discussion
This study investigated the effects of acquired vestibular pathology on the organization of open field behavior under dark and light conditions at two time points. Under dark conditions, no group differences were observed in general measures of behavior, nor were these measures observed to vary as a function of time. In contrast, group differences were observed in stop and progression measures. For example, within-sample stop clustering and change in heading measures significantly varied between groups during both dark testing sessions. Vestibular mice exhibited more concentrated within-sample stop clustering and larger changes in heading compared to control mice. Group differences in progression circuity were observed at both sessions with vestibular mice exhibiting significantly greater path circuity; however, during the second dark session the magnitude of this group difference decreased as progression class became shorter. Under light conditions, no group differences were observed in measures of general behavior, nor were these measures observed to vary as a function of time. Like dark conditions, within-sample stop clustering and change in heading significantly varied between groups during both light sessions. Vestibular mice exhibited more concentrated within-sample stop clustering and larger changes in heading compared to control mice. Finally, vestibular mice exhibited greater progression circuity compared to control mice during light testing which depended on the progression class length during both testing sessions. The following sections will consider the factors that may contribute to group differences in performance observed under dark and light conditions across both time points.
Role of vestibular system in spatial orientation
Previous work has reported consistent movement patterns associated with loss of spatial orientation. For example, early work documented that blindfolded humans exhibited an increased tendency to follow a spiral trajectory as the length of the path walked or swam increased (Schaeffer 1928). In addition, humans navigating without access to distinct environmental cues (such as in a dense forest or desert) exhibited a similar spiraling tendency (Souman, Frissen et al. 2009). Finally, emergence of spiraling behavior in blindfolded humans was associated with poorer estimation of direction to the point movement started (Wallace, Choudhry et al. 2006). In the current study, vestibular mice exhibited larger changes in heading and more circuitous progressions. This pattern of mouse behavior parallels spiraling behavior in humans. Therefore, it is possible that the effects of vestibular system damage on organization of open field behavior reflects an impaired ability to maintain spatial orientation.
Spatial orientation depends on an animal’s accurate representation of current position and using available sources of information to update this representation after movement has occurred. This online information processing likely depends on electrophysiological signals generated by self-movement cues. First, neurons within the head direction cell network exhibit firing of action potentials that are tuned to specific directions within an environment (for a review see (Wiener and Taube 2005). Environmental cues typically have stimulus control over head direction signal tuning (Taube, Muller et al. 1990); however, this signal tuning has been observed to persist in the absence of visual cues (Goodridge, Dudchenko et al. 1998) and in novel environments (Stackman, Golob et al. 2003). The vestibular system provides a crucial component of these self-movement cues, as indicated by unstable or otherwise degraded head direction cell activity in mice with dysfunctional otolith organs (Yoder and Taube 2009), semicircular canals (Valerio and Taube 2016), canal blockade (Muir, Brown et al. 2009), or following bilateral chemical labyrinthectomy (Stackman and Taube 1997). Next, rhythmic slow-wave activity (3–12 Hz), or theta rhythm, recorded from the hippocampal formation has been posited to represent movement extent (Bland and Colom 1993). This is based on observations that hippocampal theta precedes the initiation of voluntary movements (Oddie and Bland 1998) and that theta frequency varies with movement magnitude (Whishaw and Vanderwolf 1973). Interestingly, bilateral vestibular damage has been shown to disrupt movement-elicited hippocampal theta rhythm (Russell, Horii et al. 2006), suggesting that this type of theta rhythm is heavily influenced by vestibular system activity. Finally, hippocampal place cells have firing characteristics that are tuned to specific regions of an environment, or place fields (O'Keefe and Nadel 1978). The location of these place fields has been shown to be controlled by environmental stimuli (Muller and Kubie 1987; O'Keefe and Burgess 1996); however, place field stability has also been observed under dark conditions (Quirk, Muller et al. 1990), suggesting that self-movement cues are sufficient to maintain place field specificity. Recent work has demonstrated that temporary inactivation of the vestibular system results in disrupted location tuning of place cells in both dark and light conditions (Stackman, Clark et al. 2002). Place field tuning has been observed to be less stable and located closer to environmental stimuli in otoconia-deficient mice (Harvey, Rutan et al. 2018). Vestibular signals are thus necessary for place cell activity, and vision cannot completely compensate for the loss of vestibular signals. In the current study, acquired vestibular pathology was associated with increased density of within-sample stop clustering, suggesting that disrupted place cell function in vestibular mice is not related to an impaired ability to perceive location. In addition, there is no evidence that olfactory cues were used to perceive home base location (Hines and Whishaw 2005). Therefore, group differences in within-sample stop clustering may reflect higher tendency of vestibular mice to engage in circling behavior, as observed in Usher mice (Donaldson, Jennings et al. 2018). Alternatively, vestibular pathology has been associated with anxiety related disorders (Furman and Jacob 2001; Perna, Dario et al. 2001; Kalueff, Ishikawa et al. 2008; Coelho and Balaban 2015). Therefore, it is possible that acquired vestibular pathology increased anxiety levels in the open field and was manifested as multiple returns to the home base, thus an increased density of within-sample stop clustering. Future studies are needed to investigate the effects of anxiolytic therapies on within-sample stop clustering in mice with vestibular pathology.
Manipulating access to environmental cues has provided an opportunity to further characterize the role of the vestibular system in maintaining spatial orientation. Rats and mice accurately carry a food item to a refuge that is hidden below the surface of a table, independent of access to visual environmental cues. Rodents with acquired (Wallace, Hines et al. 2002; Zheng, Darlington et al. 2006; Zheng, Goddard et al. 2009) or genetic (Yoder, Goebel et al. 2015) vestibular pathology carried the food item directly back to the refuge when provided access to visual environmental cues. In contrast, under dark conditions, both types of vestibular pathology resulted in a highly circuitous return path to the refuge, consistent with impaired self-movement cue processing. In the current study, significant group differences were observed in within-sample stop clustering, change in heading, and progression path circuity under both dark and light conditions. Specifically, the effect size associated with the main effect of group decreased from dark to light conditions by 50% for within-sample stop clustering and decreased by 20% for change in heading. In addition, the average group effect size for progression path circuity decreased from dark to light conditions by 19% during the early session and 44% during the late session. Importantly, the vestibular mice made significantly more circuitous progressions for all progression lengths in early dark trials and for medium and long progression lengths during late dark trials, whereas they only made more circuitous progressions for long progressions during light trials at either time point. This finding suggests that acquired vestibular dysfunction impairs the ability to move in a straight line when relying on path integration alone, but vision can compensate for this loss during relatively simple, short trajectories. Thus, this pattern is consistent with bilateral chemical labyrinthectomies sparing the use of environmental cues to guide some aspects of movement; however, deficits in processing self-movement cues may influence encoding relationships between environmental cues. For example, humans with bilateral vestibular loss exhibit significant performance impairments on a virtual reality version of the Morris Water task (Brandt, Schautzer et al. 2005) and on a triangle-completion task (Xie, Bigelow et al. 2017). Further work is needed to determine whether vestibular pathology influences encoding and the nature of the representation of environmental cues.
Vestibular plasticity or compensatory mechanisms
In general, the interval between bilateral chemical labyrinthectomies and testing session did not significantly influence measures of behavioral organization; however, progression path circuity was an exception to this pattern. During the 3-week dark session, group differences in progression path circuity were independent of the specific class of progression length. In contrast, during the 7-week dark session, groups differences depended on the specific class of progression length. Specifically, both groups exhibited equivalently low levels of path circuity on short progressions. The vestibular group exhibited similar improvement in progression path circuity between 4-week and 8-week testing sessions under light conditions. Compensatory mechanisms may have contributed to this improvement in performance observed across early and late sessions. For example, despite all vestibular mice failing the swim test, spared vestibular function may have been sufficient to compensate for the loss of function. Previous work has shown that unilateral vestibular damage disrupted performance on a food carrying task under dark conditions at 3 months, whereas significant improvements in performance were observed under dark testing at 6 months (Zheng, Darlington et al. 2006; Zheng, Goddard et al. 2009). In the current study, improvements in short progression class circuity were observed at an earlier time point (approximately 2 months); however, persistent disruptions in progression circuity were observed in the long progression class. It is possible that persistent disruptions in progression circuity, within-sample stop clustering, and change in heading may have abated, had testing been extended six months past the bilateral chemical labyrinthectomy. This work provides a foundation to investigate the time scale and mechanisms of compensation associated with acquired vestibular pathology.
Genetic models of vestibular pathology do not exhibit a capacity for compensation equivalent to that of acquired pathology. For example, disruptions in progression path circuity and change in heading under dark conditions are observed in genetic otoconia deficient tilted mice at 3- and 8 months of age (Blankenship, Cherep et al. 2017). In addition, these same measures were disrupted in the genetic harmonin deficient Usher mice, and these deficits persisted across 2- and 6-month testing sessions under dark conditions (Donaldson, Jennings et al. 2018). The age range and multiple testing sessions represented in both studies afford sufficient opportunity for the development of compensatory mechanisms. The fact that these deficits persisted over time suggests that the loss of vestibular function early in development has an enduring effect on brain structures (i.e., hippocampal formation) that are integral in processing self-movement information (Maaswinkel, Jarrard et al. 1999; Wallace and Whishaw 2003; Winter, Köppen et al. 2013). In contrast, improved performance was observed in the current study with acquired vestibular pathology mice and previous work with rats (Zheng, Darlington et al. 2006). Therefore, any compensatory mechanisms associated with acquired vestibular pathology may not be sufficient to ameliorate the widespread developmental effects associated with congenital vestibular dysfunction. Future work using chemogenic techniques to target the vestibular nuclei may provide more insight into vestibular system neuroplasticity that is associated with acquired versus genetic models of vestibular pathology.
Possible limitations
Group differences in behavioral organization were attributed to impaired processing of self-movement cues; however, several alternative explanations should be considered. First, bilateral chemical labyrinthectomies involve puncturing the tympanic membrane and infusing sodium arsanilate. Considering that both vestibular and auditory systems may have been damaged, group differences in behavior may reflect an impaired ability to use auditory cues to maintain spatial orientation. For example, mice can be trained over multiple trials to move toward a sound source to locate a food item (Watanabe and Yoshida 2007). However, beacon homing appears to be the limit of rodents’ ability to use auditory cues to maintain spatial orientation. Specifically, auditory cues were not sufficient to guide place learning in the Morris water task under dark conditions (Rossier, Haeberli et al. 2000), and these cues failed to establish stimulus control over head direction cells preferred firing direction (Goodridge, Dudchenko et al. 1998). It is also important to note that self-movement cues dominate over auditory cues when rats carry food to a refuge when access to visual environmental cues has been limited by blindfold (Maaswinkel and Whishaw 1999). Furthermore, conditional genetic vestibular, but not auditory pathology, has been shown to influence open field behavior (Antoine, Vijayakumar et al. 2017). These observations discount the role of auditory cue impairments in mediating disruptions in behavioral organization in mice with acquired vestibular dysfunction.
Another possible limitation in attributing group differences to impaired processing of self-movement cues was the frequency of falling off the table. Specifically, during the 3-week dark session, vestibular mice were more likely to fall off the table. The increase in falls during this session may reflect a balance deficit in the vestibular group. Alternatively, it is possible that falling off the table, being placed at the center of the table, or a combination of these factors affected spatial orientation. Although two-minutes elapsed prior to capturing movement for the next sample, persistently impaired spatial orientation may have contributed to the observed disruption in behavior organization in the vestibular group. This possibility was investigated by correlating the number of falls from 3-week dark sessions with behavioral measures sensitive to vestibular pathology (Blankenship, Cherep et al. 2017; Donaldson, Jennings et al. 2018). Neither change in heading [r(12) = 0.29, p = 0.32] nor progression path circuity [r(12) = 0.16, p = 0.60] were significantly associated with the number of falls. Upon further inspection of the vestibular mouse data (Table 5) it was noted that a vestibular mouse with the most falls exhibited the most direct progressions and a relatively low change in heading values. Furthermore, two mice with the lowest path circuity values had either 1 or 0 falls. Finally, significant group differences in change in heading and progression path circuity were observed during subsequent sessions under dark and light conditions, despite almost no observed falls. These observations do not provide evidence that supports a relationship between fall frequency and disruption in behavioral organization.
Conclusion
The current study investigated the impact of bilateral chemical labyrinthectomy on the organization of mouse behavior under dark and light conditions at approximately 3- and 7-weeks post-surgery. Acquired damage to the vestibular system spared general measures of behavior under dark and light conditions at both time points. In contrast, significant group differences were observed in within-sample stop clustering, change in heading, and progression class path circuity. Access to visual environmental cues attenuated disruptions in performance in vestibular mice; however, groups still exhibited significant differences in performance. Improvements in vestibular mice performance during the second session were restricted to the progression circuity of short and medium progression classes. These results are consistent with the vestibular system providing a crucial source of self-movement cues for maintaining spatial orientation.
References
Antoine MW, Vijayakumar S, McKeehan N, Jones SM, Hébert JM (2017) The severity of vestibular dysfunction in deafness as a determinant of comorbid hyperactivity or anxiety. J Neurosci 37(20):5144–5154
Avni R, Zadicario P, Eilam D (2006) Exploration in a dark open field: a shift from directional to positional progression and a proposed model of acquiring spatial information. Behav Brain Res 171(2):313–323
Avni R, Elkan T, Dror AA, Shefer S, Eilam D, Avraham KB, Mintz M (2009) Mice with vestibular deficiency display hyperactivity, disorientation, and signs of anxiety. Behav Brain Res 202(2):210–217
Batschelet E (1981) "Circular statistics in biology." Academic Press, 111 Fifth ave., New York, 10003, 388
Bland BH, Colom LV (1993) Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Prog Neurobiol 41(2):157–208
Blankenship PA, Cherep LA, Donaldson TN, Brockman SN, Trainer AD, Yoder RM, Wallace DG (2017) Otolith dysfunction alters exploratory movement in mice. Behav Brain Res 325:1–11
Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, Darlington C, Smith P, Strupp M (2005) Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 128(11):2732–2741
Chen Y-C, Pellis SM, Sirkin DW, Potegal M, Teitelbaum P (1986) Bandage backfall: labyrinthine and non-labyrinthine components. Physiol Behav 37(5):805–814
Clark BJ, Hamilton DA, Whishaw IQ (2006) Motor activity (exploration) and formation of home bases in mice (C57BL/6) influenced by visual and tactile cues: modification of movement distribution, distance, location, and speed. Physiol Behav 87(4):805–816
Coelho CM, Balaban CD (2015) Visuo-vestibular contributions to anxiety and fear. Neurosci Biobehav Rev 48:148–159
Donaldson T, Jennings KT, Cherep LA, McNeela AM, Depreux FF, Jodelka FM, Hastings ML, Wallace DG (2018) Antisense oligonucleotide therapy rescues disruptions in organization of exploratory movements associated with Usher syndrome type 1C in mice. Behav Brain Res 338:76–87
Donaldson T, Jennings K, Cherep L, Blankenship P, Blackwell A, Yoder R, Wallace D (2019) Progression and stop organization reveals conservation of movement organization during dark exploration across rats and mice. Behav Proc 162:29–38
Drai D, Golani I (2001) SEE: a tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav Rev 25(5):409–426
Drai D, Benjamini Y, Golani I (2000) Statistical discrimination of natural modes of motion in rat exploratory behavior. J Neurosci Methods 96(2):119–131
Eilam D, Golani I (1989) Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res 34(3):199–211
Furman JM, Jacob RG (2001) A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord 15(1–2):9–26
Golani I, Benjamini Y, Eilam D (1993) Stopping behavior: constraints on exploration in rats (Rattus norvegicus). Behav Brain Res 53(1–2):21–33
Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS (1998) Cue control and head direction cells. Behav Neurosci 112(4):749
Harvey RE, Rutan SA, Willey GR, Siegel JJ, Clark BJ, Yoder RM (2018) Linear self-motion cues support the spatial distribution and stability of hippocampal place cells. Curr Biol 28(11):1803–1810
Hines DJ, Whishaw IQ (2005) Home bases formed to visual cues but not to self-movement (dead reckoning) cues in exploring hippocampectomized rats. Eur J Neurosci 22(9):2363–2375
Kalueff AV, Ishikawa K, Griffith AJ (2008) Anxiety and otovestibular disorders: linking behavioral phenotypes in men and mice. Behav Brain Res 186(1):1–11
Maaswinkel H, Whishaw IQ (1999) Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res 99(2):143–152
Maaswinkel H, Jarrard LE, Whishaw IQ (1999) Hippocampectomized rats are impaired in homing by path integration. Hippocampus 9(5):553–561
Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, Taube JS (2009) Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla. J Neurosci 29(46):14521–14533
Muller RU, Kubie JL (1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7(7):1951–1968
Nemati F, Whishaw IQ (2007) The point of entry contributes to the organization of exploratory behavior of rats on an open field: an example of spontaneous episodic memory. Behav Brain Res 182(1):119–128
Oddie SD, Bland BH (1998) Hippocampal formation theta activity and movement selection. Neurosci Biobehav Rev 22(2):221–231
O’Keefe J, Burgess N (1996) Geometric determinants of the place fields of hippocampal neurons. Nature 381(6581):425–428
O'Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford
Ossenkopp K-P, Hargreaves EL (1993) Spatial learning in an enclosed eight-arm radial maze in rats with sodium arsanilate-induced labyrinthectomies. Behav Neural Biol 59(3):253–257
Perna G, Dario A, Caldirola D, Stefania B, Cesarani A, Bellodi L (2001) Panic disorder: the role of the balance system. J Psychiatr Res 35(5):279–286
Quirk GJ, Muller RU, Kubie JL (1990) The firing of hippocampal place cells in the dark depends on the rat’s recent experience. J Neurosci 10(6):2008–2017
Rossier J, Haeberli C, Schenk F (2000) Auditory cues support place navigation in rats when associated with a visual cue. Behav Brain Res 117(1–2):209–214
Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK (2006) Lesions of the vestibular system disrupt hippocampal theta rhythm in the rat. J Neurophysiol 96(1):4–14
Schaeffer A (1928) Spiral movement in man. J Morphol 45(1):293–398
Souman JL, Frissen I, Sreenivasa MN, Ernst MO (2009) Walking straight into circles. Curr Biol 19(18):1538–1542
Stackman RW, Taube JS (1997) Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci 17(11):4349–4358
Stackman RW, Clark AS, Taube JS (2002) Hippocampal spatial representations require vestibular input. Hippocampus 12(3):291–303
Stackman RW, Golob EJ, Bassett JP, Taube JS (2003) Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol 90(5):2862–2874
Taube JS, Muller RU, Ranck JB (1990) Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10(2):420–435
Tchernichovski O, Golani I (1995) A phase plane representation of rat exploratory behavior. J Neurosci Methods 62(1–2):21–27
Thompson SM, Berkowitz LE, Clark BJ (2018) Behavioral and neural subsystems of rodent exploration. Learn Motiv 61:3–15
Valerio S, Taube JS (2016) Head direction cell activity is absent in mice without the horizontal semicircular canals. J Neurosci 36(3):741–754
Wallace DG, Whishaw IQ (2003) NMDA lesions of Ammon’s horn and the dentate gyrus disrupt the direct and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the hippocampus in dead reckoning. Eur J Neurosci 18(3):513–523
Wallace DG, Hines DJ, Pellis SM, Whishaw IQ (2002) Vestibular information is required for dead reckoning in the rat. J Neurosci 22(22):10009–10017
Wallace DG, Choudhry S, Martin MM (2006) Comparative analysis of movement characteristics during dead-reckoning-based navigation in humans and rats. J Comp Psychol 120(4):331
Watanabe S, Yoshida M (2007) Auditory cued spatial learning in mice. Physiol Behav 92(5):906–910
Whishaw I, Vanderwolf CH (1973) Hippocampal EEG and behavior: change in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats. Behav Biol 8(4):461–484
Whishaw IQ, Cassel JC, Majchrzak M, Cassel S, Will B (1994) “Short-stops” in rats with fimbria-fornix lesions: Evidence for change in the mobility gradient. Hippocampus 4(5):577–582
Wiener SI, Taube JS (2005) Head direction cells and the neural mechanisms of spatial orientation, MIT Press
Winter SS, Köppen JR, Ebert TB, Wallace DG (2013) Limbic system structures differentially contribute to exploratory trip organization of the rat. Hippocampus 23(2):139–152
Xie Y, Bigelow RT, Frankenthaler SF, Studenski SA, Moffat SD, Agrawal Y (2017) Vestibular loss in older adults is associated with impaired spatial navigation: data from the triangle completion task. Front Neurol 8:173
Yoder RM, Kirby SL (2014) Otoconia-deficient mice show selective spatial deficits. Hippocampus 24(10):1169–1177
Yoder RM, Taube JS (2009) Head direction cell activity in mice: robust directional signal depends on intact otolith organs. J Neurosci 29(4):1061–1076
Yoder RM, Goebel EA, Köppen JR, Blankenship PA, Blackwell AA, Wallace DG (2015) Otolithic information is required for homing in the mouse. Hippocampus 25(8):890–899
Zheng Y, Darlington CL, Smith PF (2006) Impairment and recovery on a food foraging task following unilateral vestibular deafferentation in rats. Hippocampus 16(4):368–378
Zheng Y, Goddard M, Darlington CL, Smith PF (2009) Long-term deficits on a foraging task after bilateral vestibular deafferentation in rats. Hippocampus 19(5):480–486
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Data availability statement
Data will be made available on reasonable request.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Banovetz, M.T., I Lake, R., Blackwell, A.A. et al. Effects of acquired vestibular pathology on the organization of mouse exploratory behavior. Exp Brain Res 239, 1125–1139 (2021). https://doi.org/10.1007/s00221-020-06032-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-06032-1