Abstract
Anthocyanins (ACNs) are water-soluble pigments in various fruits and vegetables known for their high antioxidant activity. They are used as natural food colorants and preservatives and have several medicinal benefits. However, their application in functional foods and nutraceuticals is often compromised by their low stability to heat, oxygen, enzymes, light, pH changes, and solubility issues. Spray drying has emerged as an effective microencapsulation technique to enhance the shelf life, quality, and stability of ACNs. This manuscript reviews the latest scientific developments in spray drying microencapsulation of ACNs-rich fruit extracts. Process optimization and the stability and physicochemical properties of the spray-dried, microencapsulated ACNs-rich powders are discussed. This review also covers functional food and nutraceutical applications and introduces novel encapsulation methods, such as freeze-drying, supercritical carbon dioxide (SC-CO2), coacervation, drum drying, and electrospraying, highlighting their potential in improving the utility of ACNs-rich fruit extracts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the present era of optimal nutrition, the growing demand for healthier foods has led to the consumption of natural antioxidant-containing foods, such as polyphenol-rich fruits and vegetables. Anthocyanins (ACNs) are water-soluble phenolic compounds renowned for their potent natural antioxidant properties. Their unique structure, characterized by a flavonoid backbone, multiple hydroxyl groups, conjugated double bonds, chelation sites, glycosylation, and varied substitution patterns, collectively contributes to their exceptional antioxidant properties. These structural attributes enable ACNs to effectively neutralize free radicals, inhibit metal ion-induced oxidative reactions, and protect cells and tissues from oxidative damage, making them valuable natural antioxidants (Enaru et al., 2021; Tarone et al., 2020).
ACNs are responsible for plants’ orange, red, violet, and blue colors and thus hold great potential as natural colorants (Azman et al., 2022a, b). Grapes, blackcurrants, blueberries, and bilberries are among the most popular ACNs-rich fruits. In contrast, red cabbages (Machado et al., 2022), eggplants (Demiray et al., 2023), and red onions (Ali et al., 2016) are typical examples of ACNs-rich vegetables. In addition to antioxidants, ACNs provide a broad spectrum of therapeutic effects and biological activities, including anticarcinogenic, inflammatory, immune-stimulating, antibacterial, antiallergic, and antiviral properties (Nawawi et al., 2023a; Riberio et al., 2019; Salehi et al., 2020). However, the biological properties and nutraceutical value of ACNs are often compromised by their instability. The stability of ACNs is affected by multiple factors such as light, temperature, pH, enzymatic reactions, oxidation, presence of metal ions, humidity, microorganisms, moisture, solvents, and the presence of co-pigments, causing degradation or partial loss of these compounds (Azman et al., 2022a, b; Feitosa et al., 2023; Nawawi et al., 2023a; Nawawi et al., 2023b). In this regard, different delivery processes have been developed to improve the stability of ACNs and coloring pigments, such as microencapsulation, nanoencapsulation, and protein complex formation (Feitosa et al., 2023).
Microencapsulation is a viable alternative that efficiently preserves thermally sensitive compounds such as phenolic compounds (Abdel‐Aty et al., 2022; Gawalek, 2022). This technique entraps bioactive compounds within the encapsulating agent and transforms liquids into powders for easier handling (Rocha et al., 2019; Singh et al., 2023a). Encapsulating agents such as maltodextrin (MD), gum arabic (GA), carrageenan, alginate, waxes, and phospholipids are added to facilitate powder production, protect bioactive compounds from oxygen, light, or other unfavorable conditions, and increase stability (Li et al., 2017; Ligarda-Samanez et al., 2023).
Many investigations on the microencapsulation of ACNs-rich fruit extracts, such as berries, pomegranates, jaboticaba, and cherries, have focused on storage, color stability, and the quality of the final fruit powder (Gawalek, 2022; Halahlah et al., 2023; Mahdavi et al., 2016b; Shwetha and Preetha, 2016). Spray drying, freeze-drying, and supercritical carbon dioxide (SC–CO2) technology are commonly used microencapsulation techniques, and spray drying is the most popular among food industries owing to its cost-effectiveness, efficiency, and high yield of good-quality powder products (Ravichandran et al., 2023; Da Rosa et al., 2019). Over the years, various parameters of spray-drying microencapsulation techniques have been studied for ACNs from various fruit types. New updates are required to determine which parameters can be applied to best suit specific fruits.
Therefore, the primary goal of this review was to assess the current trends and advances in spray drying microencapsulation of ACNs-rich fruit extracts. The effects of different parameters, such as types of encapsulating agents, the ratio of encapsulating agent to feed, drying temperature, and feed flow rate, on the physiochemical and stability characteristics and functional food quality of the end-use ACNs powder product are discussed. Furthermore, an overview of innovative microencapsulation techniques, such as freeze-drying, SC-CO2, coacervation, drum drying, and electrospraying, is provided.
An overview of ACNs
The word ACNs has been coined from two Greek words, namely Anthos (flower) and kyanos (blue) (Koop et al., 2022). As a class of polyphenols and sub-class of flavonoids, ACNs are widely distributed in different parts, especially leaves, fruits, and flowers of various plants belonging to the Rosaceae, Vitaceae, Saxifragaceae, Ericaceae, Cruciferae, Fabaceae and Caprifoliaceae families, among others (Li et al., 2021; Mazza and Miniati, 2018). ACNs tend to be located in the cells of plant vacuoles and cell walls and can be acquired through extraction (Ijod et al., 2022) (Fig. 1). ACNs yield in fruits depends on several factors such as processing and extraction methods, growing location and weather, fruit maturity levels, and storage conditions before analysis (Mazza and Miniati, 2018; Nawawi et al., 2023a).

Adapted from Nistor et al. (2022) with modification
ACNs move from the synthesis site, the endoplasmic reticulum (ER), to the vacuole for storage. ER-derived vesicles facilitate anthocyanin transport to the vacuole, where they bind to the membrane via soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) and release ACNs during the micro (1) and macro (2) autophagy processes. Several membrane proteins (multidrug and toxic compound extrusion (MATE), ATP-binding cassette (ABC), and bilitranslocase (BTL-like transporters) aid in the transport of ACNs into vacuoles and their sequestration in vacuolar inclusions (AVIs) in the membrane transporter-mediated pathway (3), Glutathione S-transferases (GSTs) mediate the conjugation of ACNs to generate the glutathione-ACNs conjugate, which serves as an intact and efficient means of transport from the ER to the vacuole.
The concentration of ACNs varies from 0.1 to 1.0% across various plant materials (Ercoli et al., 2021). Vegetables (e.g., red potato, purple sweet potato, eggplant, carrot, purple corn, red onion, and red cabbage) and fruits (e.g., berries, cherries, blood oranges, blackcurrants, pomegranate, grapes, plums, and apples) are essential sources of ACNs for human nutrition (Karacabey et al., 2023; Mohammed and Khan, 2022; Tan et al., 2023). Berries, grapes, blackcurrants, and some tropical fruits have been identified as abundant sources of ACNs (Khoo et al., 2017). Due to their broad range of nutra-pharmaceutical attributes and chemo-preventive effects, as evidenced by clinical trials, ACNs have emerged as promising natural compounds with the potential to replace synthetic additives or colorants (Thakur et al., 2023).
ACNs consist of two aromatic rings attached to three carbons in an oxygenated heterocycle and a chromane ring with a second aromatic ring (Tarone et al., 2020) (Fig. 2). ACNs have sugar at R3 positions called oligosaccharide side chains, normally in a single glucoside unit. The amount of hydroxyl and methoxy groups indicates the level of intensity and type of color of the ACNs. The higher amounts of hydroxyl and methoxy groups contribute to the extracts’ intense bluish and redness colors, respectively (Enaru et al., 2021).

Adapted from Kozłowska and Dzierżanowski (2021) with modification
Microencapsulation of ACNs extracts from various food sources and general chemical structure of ACNs and their anthocyanidins. R3 = sugar (glucose, arabinose, galactose, etc.).
ACNs are also known as the glycosylated (aglycone) forms of anthocyanidins. Chemically, anthocyanidins are sugar-free counterparts of ACNs. As natural dyes, these compounds are responsible for the color of many fruits (Khoo et al., 2017). There are six main types of glycosides derivative ACNs: pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin. Interestingly, pelargonidin displays a red-colored pigment in fruits and berries but appears orange in flowers. Besides, cyanidin appears reddish-purple or magenta, while delphinidin causes a blue-reddish or purple pigment in plants. Examples of methylated anthocyanidins include peonidin, malvidin, and petunidin. Peonidin contributes to the magenta color in grapes, berries, and red wines. Also, malvidin displays as a darker rusty red pigment in red wine, while petunidin appears as a dark red or purple pigment in blackcurrants and purple petals of the flower (Khoo et al., 2017). These color variations can result from the complex interplay between pH, co-pigments, metal ions, genetics, environmental factors, chemical modifications, and concentration levels (Enaru et al., 2021).
Stability of ACNs
ACNs, which are hydrophilic, are highly unstable compounds and are quickly degraded due to different factors. The stability of ACNs is influenced by their concentration, pH, storage temperature, chemical structure, the presence of enzymes, proteins, flavonoids, metal ions, oxygen, and light (Enaru et al., 2021; Jafari et al., 2016). Their stability is also affected by the presence of hydroxyl or methoxy functional groups in the structure, as the presence of these clusters decreases the stability of the compound in an organic solvent or aqueous solution (Khoo et al., 2017).
The quality of ACNs degrades during processing and storage, thereby reducing the effectiveness of their potential role in the food and pharmaceutical industries. Therefore, preventive measures must be employed to maintain the quality of these compounds, particularly during thermal processing (Ijod et al., 2022). The degradation of ACNs occurs when they are exposed to temperatures exceeding 60 °C for prolonged periods, typically exceeding 60 min. Such conditions can break covalent bonds within ACN molecules and induce oxidation processes, resulting in a loss of color and potential health benefits (Ali et al., 2016). In blanched purple potatoes (95–97 °C/2 min), a 63% loss in total monomeric ACNs was recorded compared to that in fresh potatoes (Karacabey et al., 2023). Also, hot drying (60℃, 10 h), hot water blanching (5 min), and steaming (100℃, 5 min) of red cabbages resulted in ACNs losses of 60, 23 and 13%, respectively (Tan et al., 2023).
Improving ACNs stability: chemical and biological approaches
The therapeutic applications of ACNs are often limited because of their reduced stability and low solubility in aqueous and organic media. Interestingly, ACNs can be transformed into acylated or glycosylated derivatives using enzymatic, chemical, or chemoenzymatic approaches. For example, converting ACNs into bioconjugates via fatty acid (FAs) acylation may offer the opportunity to positively modify these compounds’ physicochemical properties and biological functionalities (Khoo et al., 2017). Bioconjugates, a novel class of hybrid materials consisting of a synthetic macromolecule linked to a biomolecule/biological entity such as peptides or proteins, vitamins, and nucleic acids, are gaining increasing importance in the fields of medicine, biotechnology, and nanotechnology (Li and Mahato, 2017).
The stability of ACNs can also be improved through intramolecular copigmentation, wherein the color intensity of anthocyanidins/ACNs is increased or reinforced in the presence of other flavonoids as cofactors or copigments (Azman et al., 2022a, b). In this phenomenon, due to color intensification, an increase in color intensity with spectral shifts towards higher wavelengths can be observed with the addition of a copigment to acidic (preferably), neutral, and even slightly alkaline ACNs aqueous solutions.
Improving ACNs stability: physical approaches
In addition to bioconjugation and copigmentation, the stability and functional properties of ACNs can be improved using microencapsulation. Microencapsulation is a viable alternative for enhancing the stability and practical uses of ACNs on an industrial scale. In this process, ACNs, being the core material (active agent), are entrapped within another substance (wall material/coating material) or encapsulants such as starches, gelatin, GA, and MD (Estupiñan-Amaya et al., 2023; Mahdavi et al., 2016a; Rocha et al., 2019).
Optimization of various parameters (type and concentration of the encapsulating agent, concentration of the active agent, ratio of coating/active material, process time, temperature, etc.) is required to establish the best conditions for microencapsulation, offering better yield and good quality of the end-use powdered product, which has been extensively reviewed by Tarone et al. (2020). In this context, conventional optimization methods are now being replaced by modern approaches, such as response surface methodology (RSM) and in silico (computer simulation) modeling studies, to devise an optimized microencapsulation process (Machado et al., 2022; Mahdavi et al., 2016a; Tarone et al., 2020).
Microencapsulation provides many advantages to the final product by diminishing the surroundings and protecting bioactive compounds/ACNs from side effects caused by air, light, moisture, and heat. This process can deteriorate the vapor of the inside material to the outside environment and modify the physical characteristics to make ACNs or related bioactive materials more convenient to use. Also, microencapsulation is efficient in masking the inside material's flavor and forming two phases when mixed with liquid or semi-solid products (Ray et al., 2016). Powdered particle size can be categorized as macro (> 5000 µm), micro (1–5000 µm), and nano (< 1 µm) (Jafari et al., 2008).
Spray drying microencapsulation
Spray drying, due to its versatility, cost-effectiveness, and ease of operation, is the most common and widely applicable technique for the encapsulation of bioactive such as ACNs-rich extracts (Da Rosa et al., 2019; Ravichandran et al., 2023) (Fig. 2). Spray drying process is the traditional method for forming powders from semi-solid or liquid forms. Most of the 15,000 industries have used spray dryers to form products, such as luminescence materials, oxides, chemicals, fertilizers, and dried foods (Nandiyanto et al., 2019).
Additionally, it is a process in which the industry can manage acceptable levels of deterioration and decomposition of volatile compounds such as fruit juice. Spray drying, which involves encapsulation by creating protective ‘walls’ around sensitive ingredients, converts liquids into solids and enhances their shelf-life and color stability while safeguarding them against oxidation (Vasile et al., 2023). The reduced volume resulting from spray drying also simplifies the handling and storage of the compounds.
The principles of spray drying include preparation, homogenization, atomization, dispersion, and dehydration of the liquid solution. A critical issue during this process is wall deposition, which may affect the quality and quantity of the product that needs to be achieved. The occurrence of wall deposition depends on the spray dryer’s type, size, and operating parameters. Appropriate measures and controls are needed to avoid wall deposition, thus preventing the high maintenance cost and lowering the powder yield (Tarone et al., 2020). The efficiency of spray drying depends on the selection of parameters, such as the encapsulating agent and its concentration added to the feed (active agent), inlet and outlet temperatures, atomization speed or pressure, and feed flow rate (Gawalek, 2022; Pan et al., 2022; Vasile et al., 2023). These factors must be controlled and manipulated for acceptable physical properties and a higher powder yield.
Encapsulating agents
The limitation of the spray drying technique is the use of high temperatures for drying and air access (Bednarska and Janiszewska‐Turak, 2019), which may decompose thermally sensitive ACNs (Gawalek, 2022). Therefore, encapsulating agents are introduced in the spray drying to facilitate powder production, especially for ripe fruits with high °Bx value. This high °Bx value corresponded to a low glass transition temperature (Tg). When ripe fruits dehydrate above Tg, they may exhibit stickiness and adhere to dryer walls, resulting in a reduction in the final yield of the product (Zotarelli et al., 2017). To prevent stickiness, one option is to control the glass transition temperature so that it remains below Tg + 20 °C. High molecular weight encapsulating agents can also improve the product’s glass transition (Machado et al., 2022).
Encapsulating agents can be divided into groups of carbohydrates, proteins, or a combination of both. Carbohydrates act as a protective barrier from the external environment to the inside material, such as gums, starch, modified starches, dextrins, and cellulose (Halahlah et al., 2023; Lacerda et al., 2016; Villacrez et al., 2013). The most essential characteristics of this group are their emulsifying activity and solubility properties. These agents should exhibit characteristics such as low viscosity, non-hygroscopic, bland flavor/tasteless, non-reactive with core materials, soluble in aqueous solvents, inexpensive, food-grade, flexible, rigid, thin, and pliable (Tan et al., 2015).
MD has good water solubility and low viscosity and is produced by enzymatic or acid hydrolysis of starch (Lacerda et al., 2016). The low emulsifying properties of MD can be counteracted by substituting it with sodium octenyl succinate (OSA) starch. OSA starch introduces lipophilic elements, resulting in amphiphilic properties that enhance emulsification (Lacerda et al., 2016; Sweedman et al., 2013). Another encapsulating agent in carbohydrates is inulin, which is a polysaccharide. This polysaccharide consists of fructose units linked by β-(2,1) bonds with glucose in the chain. Inulin is derived from chicory and has dietary fiber and prebiotic effects on consumers (El-Kholy et al., 2020; Lacerda et al., 2016).
Examples of protein types include whey protein isolates (WPI) and soy protein isolates (SPI) (Robert and Fredes, 2015). These proteins excel as encapsulating agents because of their distinct attributes and adaptability. Whey protein isolates sourced from whey, a byproduct of cheese production, are renowned for their high nutritional quality as complete proteins containing all essential amino acids. They also exhibit exceptional emulsifying properties, stabilize emulsions, and efficiently encapsulate lipophilic compounds. Moreover, WPI has a low allergenicity, making it suitable for a wide array of applications, and its neutral flavor accommodates the encapsulation of diverse ingredients. On the other hand, SPI, derived from soybeans, offers versatility by encapsulating a broad spectrum of ingredients, including flavors, vitamins, minerals, and lipids. With its high protein content, SPI enhances the nutritional value of encapsulated products. In contrast, its functional properties, such as emulsification and film formation, further contribute to its effectiveness in encapsulation applications. Being gluten-free and sustainably sourced from soybeans, SPI aligns with dietary preferences and environmentally conscious practices (Bian et al., 2022).
The selection of encapsulating agents depends on the solubility of the bioactive compound of interest, which is either hydro- or lipo-soluble. GA is the best encapsulating agent for liposoluble bioactive compounds, and hydro-soluble compounds such as ACNs and MD with different dextrose equivalent (DE) values, GA, or modified starch are commonly used. Due to its low hygroscopicity, the prominent MD used in spray drying is MD 10-DE (De Souza et al., 2015).
Encapsulating agents from natural polymers can prevent the degradation of ACNs and aid in their delivery to the human body for nutraceutical applications (Vergara et al., 2020). Combining OSA starch, inulin, and MD as encapsulating agents with an encapsulating carbohydrates (EC) ratio of 2:1:1 (2/3:1/6:1/6) produced jussara pulp microparticles with favorable properties. Specifically, these microparticles exhibited excellent color, antioxidant activity, and ACNs content, making them a promising encapsulation approach for preserving the quality and functionality of the jussara pulp (Lacerda et al., 2016).
The production of ACNs-rich powders from various fruit sources using spray drying is summarized in Table 1. Overall, the SPI encapsulation technique was highly efficient for polyphenols (catechins, ellagitannins, gallitannins, and quercetin glycosides). This is due to the nature of the charge of bioactive compounds, where polyphenols and ACNs have negative and positive charges, respectively. Also, the polyelectrolyte structure (density and charge type) contributes to the interaction of SPI with the bioactive polymer. Meanwhile, MD was more effective in entrapping ACNs owing to the production of larger and smoother particles of fruit powder than GA, which produced smaller particles and wrinkled surfaces, thus making it easy to be exposed to oxygen (Ferrari et al., 2013).
Specific inlet and outlet temperatures are needed to maximize encapsulation efficiency and prevent the acceleration of ACNs, polyphenols, and antioxidant degradation (Gawalek, 2022). Higher inlet temperatures may produce lower-quality ACNs powder, such as a dense surface layer, thereby reducing the efficiency of powder reconstitution (Jafari et al., 2017). In some instances, spray drying using a high inlet temperature and aspiration rate may contribute to the high yield of microparticles. Consequently, this can reduce the stickiness of fruit powder on the cyclone wall (Yingngam et al., 2018). A more significant loss of ACNs was detected when the air inlet temperature was higher than 180℃ (Gawalek, 2022). A higher inlet temperature with a lower concentration of encapsulating agents below 30% (w/w) leads to decreased ACNs stability due to non-enzymatic browning and pro-anthocyanidin deterioration (De Souza et al., 2015).
A combination of encapsulating agents, such as protein-based products, protects ACNs better than a single encapsulating agent. Combining encapsulating agents is assumed to create a strong interaction, leading to a synergistic effect in improving stability. The temperature and presence of light also influence the stability of ACNs during storage. Low temperatures and dark conditions during storage improve the stability of ACNs and prolong their shelf-life. Overall, optimizing spray drying conditions, particularly drying temperature, is crucial to ensure polyphenol encapsulation, as presented in Table 1.
Physicochemical, functional food, and nutraceutical attributes of encapsulated ACNs–rich powder
Moisture content, water activity, and particle size are the most critical parameters for producing microencapsulated ACNs powders. The low moisture content of microencapsulated fruit powder is vital to achieve excellent stickiness, flowability, and storage stability and prevent microbial growth. This is due to the higher water activity, which provides more free water space for microbial growth. Notably, the water activity level of the microencapsulated powder produced by spray drying is below 0.3, effectively inhibiting microbial growth (Todorović et al., 2022).
Furthermore, the particle size of the powder affects the texture and nutritional properties of the food product. The optimal size range for microencapsulated powder produced by spray drying is 10−100 μm (Da Rosa et al., 2019). Solubility is also salient because it provides desirable properties such as dispersibility, solubility, and wettability. These properties contribute to the rehydration of food ingredients; thus, a low moisture content of the powder is desired (Mahdavi et al., 2016a). As shown in Fig. 3, good solubility of the microencapsulated pomegranate powder was achieved when GA was used as an encapsulating agent. However, its optical properties are poor compared to encapsulation with waxy starch and MD and without cellulose (Yousefi et al., 2010).
Effect of encapsulating agent on the microstructure of microencapsulated ACNs powder with (a) GA (12%, w/v), (b) waxy starch (12%, w/v), and (c) MD (12%, w/v) without cellulose from Yousefi et al. (2010)
Santos et al. (2019) found that using MD in blackberry spray drying efficiently preserved the physicochemical characteristics of the end-use powder product. This was due to the MD characteristics having a less hygroscopic nature, which resulted in improved ACNs retention, low moisture content, and excellent powder reconstitution. With these properties, this spray-dried blackberry powder is easily soluble in water and can be effectively applied to produce juice fruit powder.
The microencapsulation of ACNs provides multiple benefits to human health. For example, the addition of encapsulated ACNs powders into yogurt, ice cream, or other desserts can serve as a prebiotic, assisting in addressing digestive system issues. Using inulin to encapsulate ACNs in cornelian cherry fruit extract has an anti-diabetic effect at 1 mg/mL. It is suitable as a food ingredient for diabetic consumers (Enache et al., 2020). Besides, this product suits a vegan diet and consumers with lactose intolerance and diabetic problems (Dias et al., 2020; Enache et al., 2020). In the simulated gastrointestinal studies by da Rosa et al. (2019), microencapsulated blueberry extracts successfully improved ACNs digestion than unencapsulated blueberry extracts. Oancea et al. (2018) also reported that using WPI as an encapsulating agent could facilitate the release of ACNs into the intestine. The presence of various enzymes and different pH levels in the human digestive system makes it a complex system. This affects the stability and bioavailability of the ACNs. Combining encapsulating agents, as opposed to single encapsulating agents, is a successful approach. The synergistic effects of these encapsulating agents improve the stability and bioavailability of the ACNs. This implementation enables a controlled-release mechanism that efficiently administrates ACNs to targeted organs or systems, thereby promoting the overall improvement of human health (Fig. 4) (Da Rosa et al., 2019; Enache et al., 2020; Mansour et al., 2020; Oancea et al., 2018). Given these potential health advantages, producing microencapsulated ACNs powder with high solubility is essential and possesses sustained-released properties for its effective use in dry mixes or instant health foods.
Other innovative microencapsulation techniques
In addition to spray drying, various microencapsulation approaches have been studied and verified for their efficiency in encapsulating ACNs. Promising techniques for ACNs include freeze-drying, SC–CO2, coacervation, drum-drying, and electrospraying microencapsulation. Each approach has unique benefits regarding quality, compound preservation, and encapsulation effectiveness. The following section will discuss their principles, benefits, and prospective uses for protecting the durability and efficacy of ACNs in detail.
Freeze-drying
The freeze-drying technique (Fig. 5a) relies on the sublimation of water from frozen material and has been explored as an alternative approach for encapsulating ACNs (Fredes et al., 2018). This process involves freezing, sublimation, desorption, and storage (Bhatta et al., 2020). The sublimation phase efficiently extends shelf life and preserves the quality of heat-sensitive food materials. As a simple technique for encapsulating water-soluble essences such as ACNs and other natural aromas or medications, freeze-drying is one of the most convenient methods for drying thermosensitive substances that are unstable in aqueous solution (Azarpazhooh et al., 2018; Estupiñan-Amaya et al., 2020) (Table 2).

Adapted from Bigazzi et al. (2020) with modifications
Schematic diagram of (a) a typical freeze-drying mechanism, (b) supercritical conditions, (c) ACNs coacervation, (d) double drum drying mechanism, (e) a typical electrospraying mechanism.
As shown in Table 2, MD frequently provided excellent ACNs retention and stability results, particularly at low DE values. A low DE generates low hygroscopicity in the ACNs-rich powder, which minimizes moisture absorption and accelerates powder deterioration. Moreover, MD is highly soluble, blending effortlessly with water and producing potent combinations when combined with ACNs.
The concentration of the encapsulating agent affects ACNs stability, as a high amount of the encapsulating agent serves as a solid wall to protect the core molecule. Regular integration of encapsulating agents improves ACNs retention during storage. This combination initiates the construction of a dual-property wall that regulates compound delivery while simultaneously increasing compound stability. This synergistic effect was observed when MD was combined with the different encapsulation agents.
Supercritical carbon dioxide (SC-CO 2 )
SC-CO2 is an emerging method that is inert, non-toxic, non-flammable, low cost, environmentally friendly, versatile, and free from toxic excess in the yield of the product formed (Fig. 5b) (Da Fonseca Machado et al., 2018). SC-CO2 has excellent solvents such as carbon dioxide (CO2), ethane, water, propane, and dimethyl ether, which can be categorized as having gas-like low viscosity, intermediate diffusivity, and liquid-like high density. This technique may overcome the disadvantages of the conventional spray drying technique by applying the processing medium with the conditions above its critical point, 31.1 °C and 7.4 mPa (Jang and Koh, 2023), to precipitate and encapsulate the ACNs (Da Fonseca Machado et al., 2018). A supercritical fluid is above its critical point where gas and liquid exist in the equilibrium phase, and this fluid is known as a pure substance (Wang et al., 2020). This technique's efficiency depends on the active ingredient's thermodynamic properties, encapsulating agents, suitable co-solvents, and the materials used.
As shown in Table 2, the ACNs exhibited more excellent stability when encapsulated in polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP) using CO2 as the solvent and ethanol as a co-solvent. It is worth noting that these two solvents are generally recognized as safe (GRAS). The improved ACNs retention during SC–CO2 encapsulation can be attributed to the solubility of PEG and PVP in ethanol. PVP and PEG are both soluble in ethanol, which facilitates their combination with ACNs to create a stable solution before encapsulation.
Coacervation
Coacervation microencapsulation involves the separation of one or more hydrocolloids from the original solution. Following this separation, the newly created coacervate phase surrounds and encapsulates the active ingredient, which is either suspended or emulsified within the same reaction medium (Fang and Bhandari, 2010) (Fig. 5c). Coacervation encapsulation can be accomplished using a single colloidal solute such as gelatin, or through a more intricate method involving substances such as gelatin and gum acacia. Although complex coacervation often lacks specific shapes and is deemed an expensive method for encapsulating food items, it is important to weigh its potential advantages. Specifically, they can be valuable for encapsulating sensitive and high-value functional ingredients, including ACNs (Devi et al., 2023).
Devi et al. (2023) also found that using the dual emulsion method followed by complex coacervation with gelatin and acacia gum enhanced the microencapsulation of ACNs from black rice bran. This approach improved the encapsulation efficiency and thermal stability and ensured better stability of ACNs during storage at both 7℃ and 37℃. The microcapsules exhibited decreased moisture content, hygroscopicity, and solubility. Additionally, their appearance was characterized by smooth, circular, or intact surfaces and firm and agglomerated structures. The findings in Table 2 indicate that the coacervation formulation effectively extended the shelf life of ACNs, especially under high-temperature conditions, compared to ACNs that were not encapsulated within a coacervation complex. This indicates that the coacervation formulation formed between the different encapsulating agents strengthens their heat stability, thereby improving the protection of the core materials. Also, the selection of gum-based materials significantly influences the complexity and stability of coacervation formulations. This factor is crucial for dealing with sensitive compounds.
Drum drying
Drum drying is widely employed in food and chemical sectors to produce powdered or granular substances. This process involves spreading a liquid or slurry in a thin, even layer on the surface of a heated revolving drum to dry the material. The material underwent drying upon contact with the internally heated surface of the rotating drum (Fig. 5d) (Sakulnarmrat et al., 2021b; Sakulnarmrat and Konczak, 2022). Encapsulating agents are often used to protect ACNs during microencapsulation. Meanwhile, Senevirathna et al. (2021) produced purple sweet potato powder with the addition of citric acid rather than encapsulating it with encapsulating agents. In comparison to the control, the powder made with 0.6% citric acid had a higher concentration of ACNs, antioxidant activity, and an intense red color. As a result, it can be determined that factors such as steam pressure, drum rotation speed, and citric acid content influence the powder quality, which can be optimized using RSM.
Based on the findings in Table 2, it can be summarized that even though a similar combination of encapsulating agents was incorporated during drum drying, the encapsulation efficiency was different, probably because of the different sources of ACNs, which possess different properties and interactions with encapsulating agents. Therefore, optimization must be performed to select the best concentration of both encapsulating agents to address this issue.
Electrospraying
Electrospraying encapsulation involves the formation of nanodroplets by using a high-voltage electric field. The voltage, solution feed rate, solution properties, humidity, temperature, and separation from the needle tip to the collector are only a few variables that might influence the ultimate output (Atay et al., 2018). The electrospraying equipment included a syringe pump, voltage power supply, collector, and syringe (Fig. 5e). The electrospraying process requires injecting a syringe-fed mixture of ACNs and encapsulating agents into a liquid medium before an electric field is applied at the nozzle. Therefore, it overcomes the surface tension and produces a cone-shaped droplet called a Taylor cone. Increasing the electric field causes the Taylor cone to become fragile and expels tiny droplets of encapsulated ACNs. Therefore, the solvent evaporates in the air, causing the droplets to coagulate as small particles and accumulate in the collector (Atay et al., 2018; González-Cruz et al., 2020).
The solution properties, including viscosity, surface tension, pH, and electrical conductivity, are crucial elements that must be considered to ensure that ACNs can undergo electrospraying. Improper solution properties cause undesirable particles to form, eventually reducing encapsulation efficiency (Atay et al., 2018). As reported by González-Cruz et al. (2020), the addition of 10 – 20% zein successfully encapsulated ACNs from blueberries; further increases in concentration caused instability and clogging in the nozzle. Adding ACNs to 10 – 30% agave fructans or WPI resulted in instability during the electrospraying process. Based on Table 2, it can be inferred that various factors must be considered during the encapsulation of ACNs by electrospraying. Using a combination of encapsulating agents has improved protective properties and the controlled release of ACNs. Nonetheless, when employing a single encapsulating agent, the concentration and molecular weight of the agent play critical roles in achieving high-quality encapsulated ACNs.
Encapsulation efficiency across various microencapsulation techniques
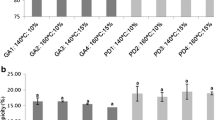
The encapsulation efficiency (EE) is a crucial parameter that determines the ability of the microencapsulation process to protect targeted compounds. The data presented by various authors in Table 1 and Table 2 for various microencapsulation techniques employed for encapsulating ACNs were used to illustrate the potential of their encapsulation efficiency (Fig. 6). SD and drum drying consistently showed high EE values ranging from 91.14%–99.80% and 98.85%–98.86%, respectively. In contrast, FD exhibited slightly more variability in EE, ranging from 85.00 to 98.33%. Although FD yielded a high EE in some studies (98.33%), some authors reported values lower than 90%, indicating an inconsistency in achieving at least 90% EE. Other techniques, such as SC-CO2, showed an EE higher than 90%. Coacervation and electrospraying display varying degrees of efficiency, indicating the influence of the process parameters and formulation characteristics. The aforementioned techniques had the lowest EE, ranging from 44.77%–86.00% and 52.65%–76.90%, respectively. Considering the EE data, SD is the best and most promising choice for ACNs encapsulation, owing to its consistently high efficiency, relatively straightforward process, and cost-effectiveness.
Percentage encapsulation efficiency (EE) of various encapsulated ACN powders by referring to the optimal operation of different encapsulation techniques as reported by different authors. The straight line indicates 90% of EE. Different bar colors indicate the different techniques. Yellow, spray drying; blue, freeze-drying; purple, SC-CO2; orange, coacervation; green, drum drying; dark red, electrospraying; numbers 1–17 referred to different authors. 1 – Fredes et al. (2018), 2 – Yingngam et al. (2018), 3 – Ribeiro et al. (2019), 4 – Xue et al. (2019), 5 – Pan et al. (2022), 6 – Laureanti et al. (2023), 7 – Zahed et al. (2023), 8 – Jang and Koh (2023), 9 – Nguyen et al. (2022), 10 – Xue et al. (2019), 11 – Santos et al. (2013), 12 – Gharanjig et al. (2020) 13 – Sarkar et al. (2020), 14 – Sakulnarmrat and Konczak (2022), 15 – Sakulnarmrat et al. (2021b), 16 – Atay et al. (2018), 17 – González-Cruz et al. (2020)
Current status of product application with microencapsulation techniques
The increasing awareness and demand for healthy products among consumers requires the food industry and researchers to determine a solution for incorporating and protecting bioactive compounds, such as ACNs, in products. Therefore, microencapsulation is a promising solution for protecting ACNs. Many researchers have compared and determined the effects of different parameters and microencapsulation techniques on the stability of ACNs after incorporation into the product (Mihalcea et al., 2020; Sakulnarmrat et al., 2021a; Santos et al., 2022). For instance, Sakulnarmrat and Konczak (2022) incorporated ACNs from lamduan into gummy jellies after double-drum drying with different encapsulating agents. The combination of MD and GA (60:40) was selected as the best combination and applied to gummy jellies. The shelf-life stability of the gummy jellies was studied for eight weeks at different temperatures (25℃ and 35℃). The highest lamduan encapsulated powder (30 g/kg) added to gummy jellies showed the most extended shelf life and retention of ACNs at both temperatures after eight weeks of storage. These findings corroborated the idea that encapsulating ACNs before application to food products retained and enhanced their stability and functionality. Other examples of ACNs sources, microencapsulation techniques, and their applications in various products are shown in Table 3.
Advantages and disadvantages of spray drying compared to other methods
Like any other method, spray drying has unique strengths and limitations compared to alternative techniques. It is quicker, more affordable, versatile, and appropriate for large-scale production than freeze-drying, SC–CO2, coacervation, drum drying, and electrospraying microencapsulation. However, the potential deterioration of heat-sensitive substances and low encapsulation efficiency during the encapsulation process are noteworthy disadvantages of spray drying. However, the choice of method always depends on the specific properties of the product being dried and the desired characteristics of the final dried product.
Conventional spray drying is the most popular and practically viable technique for microencapsulating ACNs and other bioactive components. High-quality powders with low moisture content, water activity, particle size, and morphology can be produced using spray drying. The selection of encapsulating agents may vary from case to case and mainly depends on the nature of the feed material/active agent to be microencapsulated. Various encapsulating agents are used on specific fruits at specific concentrations or ratios. It can be concluded that the best encapsulating agent is MD, with an inlet temperature of < 180℃. This was due to MD characteristics with low viscosity, moisture, hygroscopicity, and good solubility. These can help reconstitute and entrap bioactive compounds from deterioration during processing. Thus, it can easily be used as a food ingredient. Although there are many emerging technologies for microencapsulation, spray drying is still widely practiced in most industries due to its low operation cost, high yield, speed, and efficiency.
Overall, spray drying is more effective than other techniques for microencapsulation due to its ability to encapsulate bioactive compounds rapidly and individually. However, ACNs extraction requires longer, and the solvents are expensive and risky. Moreover, it is recommended that appropriate encapsulating agents for specific fruits be used to increase the stability of ACNs-rich powder, which is costly. Storage surroundings are also necessary for stabilizing the ACN compound; thus, suitable storage, such as vacuum-pack packaging, can be used to prevent oxidation. Therefore, it is recommended to add an antioxidant agent, such as tocopherol, into the fruit extract and encapsulating agents before the spray drying. Studies have focused on powdered products' physicochemical and nutritional characteristics derived from the spray-drying microencapsulation of ACNs-rich fruits. However, functional foods and nutraceutical qualities are frequently overlooked. Therefore, there is a need to analyze and explore the functional food and nutra-pharmaceutical prospects of ACNs-rich microencapsulated powdered products for specific food applications. Moreover, it can be noted that different process variables for spray drying microencapsulation of ACNs-rich fruits have been optimized by the researchers using conventional methods. However, with new software development, modern tools such as RSM and computer simulation techniques are needed to allow rapid evaluation and optimization of the plans and design of the spray drying-based microencapsulation process. Using artificial neural networking (ANN) to validate such designs can provide more value for optimizing such processes.
Abbreviations
- ABC:
-
ATP-binding cassette
- ACNs:
-
Anthocyanins
- ANN:
-
Artificial neural networking
- AVIs:
-
Vacuolar inclusions
- BTL:
-
Bilitranslocase
- CHI:
-
Chitosan
- CMC:
-
Carboxymethylcellulose
- CO2 :
-
Carbon dioxide
- CSG:
-
Cress seed gum
- DE:
-
Dextrose equivalents
- EC:
-
Encapsulating carbohydrates
- ER:
-
Endoplasmic reticulum
- FAs:
-
Fatty acids
- FD:
-
Freeze-drying
- GA:
-
Gum arabic
- GGM:
-
Galactoglucomannan
- GRAS:
-
Generally recognized as safe
- GSTs:
-
Glutathione S-transferases
- GX:
-
Glucuronoxylan
- HMP:
-
High methyl pectin
- HPH:
-
High-pressure homogenization
- IN:
-
Inulin
- IG:
-
Ionic gelation
- kDa:
-
Kilodalton
- KON:
-
Konjac
- MATE:
-
Multidrug and toxic compound extrusion
- MC:
-
Modified chitosan
- MD:
-
Maltodextrin
- mPa:
-
Millipascal
- Mw:
-
Molecular weight
- OSA:
-
Sodium octenyl succinate
- PAG:
-
Prosopis alba exudate gum
- PEC:
-
Pectin
- PEG:
-
Polyethylene glycol
- PVP:
-
Polyvinylpyrrolidone
- RBC:
-
Rice bran concentrate
- RSM:
-
Response surface methodology
- SA:
-
Sodium alginate
- SC-CO2 :
-
Supercritical carbon dioxide
- SMP:
-
Soymilk powder
- SNARE:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- SPI:
-
Soy protein isolates
- SPP:
-
Soy protein powder
- TA:
-
Total anthocyanins
- Tg:
-
Glass transition temperature
- TP:
-
Total phenolics
- TS:
-
Tapioca starch
- WPI:
-
Whey protein isolates
- WS:
-
Waxy starch
- XG:
-
Xanthan gum
- ZG:
-
Zedo gum
- °Bx:
-
Brix
References
Abdel‐Aty AM, Barakat AZ and Mohamed SA. Garden cress gum and maltodextrin as microencapsulation coats for entrapment of garden cress phenolic-rich extract: improved thermal stability, storage stability, antioxidant and antibacterial activities. Food Science and Biotechnology. 32:47-58 (2022)
Ali O-H, Al-Sayed HMA, Yasin NMN and Afifi E. Effect of different extraction methods on stablity of anthocyanins extracted from red onion peels (Allium cepa) and its uses as food colorants. Egyptian Journal of Nutrition. 47:1-24 (2016)
Almeida RF, Gomes MHG and Kurozawa LE. Rice bran protein increases the retention of anthocyanins by acting as an encapsulating agent in the spray drying of grape juice. Food Research International. 172:113237 (2023)
Atay E, Fabra MJ, Martínez‐Sanz M, Gómez‐Mascaraque LG, Altan A and López‐Rubio A. Development and characterization of chitosan/gelatin electrosprayed microparticles as food grade delivery vehicles for anthocyanin extracts. Food Hydrocolloids. 77:699-710 (2018)
Azarpazhooh E, Sharayei P, Zomorodi S and Ramaswamy HS. Physicochemical and phytochemical characterization and storage stability of freeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food and Bioprocess Technology. 12:199-210 (2018)
Azman EM, Nor NDM, Charalampopoulos D and Chatzifragkou A. Effect of acidified water on phenolic profile and antioxidant activity of dried blackcurrant (Ribes nigrum L.) pomace extracts. LWT. 154:112733 (2022a)
Azman EM, Nurhayati Y, Chatzifragkou A and Charalampopoulos D. Stability enhancement of anthocyanins from blackcurrant (Ribes nigrum L.) pomace through intermolecular copigmentation. Molecules. 27:5489 (2022b)
Bąkowska-Barczak A and Kolodziejczyk PP. Black currant polyphenols: Their storage stability and microencapsulation. Industrial Crops and Products. 34:1301-1309 (2011)
Bednarska MA and Janiszewska‐Turak E. The influence of spray drying parameters and carrier material on the physico-chemical properties and quality of chokeberry juice powder. Journal of Food Science and Technology. 57:564-577 (2019)
Bhatta S, Janežić TS and Ratti C. Freeze-drying of plant-based foods. Foods. 9:87 (2020)
Bian X, Xing T-L, Yang Y, Fan J, Ma C, Liu X, Wang Y, He Y, Wang L, Wang B and Zhang N. Effect of soy protein isolate on physical properties of quinoa dough and gluten‐free bread quality characteristics. Journal of the Science of Food and Agriculture. 103:118-124 (2022)
Bigazzi W, Penoy N, Évrard B and Piel G. Supercritical fluid methods: an alternative to conventional methods to prepare liposomes. Chemical Engineering Journal. 383:123106 (2020)
Da Fonseca Machado AP, Rezende CA, Rodrigues RAF, Barbero GF, De Tarso Vieira E Rosa P and Martínez J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technology. 340:553-562 (2018)
Da Rosa JR, Nunes GS, Motta MH, Fortes JP, Weis GCC, Hecktheuer LHR, Müller EI, Menezes C and Da Rosa CS. Microencapsulation of anthocyanin compounds extracted from blueberry (Vaccinium spp.) by spray drying: characterization, stability and simulated gastrointestinal conditions. Food Hydrocolloids. 89:742-748 (2019)
De Araújo Santiago MCP, Nogueira RI, Paim DRSF, Gouvêa ACMS, De Oliveira Godóy RL, Peixoto FM, Pacheco S and Freitas SP. Effects of encapsulating agents on anthocyanin retention in pomegranate powder obtained by the spray drying process. LWT. 73:551-556 (2016)
De Souza VB, Thomazini M, Balieiro JCC and Fávaro-Trindade CS. Effect of spray drying on the physicochemical properties and color stability of the powdered pigment obtained from vinification byproducts of the Bordo grape (Vitis labrusca). Food and Bioproducts Processing. 93:39-50 (2015)
Rocha J de CG, De Barros FAR, Perrone ÍT, Viana KWC, Tavares GM, Stephani R and Stringheta PC. Microencapsulation by atomization of the mixture of phenolic extracts. Powder Technology. 343:317-325 (2019)
Demiray E, Gerbağa A, Karatay SE and Dönmez G. Sequential anthocyanin extraction and ethanol production from eggplant peel through biorefinery approach. Bioenergy Research. 17:383-391 (2023)
Deng W, Li X, Ren G, Bu Q, Ruan Y, Ye F and B L. Stability of purple corn anthocyanin encapsulated by maltodextrin, and its combinations with gum arabic and whey protein isolate. Foods. 12:2393 (2023)
Devi LM, Das AB and Badwaik LS. Effect of gelatin and acacia gum on anthocyanin coacervated microcapsules using double emulsion and its characterization. International Journal of Biological Macromolecules. 235:123896 (2023)
Dias S, Castanheira EMS, Sousa SF and Andrade PB. Natural pigments of anthocyanin and betalain for coloring soy-based yogurt alternative. Foods. 9:771 (2020)
El-Kholy WM, Aamer RA and Ali ANA. Utilization of inulin extracted from chicory (Cichorium intybus L.) roots to improve the properties of low-fat synbiotic yoghurt. Annals of Agricultural Sciences. 65:59-67 (2020)
Enache IM, Vasile A, Enachi E, Barbu V, Stănciuc N and Vizireanu C. Co-microencapsulation of anthocyanins from cornelian cherry fruits and lactic acid bacteria in biopolymeric matrices by freeze-drying: evidences on functional properties and applications in food. Polymers. 12:906 (2020)
Enaru B, Drețcanu G, Pop T, Stănilă A and Diaconeasa Z. Anthocyanins: factors affecting their stability and degradation. Antioxidants. 10:1967 (2021)
Ercoli S, Cartes J, Cornejo P, Tereucán G, Winterhalter P, Contreras B and Ruíz A. Stability of phenolic compounds, antioxidant activity and colour parameters of a coloured extract obtained from coloured-flesh potatoes. LWT. 136:110370 (2021)
Estupiñan‐Amaya M, Fuenmayor CA and López‐Córdoba A. New freeze-dried andean blueberry juice powders for potential application as functional food ingredients: effect of maltodextrin on bioactive and morphological features. Molecules. 25:5635 (2020)
Estupiñan‐Amaya M, Fuenmayor CA and López‐Córdoba A. Exploring the potential of wild andean blueberries for powdered juice production through spray drying. Foods. 12:2348 (2023)
Fang Z and Bhandari B. Encapsulation of polyphenols – a review. Trends in Food Science and Technology. 21:510-523 (2010)
Feitosa BF, Decker BLA, De Brito ES, Rodrigues S and Mariutti LRB. Microencapsulation of anthocyanins as natural dye extracted from fruits – A systematic review. Food Chemistry. 424:136361 (2023)
Ferrari CC, Germer SPM, Alvim ID and Aguirre JM. Storage stability of spray-dried blackberry powder produced with maltodextrin or gum arabic. Drying Technology. 31:470-478 (2013)
Fredes C, Becerra C, Parada J and Robert P. The microencapsulation of Maqui (Aristotelia chilensis (Mol.) Stuntz) juice by spray-drying and freeze-drying produces powders with similar anthocyanin stability and bioaccessibility. Molecules. 23:1227 (2018)
García-Chacón JM, Rodríguez-Pulido FJ, Heredia FJ, González-Miret ML and Osorio C. Characterization and bioaccessibility assessment of bioactive compounds from Camu-camu (Myrciaria dubia) powders and their food applications. Food Research International. 176:113820 (2024)
Gawałek J. Spray drying of chokeberry juice—antioxidant phytochemicals retention in the obtained powders versus energy consumption of the process. Foods. 11:2898 (2022)
Gharanjig H, Gharanjig K, Farzi G, Hosseinnezhad M and Jafari SM. Novel complex coacervates based on Zedo gum, cress seed gum and gelatin for loading of natural anthocyanins. International Journal of Biological Macromolecules. 164:3349-3360 (2020)
González‐Cruz EM, Calderón‐Santoyo M, Barros‐Castillo JC and Ragazzo‐Sánchez JA. Evaluation of biopolymers in the encapsulation by electrospraying of polyphenolic compounds extracted from blueberry (Vaccinium corymbosum L.) variety Biloxi. Polymer Bulletin. 78:3561-3576 (2020)
Halahlah A, Räikkönen H, Piironen V, Valoppi F, Mikkonen KS and Ho TB. Wood hemicelluloses as sustainable wall materials to protect bioactive compounds during spray drying of bilberries. Powder Technology. 415:118148 (2023)
Hoang NTN, Nguyen N, Nguyen L, Le ATH and Đao ĐTA. Research on optimization of spray drying conditions, characteristics of anthocyanins extracted from Hibiscus sabdariffa L. flower, and application to marshmallows. Food Science & Nutrition. 12:2003-2015 (2024)
Ijod G, Musa FN, Anwar F, Suleiman N, Adzahan NM and Azman EM. Thermal and nonthermal pretreatment methods for the extraction of anthocyanins: A review. Journal of Food Processing and Preservation. 46:e17255 (2022)
Jafari SM, Assadpoor E, He Y and Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Drying Technology. 26:816-835 (2008)
Jafari SM, Mahdavi-Khazaei K and Kakhki AH. Microencapsulation of saffron petal anthocyanins with cress seed gum compared with Arabic gum through freeze drying. Carbohydrate Polymers. 140:20-25 (2016)
Jafari SM, Ghalenoei MG and Dehnad D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technology. 311:59-65 (2017)
Jang Y and Koh E. Characterisation and storage stability of aronia anthocyanins encapsulated with combinations of maltodextrin with carboxymethyl cellulose, gum arabic, and xanthan gum. Food Chemistry. 405:135002 (2023)
Karacabey E, Bardakçi MS and Baltacıoğlu H. Physical pretreatments to enhance purple-fleshed potatoes drying: effects of blanching, ohmic heating and ultrasound pretreatments on quality attributes. Potato Research. 66:1117-1142 (2023)
Khoo HE, Azlan A, Tang ST and Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food and Nutrition Research. 61:1361779 (2017)
Koop BL, Da Silva MN, Da Silva FD, Lima KTDS, Soares LS, De Andrade CJ, Valencia GA and Monteiro AR. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: sources, classification and enhanced stabilization by encapsulation and adsorption. Food Research International. 153:110929 (2022)
Kozłowska A and Dzierżanowski T. Targeting inflammation by anthocyanins as the novel therapeutic potential for chronic diseases: An update. Molecules. 26:4380 (2021)
Lacerda ECQ, Calado V, Monteiro M, Finotelli PV, Torres AG and Perrone D. Starch, inulin and maltodextrin as encapsulating agents affect the quality and stability of jussara pulp microparticles. Carbohydrate Polymers. 151:500-510 (2016)
Laureanti EJG, Paiva TS, De Matos Jorge LM and Jorge RMM. Microencapsulation of bioactive compound extracts using maltodextrin and gum arabic by spray and freeze-drying techniques. International Journal of Biological Macromolecules. 253:126969 (2023)
Li F and Mahato RI. Bioconjugate therapeutics: current progress and Future perspective. Molecular Pharmaceutics. 14:1321-1324 (2017)
Li Y, Tang B, Chen J and Lai P. Microencapsulation of plum (Prunus salicina Lindl.) phenolics by spray drying technology and storage stability. Food Science and Technology. 38:530-536 (2017)
Li B, Wang L, Bai W, Chén W, Chen F and Shu C. Dietary sources of anthocyanins. pp. 19-51. In: Anthocyanins. Springer, Singapore (2021)
Ligarda-Samanez CA, Choque-Quispe D, Moscoso-Moscoso E, Palomino-Rincón H, Taipe-Pardo F, Landa JPA, Arévalo-Quijano JC, Muñoz-Saenz JC, Quezada URQ, Huamán-Carrión ML, Gómez EG, León RS, Alipio RAL, Muñoz-Saenz JM and Gutiérrez R. nanoencapsulation of phenolic extracts from native potato clones (Solanum tuberosum spp. andigena) by spray drying. Molecules. 28:4961 (2023)
Machado MH, Da Rosa Almeida A, De Oliveira Brisola Maciel MV, Vitorino VB, Bazzo GC, Da Rosa CG, Sganzerla WG, Mendes C and Barreto PLM. Microencapsulation by spray drying of red cabbage anthocyanin-rich extract for the production of a natural food colorant. Biocatalysis and Agricultural Biotechnology. 39:102287 (2022)
Mahdavi SA, Jafari SM, Assadpoor E and Dehnad D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum arabic and gelatin. International Journal of Biological Macromolecules. 85:379-385 (2016a)
Mahdavi SA, Jafari SM, Assadpour E and Ghorbani M. Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. Journal of Food Engineering. 181:59-66 (2016b)
Mansour M, Salah M and Xu X. Effect of microencapsulation using soy protein isolate and gum arabic as wall material on red raspberry anthocyanin stability, characterization, and simulated gastrointestinal conditions. Ultrasonics Sonochemistry. 63:104927 (2020)
Mazuco RA, Cardoso PMM, Bindaco ÉS, Scherer R, Castilho RO, Faraco AAG, Ruas FG, De Oliveira JP, Guimarães MCC, De Andrade TU, Lenz D, Braga FC and Endringer DC. Maltodextrin and gum arabic-based microencapsulation methods for anthocyanin preservation in Juçara Palm (Euterpe edulis Martius) fruit pulp. Plant Foods for Human Nutrition. 73:209-215 (2018)
Mazza G, Miniati E. Anthocyanins in fruits, vegetables, and grains. 1st Ed. CRC Press, Boca Raton, Florida. pp. 29-314 (2018)
Mihalcea L, Barbu V, Enachi E, Andronoiu DG, Râpeanu G, Stoica M, Dumitrașcu L and Stănciuc N. Microencapsulation of red grape juice by freeze drying and application in jelly formulation. Food Technology and Biotechnology. 58:20-28 (2020)
Mirzaei M, Emam‐Djomeh Z and Askari G. Spray‐drying microencapsulation of anthocyanins of black seedless barberry (Berberis vulgaris). Journal of Food Processing and Preservation. 45:e15858 (2021)
Mohammed HA and Khan RA. Anthocyanins: traditional uses, structural and functional variations, approaches to increase yields and products’ quality, hepatoprotection, liver longevity, and commercial products. International Journal of Molecular Sciences. 23:2149 (2022)
Nandiyanto ABD, Ogi T, Wang W, Gradoń L and Okuyama K. Template-assisted spray-drying method for the fabrication of porous particles with tunable structures. Advanced Powder Technology. 30:2908-2924 (2019)
Nawawi NIM, Ijod G, Abas F, Ramli NS, Adzahan NM and Azman EM. Influence of different drying methods on anthocyanins composition and antioxidant activities of mangosteen (Garcinia mangostana L.) pericarps and LC-MS analysis of the active extract. Foods. 12:2351 (2023a)
Nawawi NIM, Ijod G, Senevirathna SSJ, Aadil RM, Yusof NL, Yusoff MM, Adzahan NM and Azman EM. Comparison of high pressure and thermal pasteurization on the quality parameters of strawberry products: a review. Food Science and Biotechnology. 32:729-747 (2023b)
Nguyen Q, Dang T, Nguyen T and Nguyen N. Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of different carriers on selected physicochemical properties and antioxidant activities of spray-dried and freeze-dried powder. International Journal of Food Properties. 25:359-374 (2022)
Nistor M, Pop R, Daescu A, Pintea A, Socaciu C and Rugină D. Anthocyanins as key phytochemicals acting for the prevention of metabolic diseases: an overview. Molecules. 27:4254 (2022)
Nthimole CT, Kaseke T and Fawole OA. Micro-encapsulation and characterization of anthocyanin-rich raspberry juice powder for potential applications in the food industry. Processes. 10:1038 (2022)
Oancea AMS, Hasan MdM, Vasile A, Barbu V, Enachi E, Bahrim G, Râpeanu G, Silvi S and Stănciuc N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT. 95:129-134 (2018)
Pan L, Chen L-P, Wu C, Wang J, Luo S, Luo J and Zheng Z. Microencapsulation of blueberry anthocyanins by spray drying with soy protein isolates/high methyl pectin combination: physicochemical properties, release behavior in vitro and storage stability. Food Chemistry. 395:133626 (2022)
Ravichandran KS, Silva ES, Moncada M, Perkins-Veazie P, Lila MA, Greenlief CM, Thomas AL, Hoskin RT and Krishnaswamy K. Spray drying to produce novel phytochemical-rich ingredients from juice and pomace of American elderberry. Food Bioscience. 55:102981 (2023)
Ray S, Raychaudhuri U and Chakraborty R. An overview of encapsulation of active compounds used in food products by drying technology. Food Bioscience. 13:76-83 (2016)
Ribeiro A, Estevinho BN and Rocha F. Spray drying encapsulation of elderberry extract and evaluating the release and stability of phenolic compounds in encapsulated powders. Food and Bioprocess Technology. 12:1381-1394 (2019)
Robert P and Fredes C. The encapsulation of anthocyanins from berry-type fruits. Trends in foods. Molecules. 20:5875-5888 (2015)
Rosales-Chimal S, Navarro‐Cortez RO, Bello‐Pérez LA, Vargas‐Torres A and Palma‐Rodríguez HM. Optimal conditions for anthocyanin extract microencapsulation in taro starch: Physicochemical characterization and bioaccessibility in gastrointestinal conditions. International Journal of Biological Macromolecules. 227:83-92 (2023)
Sakulnarmrat K and Konczak I. Encapsulation of Melodorum fruticosum Lour. anthocyanin-rich extract and its incorporation into model food. LWT. 153:112546 (2022)
Sakulnarmrat K, Sittiwong W and Konczak I. Encapsulation of mangosteen pericarp anthocyanin‐rich extract by spray drying. International Journal of Food Science & Technology. 57:1237-1247 (2021a)
Sakulnarmrat K, Wongsrikaew D and Konczak I. Microencapsulation of red cabbage anthocyanin-rich extract by drum drying technique. LWT. 137:110473 (2021b)
Salehi B, Sharifi‐Rad J, Cappellini F, Reiner Ž, Zorzan D, Imran M, Şener B, Kılıç M, El-Shazly M, Fahmy NM, Al‐Sayed E, Martorell M, Tonelli C, Petroni K, Docea AO, Calina D and Maroyi A. The therapeutic potential of anthocyanins: Current approaches based on their molecular mechanism of action. Frontiers in Pharmacology. 11:1300 (2020)
Santos DT, Albarelli JQ, Beppu MM and Meireles MÂA. Stabilization of anthocyanin extract from jabuticaba skins by encapsulation using supercritical CO2 as solvent. Food Research International. 50:617-624 (2013)
Santos SSD, Rodrigues LM, Da Costa SC and Madrona GS. Antioxidant compounds from blackberry (Rubus fruticosus) pomace: microencapsulation by spray-dryer and pH stability evaluation. Food Packaging and Shelf Life. 20:100177 (2019)
Santos SSD, Paraíso CM, Romanini EB, Corrêa VG, Peralta RM, Da Costa SC, De Oliveira Santos O, Visentainer JV, Reis MHM and Madrona GS. Bioavailability of blackberry pomace microcapsules by using different techniques: An approach for yogurt application. Innovative Food Science & Emerging Technologies. 81:103111 (2022)
Sarkar R, Dutta A, Patra A and Saha S. Bio-inspired biopolymeric coacervation for entrapment and targeted release of anthocyanin. Cellulose. 28:377-388 (2020)
Senevirathna SSJ, Ramli NS, Azman EM, Juhari NH and Karim R. Optimization of the drum drying parameters and citric acid level to produce purple sweet potato (Ipomoea batatas L.) powder using response surface methodology. Foods. 10:1378 (2021)
Shaddel R, Hesari J, Azadmard‐Damirchi S, Hamishehkar H, Achachlouei BF and Huang Q. Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. International Journal of Biological Macromolecules. 107:1800-1810 (2018)
Shwetha, Priya B and Preetha R. Study on color stability and microencapsulation of anthrocyanin pigment using spray drying. Biosciences Biotechnology Research Asia. 13:1207-1214 (2016)
Silva PI, Stringheta PC, Teófilo RF and De Oliveira IRN. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. Journal of Food Engineering. 117:538-544 (2013)
Singh P, Pandey VK, Singh KN and Dar AH. Spray-freeze-drying as emerging and substantial quality enhancement technique in food industry. Food Science and Biotechnology. 33:231-243 (2023a)
Singh RK, Lukhmana N, Tahiliani S and Kong F. Microencapsulation of micronized tart cherry puree: Characterization and physicochemical assessment. Food Bioscience. 56:103321 (2023b)
Sweedman MC, Tizzotti M, Schäfer C and Gilbert RG. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydrate Polymers. 92:905-920 (2013)
Tan SP, Kha TC, Parks SE, Stathopoulos CE and Roach PD. Optimising the encapsulation of an aqueous bitter melon extract by spray-drying. Foods. 4:400-419 (2015)
Tan S, Lan X, Chen S, Zhong X and Li W. Physical character, total polyphenols, anthocyanin profile and antioxidant activity of red cabbage as affected by five processing methods. Food Research International. 169:112929 (2023)
Tarone AG, Cazarin CBB and Maróstica MR. Anthocyanins: new techniques and challenges in microencapsulation. Food Research International. 133:109092 (2020)
Thakur P, Anika OC, Suhag R, Dhiman A and Kumar S. Insights into the current status of bioactive value, postharvest processing opportunities and value addition of black carrot. Food Science and Biotechnology. 33:721-747 (2023)
Todorović A, Šturm L, Salević A, Lević S, Črnivec IGO, Prislan I, Skrt M, Bjeković A, Ulrih NP and Nedović V. Encapsulation of bilberry extract with maltodextrin and gum arabic by freeze-drying: formulation, characterisation, and storage stability. Processes. 10:1991 (2022)
Vasile FE, Archaina D, Jiménez-Guzmán J, Gutiérrez‐López GF, Alamilla‐Beltrán L and Mazzobre MF. Prosopis alba exudate gum as new carrier agent for obtaining powdered Hibiscus sabdariffa aqueous extracts by spray drying. Powder Technology. 419:118316 (2023)
Vergara C, Pino MTP, Zamora O, Parada J, Pérez RO, Uribe M and Kalazich J. Microencapsulation of anthocyanin extracted from purple flesh cultivated potatoes by spray drying and its effects on in vitro gastrointestinal digestion. Molecules. 25:722 (2020)
Vergara LP, Hackbart HCDS, Alves CJ, Reissig GN, Wachholz BS, Borges CD, Chim JF and Zambiazi RC. Encapsulation of phenolic compounds through the complex coacervation technique for the enrichment of diet chewable candies. Food Bioscience. 51:102256 (2023)
Villacrez JL, Carriazo JG and Osorio C. Microencapsulation of Andes berry (Rubus glaucus Benth.) aqueous extract by spray drying. Food and Bioprocess Technology. 7:1445-1456 (2013)
Wang W, Rao L, Wu X, Wang Y, Zhao L and Liao X. Supercritical carbon dioxide applications in food processing. Food Engineering Reviews. 13:570-591 (2020)
Weber F, Boch K and Schieber A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT. 75:72-77 (2017)
Wu G, Hui X, Mu J, Brennan MA and Brennan CS. Functionalization of whey protein isolate fortified with blackcurrant concentrate by spray-drying and freeze-drying strategies. Food Research International. 141:110025 (2021)
Xue J, Fan S, Meng Y and Guo Y. Enhanced stability of red‐fleshed apple anthocyanins by copigmentation and encapsulation. Journal of the Science of Food and Agriculture. 99:3381-3390 (2019)
Yamashita C, Chung MMS, Santos CD, Malacrida CR, Moraes ICF and Branco IG. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT. 84:256-262 (2017)
Yekdane N and Goli SAH. Effect of pomegranate juice on characteristics and oxidative stability of microencapsulated pomegranate seed oil using spray drying. Food and Bioprocess Technology. 12:1614-1625 (2019)
Yingngam B, Tantiraksaroj K, Taweetao T, Rungseevijitprapa W, Supaka N and Brantner A. Modeling and stability study of the anthocyanin-rich maoberry fruit extract in the fast-dissolving spray-dried microparticles. Powder Technology. 325:261-270 (2018)
Yousefi S, Emam‐Djomeh Z and Mousavi M. Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica Granatum L.). Journal of Food Science and Technology. 48:677-684 (2010)
Zahed N, Kenari RE and Farahmandfar R. Effect of different extraction methods on antioxidant properties and encapsulation efficiency of anthocyanin of pomegranate peel. Food Science and Nutrition. 11:3780-3787 (2023)
Zhao L, Temelli F and Chen L. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. Journal of Functional Foods. 34:159-167 (2017)
Zotarelli MF, Da Silva VM, Durigon A, Hubinger MD and Laurindo JB. Production of mango powder by spray drying and cast-tape drying. Powder Technology. 305:447-454 (2017)
Acknowledgements
The authors would like to acknowledge the financial support received from Universiti Putra Malaysia under the grant with Project No. GP-IPM/2020/9691100.
Funding
Universiti Putra Malaysia, GP-IPM/2020/9691100, Ezzat Mohamad Azman.
Author information
Authors and Affiliations
Contributions
Giroon Ijod: Formal analysis (lead); writing – original draft preparation (lead); data curation (lead); visualization (lead). Nur Izzati Mohamed Nawawi: Formal analysis (equal) and data curation (equal). Farooq Anwar: Conceptualization (equal); methodology (equal); writing – review and editing (equal). Muhamad Hafiz Abd Rahim: Conceptualization (equal); methodology (equal); writing – review and editing (equal). Mohammad Rashedi Ismail- Fitry: Conceptualization (equal); methodology (equal); writing – review and editing (equal). Noranizan Mohd Adzahan: Conceptualization (equal); methodology (equal); writing – review and editing (equal); supervision (equal). Ezzat Mohamad Azman: Conceptualization (equal); formal analysis (equal); methodology (lead); validation (lead); project administration (lead); funding acquisition (lead); writing – review and editing (equal); supervision (lead). All authors reviewed the manuscript and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ijod, G., Nawawi, N.I.M., Anwar, F. et al. Recent microencapsulation trends for enhancing the stability and functionality of anthocyanins: a review. Food Sci Biotechnol 33, 2673–2698 (2024). https://doi.org/10.1007/s10068-024-01603-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-024-01603-2