Abstract
Elderberry (Sambucus nigra L.), rich in polyphenols, has recently attracted great interest in functional food, nutraceutical, and pharmaceutical industries, due to their potential health benefits to humans. However, polyphenols are very sensitive compounds and unstable. The utilisation of encapsulated polyphenols, instead of free compounds, can overcome some of their limitations. The extraction of the polyphenols from the elderberry flowers and stems was made, followed by the microencapsulation of the extract by a spray drying process. The microparticles were characterised by size, morphology, and release profile. The microencapsulated polyphenols were completely released, with total release times that range from 600 to 1140 s. The kinetic models that have a better adjustment to the practical results are the zero order, the Korsmeyer-Peppas, and the Weibull models, with correlation coefficients that range from 0.900 to 0.999. The encapsulation efficiency was similar for all the analysed particles, being the results located in a range from 92.3 to 99.8%. After 8 months of storage, the microparticles were revaluated, being possible to conclude that the elderberry microparticles present very similar release profiles comparing with the ones obtained with fresh microparticles, which proves the successful encapsulation of the elderberry extract and the stability of the microparticles over time. This experimental work leads to a very successful encapsulation of elderberry extract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elderberry (Sambucus nigra L.) is a deciduous shrub that grows on sunlight-exposed locations in most parts of Europe, Asia, North Africa, and the USA. It can reach up to 6 m in height and develop small and white hermaphrodite flowers, that bloom in early summer, and dark purple berries, that ripen in late summer (Veberic et al. 2009). Sambucus nigra L. is widely used as both food and medicinal plant in Europe. Elderflowers are consumed as herbal tea and its gargle has benefits in respiratory tract illnesses such as cough, influenza, and inflammation in throat (Ağalar et al. 2017). Based on a study made in 2009, it is possible to conclude that the elderberry fruits are composed mostly by sugars, organic acids, anthocyanins, and quercetins (Veberic et al. 2009). These last two groups of compounds (anthocyanins and quercetins) were also detected in the elderberry extract, during a research of Dawidowicz, Wianowska, and Baraniak (Dawidowicz et al. 2006). It revealed the presence of five types of anthocyanins and three types of quercetins, being cyanidin 3-glucoside and cyanidin 3-sambubioside the most representative anthocyanins and quercetin 3-rutinoside, also called rutin, the main quercetin in the elderberry cultivars. The anthocyanins and the quercetins make part of a group of ingredients called flavonoids. In the nature, they are essential for diverse biological activities of plants (pollination, signalisation, and defence strategies) and, when ingested by human beings, they can act as potential chemo-preventive agents, neutralising the activities of the free radicals that accumulate in the human body that, in turn, can cause many dangerous diseases like cardiovascular diseases, neurodegenerative diseases (Alzheimer and Parkinson), or diabetes (Aguiar et al. 2016; Costa et al. 2015; Dawidowicz et al. 2006). Among their antioxidant capacity, polyphenols comprise a wide spectrum of biological activities, such as anti-inflammatory, anticarcinogenic, immune-stimulating, antiallergic, antibacterial, and antiviral properties, which leads to their potential use as therapeutic agents (Aliakbarian et al. 2015). Furthermore, these compounds can also be used on the modification of organoleptic properties (e.g. colour and flavour), mainly on food applications (Aliakbarian et al. 2015; Veberic et al. 2009).
In the last years, due to all these beneficial properties, elderberry have gain consumer trust. However, its direct use on industry has some obstacles, due to the low bioavailability, low water solubility, rapid catabolism and excretion, and low stability in environmental, processing, and gastrointestinal conditions of the phenolic compounds (Aliakbarian et al. 2015). They can be degraded if exposed to oxygen, light, enzymatic activities, adverse temperature and pH conditions, metal ions, and water, which leads to an alteration of their therapeutic properties (Bakowska-Barczak and Kolodziejczyk 2011). On the other hand, these factors can limit the shelf life and bioavailability of elderberry products, once they can form degradation compounds (off-flavours, off-colours, carcinogenic) (Balanc and Trifkovic 2015). These limitations make necessary to create delivery systems that ensure an efficient utilisation of this natural resource, such as the use of microencapsulation techniques.
By microencapsulation, a physical barrier is created, protecting the active substance from the external medium (Carvalho et al. 2016) and increasing its stability. K. Mahdavee Khazaei, when studying the viability of saffron petal’s particles, rich in anthocyanins, produced by freeze-drying, concluded that microencapsulation could be recommended as a suitable method for stabilising this type of compounds (Mahdavee Khazaei et al. 2014). The same conclusion was drawn by Sarekha Woranuch, when producing chitosan particles, loaded with eugenol, via an emulsion–ionic gelation crosslinking method (Woranuch and Yoksan 2013).
In the development of new microparticles, it is necessary to choose an encapsulating technology and wall material compatible with the properties of the active agent that will be incorporated in the final particles (Davidov-pardo and Mcclements 2014).
There are various techniques available for microencapsulation that can be divided into three main categories: chemical processes, physicochemical processes, and physical-mechanical processes (Estevinho et al. 2016).
Spray drying technique was selected considering that is a simple, flexible, rapid, low operating cost, and easy scaling-up technique and allows a large-scale production in continuous mode, a high encapsulation efficiency, a good stability of the finished product and easy handling, an easy control of microparticles properties, and a good retention of volatiles (Balanc and Trifkovic 2015; Estevinho and Rocha 2017; Estevinho et al. 2013a, b; Gonçalves et al. 2016). The commercialisation of powdery food ingredients is substantially more convenient than handling liquid ingredients. Dried powders are easier to handle and to preserve from contamination during storage and, moreover, they occupy reduced storage volumes (Gómez-Mascaraque et al. 2017).
Another factor with high relevance in the stability of the microparticles is the encapsulating agent that can be natural, semi-synthetic, or synthetic (Gonçalves et al. 2016). In this work, three different natural agents were selected (sodium alginate, gum arabic, and chitosan), considering their applicability in food and pharmaceutical industry.
Gum arabic is one of the most ancient and popular natural gums, being formed by complex polysaccharides and small amount of protein (Farooq et al. 2017; Gashua et al. 2016). It has been used mainly in the food industry (confectionary products, beverages, fruits) due to its emulsifying and stabilising properties (Dong et al. 2017).
The alginates are natural, linear, and anionic polysaccharides that are commercially available in form of salt, e.g. sodium alginate (Dias et al. 2017; Estevinho et al. 2014a). Sodium alginate, like all the other alginates, has numerous advantages, like its biodegradability, low toxicity, and crosslinking capability, what makes this polymer very useful on pharmaceutical and food applications (Dias et al. 2017).
Chitosan is a polycationic polymer, widely used due to its interesting intrinsic properties, such as biocompatibility and biodegradability, and to its low cost and lack of toxicity (Estevinho et al. 2014a, b). However, despite all its attractiveness, chitosan is water-insoluble, being only soluble in acidic solutions. To solve this limitation, chitosan structure can be chemically modified, introducing hydrophilic functional groups or using a depolymerisation process. These procedures allow the formation of a soluble agent in neutral aqueous solutions, with a low molecular weight, called modified chitosan. This new compound can now be used on a wide range of applications in food, pharmaceutical, and cosmetic industries, including the preservation of foods from microbial deterioration and the protection of some bioactive compounds (Estevinho et al. 2014b; Estevinho et al. 2013a, b).
Nowadays, there are some studies about the encapsulation of polyphenols, using the spray drying as encapsulation technique (Mahdavi et al. 2016a, b; Mahdavi et al. 2014). However, for the best of our knowledge, there are only 6 studies about the encapsulation of extracts of elderberry (Bryła et al. 2015; Comunian et al. 2018; Murugesan and Orsat 2011; Stănciuc et al. 2018; Strugała et al. 2018; Tan et al. 2018), and only one uses a spray drying process (Murugesan and Orsat 2011). In this research, Ramesh Murugesan and Valérie Orsat studied the encapsulation of elderberry juice by spray drying, using different operation conditions (inlet temperature and feed flow rate) and encapsulating agents (gum acacia, maltodextrin, soya protein powder, soya milk powder, and isolated soya protein). In the end, viable elderberry microparticles were produced. These particles were subjected to a characterisation procedure that involved the total phenolic content retention, the colour, and the powder recovery determination. However, several new questions appeared with this research, emerging the need to formulate new studies about the elderberry encapsulation by spray drying process.
So, in the present work, it was studied the viability of the production of elderberry particles by a spray drying procedure, using sodium alginate, modified chitosan, and gum arabic as encapsulating agents, to be possible, in the future, their incorporation into some food and pharmaceutical products. The encapsulation procedure was also performed with the major polyphenol described to be in the elderberry extract (rutin) (Hohnová et al. 2017; Vrchotová et al. 2017) and without using any bioactive compound, for the study of the influence of the active agent on the properties of the final particles to be possible. After their formation, all the microparticles were characterised by size and morphology. The mechanisms of release were studied, as well as some mathematical models used to adjust the experimental results obtained in the release studies. The release profiles of the elderberry particles were revaluated after 8 months of storage.
Materials and Methods
Reagents and Preparation of the Solutions
Sambucus Nigra L. (elderberry) was directly collected from a plantation located on the north of Portugal. The extraction of their bioactive compounds was performed using ethanol (96%) from Valente e Ribeiro, LDA (described in the “Extraction Procedure” section).
Rutin, the main polyphenol presented in Sambucus Nigra L. (elderberry) as described in literature (Hohnová et al. 2017; Vrchotová et al. 2017), was used as blank/core control and was provided by Sigma–Aldrich. The microencapsulation was performed using three different encapsulating agents (modified chitosan, sodium alginate, gum arabic), each one of them with a concentration of 1% (w/v). Water soluble chitosan (pharmaceutical grade) was obtained from China Eastar Group (Dong Chen) Co., Ltd. (Batch no. SH20091010). Water soluble chitosan was produced by carboxylation and had a deacetylation degree of 96.5%. The solution of modified chitosan at 1% (w/v) has a viscosity of 5 mPa s (25 °C). Sodium alginate (alginic acid, sodium salt) (Cat. No.180947) was from Aldrich (USA). Arabic gum (ref. 51201-1315371-24606P04) was from Fluka (Germany). All the encapsulating agent solutions were prepared in deionised water.
In the characterisation step, more precisely in the determination of total phenolic content, it was necessary to make a solution of 15% (w/v) sodium carbonate (Prolabo), i.e. with 15% (w/v) of salt and 85% (v/v) of solvent. Furthermore, for this determination, the Folin-Ciocalteu reagent was also used from Sigma–Aldrich and gallic acid also from Sigma–Aldrich.
All the solutions mentioned before were prepared with deionised water and subjected to a constant agitation (magnetic agitator – MS-H-Pro, Scansi; SM20, Stuart Scientific), until complete homogenisation, at room temperature. To avoid their exposure to the environmental conditions, these solutions were used immediately after their preparation.
Extraction Procedure
In this experimental work, elderberry was subjected to a solvent extraction with ethanol, performed in an ultrasonic bath. The extraction was optimised in previous studies and according to other studies described in the literature (Bashash et al. 2014; Boonchu and Utama-ang 2015).
In the beginning, elderberry (flowers, stems, and leaves) was milled with a mortar and pestle and, posteriorly, 2 g of this extract was mixed with 10 mL of deionised water and 40 mL of ethanol 96%. The resulting solution was agitated (magnetic agitator – MS-H-Pro, Scansi) at 560 rpm for 1 h. The next step was to put the extraction solution in the ultrasonic bath (Ultrasound – Elma S30H, Elmasonic), for 30 min. After this period, the solution was centrifugated (Centromix S - 549) for 10 min and, posteriorly, the supernatant liquid was separated from the residues accumulated in the bottom of the container. In the end, the beaker with the extract was coated with aluminium paper, due to the light sensibility of the solution.
Spray Drying Technique
Spray drying was performed using a mini spray dryer BÜCHI B-290 (Flawil, Switzerland) with a standard 0.5-mm nozzle. All the solutions were fed into the spray dryer at a flow rate of 4 mL/min (15%) and at an inlet temperature of 115 °C. Air pressure and aspiration rate were set to 5–6 bar and 100% (36 m3/h), respectively, and the process occurs without using the nozzle cleaner. The outlet temperature, a consequence of the other experimental conditions and of the solution properties, was around 58 °C. The selection of the operating conditions was based on preliminary studies (B.N. Estevinho et al. 2012; Estevinho et al. 2013a, b). Approximately 15 min before each spray drying process, 10 mL of the solution with the elderberry extract was added and mixed with the biopolymer solution to be used. In the same way, 10 mL of a rutin solution (the core control) at 0.2% (w/v) was mixed with the encapsulating agent solution. The rutin microparticles had a final concentration of 2% (rutin/total mass of the microparticles). So, in the end, these prefaces the total of nine different solutions, fed to the spray dryer chamber: three with the extract, three with the rutin, and three with only the encapsulating agents.
The procedure and the experimental conditions used during the spray drying were the same for all the samples (with the elderberry extract, with rutin (core control), and with only the encapsulating agents).
At the end of the test, the dried powders, recovered from the equipment, were collected and stored in falcon tubes, sealed, and covered with aluminium foil, at 4 °C, before further analysis.
Determination of Total Phenolic Content
The total phenolic content was evaluated following the Folin-Ciocalteu method that has been used by several other authors (Aliakbarian et al. 2015; Bakowska-Barczak and Kolodziejczyk 2011; Boonchu and Utama-ang 2015; Consoli et al. 2016). It is based on the transfer of electrons from phenolic compounds to phosphomolybdic/phosphotungstic acid complexes, in alkaline medium, which leads to the formation of blue complexes (Costa et al. 2015). This procedure uses the gallic acid as a standard. Gallic acid spectrum has only one peak, located, approximately, at 273 nm. Several standards were prepared, with concentrations between 0.001 and 1 mg/mL that were then analysed by UV-Vis spectrometry at 273 nm. The calibration curve (A = 36.540C + 0.085; A absorbance, C concentration) was obtained and presented a detection limit of 0.03 mg/mL. The final curve has a correlation coefficient of 0.998.
In this method, 100 μL of the phenolic extract to be analysed are mixed with 2 mL of H2O and 1 mL of an aqueous solution 15% NA2CO3. In the end, 200 μL of Folin-Ciocalteu reagent are added to the previous solution and the absorbance is measured at 765 nm, after 2 h of incubation at room temperature (Gao et al. 2000). A calibration curve was also prepared, using gallic acid as standard. The calibration curve (A = 4.021C + 0.736; A absorbance, C concentration) was obtained between 0.2 and 0.8 g/L with a correlation coefficient of 0.974. This calibration curve was applied to the elderberry extract solution and all the microparticles obtained during the experimental work to quantify the total phenolic content.
All the results were obtained in triplicate. The final results should be expressed at mg of gallic acid equivalents (GAE)/L. In the case of the elderberry extract in its natural form, the TPC was also expressed at mg GAE/g of plant material. The total phenolic content was evaluated for the initial particles and for the particles after 8 months of storage.
Particle Size Distribution Analysis
Particle size distribution was measured by laser granulometry using a Coulter LS 230 Particle Size Analyser (Miami, FL, USA). The different samples were characterised by number and volume average. To avoid the agglomeration of the particles during the measurements, ethanol at 99% was used as a dispersant. The results (mean sizes of the particles and the size distributions (in volume and number)) were obtained as an average of three runs of 30 s.
All the samples were analysed, namely the particles with rutin, with the elderberry extract and those prepared with only the encapsulating agents.
Scanning Electron Microscopy Evaluation
Structural analysis of the surface of the microparticles was performed by scanning electron microscopy (Fei Quanta 400 FEG ESEM/EDAX Pegasus X4M). The powder samples were previously fixed on a brass stub using a double-sided adhesive tape and then they were coated in vacuum by a thin layer of gold (electrically conductive) in a Jeol JFC 100 apparatus at Centro de Materiais da Universidade do Porto (CEMUP).
Like for all the other steps of the experimental work, all the particles obtained by spray drying were morphologically evaluated, using a magnification of × 1 000, × 10,000, × 30,000, and of × 50,000.
Controlled Release Studies
The studies of elderberry extract and rutin release profiles from the microparticles were performed in deionised water, to simulate the most common vehicle for incorporation of particles for delivery systems in the cosmetic, pharmaceutic, and food industries. Rutin was used as a standard to evaluate the release from the microparticles.
The rutin calibration curve was prepared with 10 different standards, with the following concentrations: 0.001; 0.0025; 0.005; 0.0075; 0.01; 0.02; 0.04; 0.05; 0.06; 0.08, and 0.1 g/L. They were made from a rutin mother solution, with a concentration of 0.02 mg/mL. All the standards were evaluated by UV-Vis spectrometry. The rutin spectrum presents peaks at 3 different wavelengths on 240, 270, and 370 nm. The calibration curves were prepared at all wavelengths, presenting very acceptable correlation coefficients, with values that ranged from 0.9944 to 0.9971. The coefficient of variation was less than 10% for all the standards.
Analysing the rutin spectrum, it is possible to verify that the peak of 370 nm is the most individualised, with well-defined boundaries, less interferences with the wavelength of the encapsulating agents and, for all these reasons, its calibration curve was selected as the calibration curve to evaluate the release profiles. Furthermore, this curve allowed to evaluate the samples until a higher concentration: the calibrations curves were prepared between 0.001 and 0.06 mg/mL, 0.001 to 0.08 mg/mL, and of 0.001 to 0.1 mg/mL for the peaks of 240, 270, and 370 nm, respectively.
All the release profiles were analysed in triplicate for all the microparticles obtained from spray dryer by UV-Vis spectrometry and, in the end, the final results were expressed as an average of the three measured absorbances.
The microparticles (4 mg of microparticles) were placed in a cuvette (CV10Q3500F, Thorlabs), on top of 3 mL of deionised water, with stirring at room temperature. The release profiles were evaluated in a continuous mode (intervals of 30 s) and at 370 ± 10 nm. The release profiles were evaluated for particles immediately after spray dryer production and after 8 months of storage.
For the release profiles, different mathematical models were adjusted, which allows the evaluation of the mass transport mechanisms that are involved in the release, which, in turn, helps to design a system with specific characteristics and to simulate the effect of the design parameters (geometry and composition) on the resulting release kinetics.
Results and Discussion
The main goal of the current work was the production of elderberry microparticles that could be used, in the future, in the food, pharmaceutical, and cosmetic industry, being incorporated into products like yogurts, cereals, milk, and pastas. To accomplish this objective, using three different encapsulating agents (modified chitosan, gum arabic, and sodium alginate), nine different types of particles by spray drying were produced: three with the elderberry extract, three with the rutin (main polyphenol of elderberry), and three with only the encapsulating agents, which allowed to evaluate the effect of the active agent on the microparticles properties.
The microparticles prepared during this experimental work presented a product yield (quantity of powder recovered, considering the quantity of raw materials—encapsulating and active agent—used) that ranged from 25 to 41%, corresponding the lower values to the particles with elderberry extract. This suggests that some compounds of the extract increased the adhesion of the particles to the walls of spray dryer. Similar results were obtained by Gonçalves and co-authors when encapsulating vitamin A, dissolved in coconut oil, with gum arabic: the microparticles with the active agent showed a low product yield, while the particles with only gum arabic presented values around 70% (Gonçalves et al. 2017a). The product yield obtained for all the spray drying particles do not exceed the value of 41% which is, for the technique applied and the scale used, a common and satisfactory result. In the present study, due to the small quantities of raw materials that are employed when compared to the scale of the spray dryer, several losses will occur throughout the equipment. Moreover, being the particles produced by spray drying very small (between 5 and 19 μm), the aspiration of some of them by the vacuum system can occur, once the cyclone has a low efficiency to separate small particles (Casanova et al. 2016). Another factor that increases the probability of obtaining low product yields in this type of procedure is the inlet temperature. In this case, being this parameter of 115 °C, the deposition of particles in the cylinder and in the cyclone wall of spray dryer was observed, which leads to large losses (Estevinho et al. 2016).
After the preparation of the microparticles, they were characterised in terms of size, morphology, release profile, and total phenolic content, presented in the next sections.
Determination of Total Phenolic Content
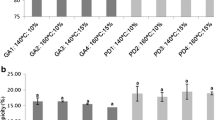
The Folin-Ciocalteu method was applied to all the microparticles recovered from the spray dryer and to the elderberry extract, before being submitted to the encapsulation procedure (Table 1).
It is possible to verify that the rutin microparticles are the samples with the highest phenolic content, with values that range from 744 to 784 mg GAE/L. The extract particles present lower values of this parameter because, unlike rutin, elderberry extract is a mixture of several compounds, having only some of them a phenolic character. The particles without an active agent are, as it was expected, the samples with the lower TPC, with values around 212–273 mg GAE/L. However, once the aim of this characterisation procedure is the determination of the total phenolic content of the active agent loaded inside the particles, to the results described before, which include the phenolic content of all the capsule compounds (active agent and encapsulating agent), the phenolic content of the empty particles was subtracted, to obtain the phenolic content of the elderberry extract and rutin, individually. So, the total phenolic content of the rutin microparticles continues to be the highest one, with values between 527 and 538 mg/L.
For the extract in its natural form, the value of this parameter is, approximately, 490 mg GAE/L, which is similar to the phenolic content of the encapsulated extract (465–498 mg GAE/L). However, subtracting to these last values, the phenolic content of the respective empty particles obtained a range from 225 to 254 mg GAE/L that is lower than the total phenolic content of the elderberry extract not encapsulated. This allows to conclude that the encapsulation procedure leads to a decrease of the phenolic content of the elderberry extract. On the other hand, it is possible to verify that the encapsulating agents increase the amount of phenolic compounds present on the microparticles. The pure extract contains 12 mg of phenolic compounds from each gram of elderberry plant, which allows concluding that only around 1.2% of the elderberry extract is composed by polyphenols.
The elderberry particles obtained by spray drying were stored for 8 months and, after this period, the total phenolic content was revaluated (Table 1). Analysing the results, it was possible to verify the existence of an increase of this parameter, being the values obtained for the particles with 8 months of storage higher than those obtained for the fresh particles (Table 1 – TPC*). This increase can be a result of some oxidation and degradation phenomena that happened to the encapsulating agents or to the extract during the storage, which may have led to the formation of sub-products. Although these compounds are degraded and, for that reason, may have lost their beneficial properties, they are also quantified as polyphenols in the FC method, which leads to false-positive values in the absorbance results, conducing to wrong total phenolic contents.
Particle Size Distribution Analysis
In food and pharmaceutical applications, there are many additional factors that need to be considered, namely the final particle shape and size of the system. The size and the surface of the microparticles depend on the encapsulating agent and the microencapsulation process used. The size and also the regular or irregular nature of the surface give a first idea about the stability of the microparticles formed. In the encapsulation by spray drying, the size is controlled by the experimental conditions used in the procedure. So, it is important to evaluate the particle size of the powders in order to adjust the conditions used during the spray drying that, in turn, influence the final purpose of the microparticles. All the microparticles prepared were analysed considering a differential volume distribution and a differential number distribution (Table 2). Analysing the particles by laser granulometry, it was possible to verify that, in the case of the differential volume distribution, the particles with the biggest mean diameter are the particles with the elderberry extract, with values of 19.3, 7.3, and 9.1 μm for the particles with modified chitosan, gum arabic, and sodium alginate, respectively. For the differential number distribution, the particles with higher dimensions vary depending on the encapsulating agent used: for the modified chitosan, the biggest particles are the ones with the extract (0.74 μm); for the gum arabic, the empty particles present the higher diameter (0.52 μm); and in the case of the sodium alginate, the particles with the biggest dimension are the rutin microparticles (1.13 μm).
Considering the differential volume distribution, the modified chitosan produced the particles with the biggest dimension, in a range from 6.1 to 19.3 μm, followed by the sodium alginate, with values around 4.7–9.1 μm, and, finally, by the gum arabic, with diameters between 4.1 and 7.3 μm. For the empty particles, the particles with the biggest diameter are the particles with gum arabic, followed by the particles with sodium alginate.
Estevinho et al. when encapsulating β-galactosidase with different biopolymers, such as modified chitosan, gum arabic, and sodium alginate, obtained very different results, once all the particles, i.e. the particles loaded with the enzyme and the particles with only the encapsulating agents, presented a constant average size, around 3 μm (Estevinho et al. 2014a).
In the differential number distribution, the particles with modified chitosan, gum arabic, and sodium alginate have an average size around 0.12–0.74, 0.11–0.52, and 0.45–1.13 μm, respectively.
The size distributions, by volume and number, of the sodium alginate microparticles, loaded with the elderberry extract, are represented in Fig. 1, as an example. For the case of the distribution by volume, the graph presents two peaks for the diameters of, approximately, 3.1 and 15.7 μm, which suggests the existence of some agglomeration effects. This aggregation phenomenon was also registered by other authors, when studied the encapsulation of different core compounds (Casanova et al. 2016; Gonçalves et al. 2017a, b).
From the analysis of the results of the particles size distribution, it was possible to verify that the particles with diameters smaller than 22.8 μm are responsible for 90% of the total volume of the sodium alginate microparticles, loaded with the elderberry extract. On the other hand, 90% of the total number of the sodium alginate microparticles, loaded with elderberry, is represented by the particles with diameters smaller than 1.4 μm.
Scanning Electron Microscopy Evaluation
For all the experiments with different encapsulating agents, spherical microparticles with a regular shape were produced (Fig. 2). In terms of size, it is possible to verify that all the samples have a heterogeneous size distribution, existing some small particles located on the middle of the particles with big dimensions.
SEM images of the microparticles with elderberry extract (1), rutin (2), and without the active agent (3), with different biopolymers: sodium alginate (A), modified chitosan (B), and gum arabic (C). Amplified 50,000 times, beam intensity (HV) 15.00 kV, distance between the sample and the lens (WD) less than 11 mm, size bars of 2 μm
In terms of the surface structure, the textural characteristics of the particles depend on the encapsulating agent used, being specific for each one of them. The particles formed with modified chitosan presented a very smooth surface and a regular shape, whatever the core compound encapsulated. The sodium alginate particles, although with also a smooth surface, have a characteristic structure with a concavity on their surface. In the case of the particles with gum arabic, they present a very rough surface, having some wrinkles and indentations in their structure. Similar results were obtained by Estevinho and co-authors, when encapsulated β-Galactosidase by spray drying, with the same biopolymers (Estevinho et al. 2014a), and vitamins B12 and C (Estevinho et al. 2016).
From the SEM images, it is possible to verify that the microparticles loaded with the elderberry extract are slightly larger than the remaining particles. On the other hand, the rutin and the empty microparticles appear to have, approximately, the same size. These results confirm the values obtained by laser granulometry.
Encapsulation Efficiency and Controlled Release Studies
The release profiles obtained for all the microparticles produced during this experimental work present two distinct zones (Fig. 3): the first one is the release zone, where a constant sustained release of the active agent occurs, which is translated by an increase of the absorbance values; the final zone corresponds to the stabilisation level, where almost all the active agent is already released. The associated deviations between the three measurements performed for each sample, represented on each release profile, are more visible in the release zone, being practically non-existent in the stabilisation level. Thus, in general, these deviations can be undervalued due to its low dimension, which proves the consistency of the measurements made during the experimental work. For all active agents, the release was total, varying the release times with the encapsulating agent used: in the case of the elderberry microparticles, the faster release occurs in the particles with modified chitosan (600 s), followed by the particles with gum arabic (1000 s); for the particles encapsulated with sodium alginate, the release time is of 1140 s.
Estevinho and co-authors, when encapsulating vitamin B12 and vitamin C with modified chitosan and sodium alginate, found similar results, concluding that modified chitosan promotes the faster release (around 10 min for the two vitamins) (Estevinho et al. 2016). So, depending on the final purpose of the microparticles, it must be chosen the most adequate encapsulating agent: if a slower release is necessary, sodium alginate should be selected as encapsulating agent; on the other hand, for a more immediate release, particles with modified chitosan should be produced.
For the release profiles represented in Fig. 3, different mathematical models were adjusted (Table 3).
The kinetic models that present a better adjustment to the practical results were the zero order, the Korsmeyer-Peppas, and the Weibull, with correlation coefficients that range from 0.900 to 0.998, from 0.987 to 0.998, and from 0.994 to 0.999, respectively (Table 4). However, from these three equations, the one that adjusts to a greater number of points (release zone and final stabilisation zone), and at the same time had the bigger correlation coefficients, is the Weibull model. This model is the most adequate to evaluate the release profiles of matrix-type microparticles, which is the case of this experimental work: the microparticles produced by spray drying are normally matrix type (the encapsulated substance distributed in the encapsulating agent) and the mechanisms involved on the release are controlled by solvent action and by diffusion (Estevinho and Rocha 2017; Gonçalves et al. 2017a). The first order and the Higuchi equations do not adjust to the experimental results. These conclusions are the same for all the analysed samples, with an exception for the gum arabic particles loaded with rutin: in this case, the Korsmeyer-Peppas and the Weibull continue being the models that adjust better to the results, presenting the last one the better fitting; however, the zero order equation was replaced by the Higuchi model that, in these samples, presents a better correlation coefficient between the adjusted equation and the experimental results.
Estevinho and Rocha, when studying the release of vitamin B12 and vitamin C from microparticles produced by spray drying, found similar results, which are related with the method used to obtain the microparticles: once the particles were of matrix type, the model that best adjusted to the experimental results was the Weibull model.
When the Korsmeyer-Peppas model is adjusted to the experimental results, the major mechanism responsible for the controlled release can be identify, based on the “n” parameter of the equation: if n < 0.43, occurs a Fickian Diffusion; if 0.43 < n < 0.85, the anomalous transport is the main transport mechanism, and results from the combination of diffusion mechanisms and swelling releases; if n ≥ 0.85, the transport is a consequence of the matrix swelling (n = 0.85 – Case II Transport; n > 0.85 – Super Case II Transport).
Analysing the values obtained for the current work (Table 4), it is possible to verify a swelling behaviour for the particles with modified chitosan and sodium alginate, and a Fickian diffusion or an anomalous transport for the gum arabic microparticles, depending on the active ingredient used.
The encapsulation efficiency (difference between the total released in the end of the release experiment and the percentage of compound released in the time zero) was similar for all the analysed microparticles, being the results located in a range from 92.3 to 99.8%. These high values are justified by the small amount of extract used during the encapsulation procedure, comparing to the amount of encapsulating agent. This leads to an almost complete encapsulation of the active agent.
Stability and Controlled Release Studies After 8-Month Storage
The microparticles stability is intimately related with the choice of the encapsulating agent and the encapsulation technique (Estevinho et al. 2015). In the spray dryer, the drying process of dispersed liquid drops is performed using hot air. This process step lasts only a few milliseconds to a few seconds; therefore, even heat-sensitive compounds can be spray dried without a significant loss (Đorđević et al. 2014). This information was confirmed by mass balance comparing the amount of active compound in the beginning of the process and obtained after spray drying and evaluated in the release studies of the fresh samples. No significant losses were obtained.
The authors used the same encapsulating agents and the same microencapsulation method to microencapsulate different compounds (vitamins and enzymes) also without significant losses. In these previous works, the stability was evaluated after 3 and 6 months and good results were obtained with core compounds losses less than 15% (Carlan et al. 2017, 2018; Estevinho et al. 2014a; Gonçalves et al. 2017a).
So, once the encapsulating agent and encapsulating technique used in this experimental work are the same of the previous works, the authors believe that a good stability of the elderberry microparticles, produced during this experimental work, can be expected. After eight months, the elderberry microparticles were revaluated and subjected to new controlled release tests. In Fig. 4, the release profiles of the initial microparticles and of the particles after 8 months of storage are represented, for the comparison of the release mechanism between the two sample types to be possible.
Analysing Fig. 4, it is possible to verify that the release profiles of the fresh microparticles and the particles with 8 months of storage, for each encapsulating and active agent, are very similar, existing only some little deviations in the release zone. The different kinetic models were adjusted to the new release profiles, and the kinetic parameters obtained are very similar to the ones obtained for the initial elderberry microparticles. The models that present a better adjustment to the experimental results are, again, the zero order, the Korsmeyer-Peppas, and the Weibull equations, with correlation coefficients that range from 0.939 to 0.994, from 0.945 to 0.997, and from 0.991 to 0.999, respectively, being the Weibull model the one that adjusted to a greater number of points. The similarity between the release profiles obtained for the elderberry microparticles with two different times of storage allowed to conclude that the release times of the different samples are practically the same in terms of percentage of release. However, the concentration of total phenolic compounds increased with the time, as already was analysed. The released values obtained for the particles with 8 months of storage were higher than those obtained for the fresh particles. This fact can be a consequence of some oxidation and degradation phenomena that happened during the storage, which lead to the formation of false-positive values in the release profiles. This phenomenon was already observed during the evaluation of the total phenolic content, whose results were also higher for the samples with 8 months of storage, relatively to the initial microparticles. In both procedures, the degradation reactions led to the formation of sub-products that were also quantified during the analyses. This happens because the evaluation of the total phenolic content by the FC method and the analysis of the release profiles by the UV/Vis kinetic tests are quantitative but not specific, counting the polyphenols and other compounds that remain in the sample, and not taking into consideration the type of polyphenol or if the antioxidant compounds continues on the sample composition or if, on the other hand, they were degraded. However, analysing the results of the TPC, it was possible to observe that these oxidation and degradation phenomena were more significant for the encapsulating agents than for the elderberry extract and rutin, allowing to suggest that these encapsulating agents lead to the increase of the “phenolic content”, as a false positive of the microparticles.
Conclusion
The main objective of the current work was to produce elderberry microparticles to be, in the future, incorporated into food, pharmaceutical, and cosmetic products. Spray drying was selected as the encapsulation technique and was performed with three different wall materials (modified chitosan, sodium alginate, and gum arabic).
The product yield values obtained after the encapsulation procedure ranged, approximately, from 25 to 41%, which is, for the technique applied and the scale that are used, a common and satisfactory result.
For the extract in its natural form, the value of the phenolic content is very higher than the results obtained for the elderberry microparticles prepared with the extract, which allows concluding that the encapsulation procedure leads to a decrease of the phenolic content of the elderberry extract.
In the case of the differential volume distribution, the particles with the biggest mean diameter are the microparticles with the elderberry extract and, for the differential number distribution, the particles of higher dimensions vary depending on the encapsulating agent used.
Spherical microparticles with a regular shape were produced, for all the analysed samples. In terms of the surface structure, the textural characteristics of the particles depends on the encapsulating agent used, being specific for each one of them.
The release studies reveal that the release was total, varying the release times with the encapsulating agent used: in the case of the elderberry particles, the faster release occurs in the particles with modified chitosan, followed by the particles with gum arabic. So, depending on the final purpose of the microparticles, it must be chosen the most adequate encapsulating agent: if a slower release is necessary, sodium alginate should be selected as encapsulating agent; on the other hand, for a more immediate release, particles with modified chitosan should be produced.
It can be concluded that the kinetic models that present a better fitting to the experimental results are the zero order, the Korsmeyer-Peppas, and the Weibull equations. However, from these three equations, the one that adjusts all the release profile (release and stabilisation zone) and, at the same time, has the bigger correlation coefficients, is the Weibull model.
The encapsulation efficiency is similar for all the analysed microparticles, the results being located in the range from 92.3 to 99.8%.
After 8-month storage, the elderberry microparticles present very similar release profiles comparing with the ones obtained with fresh microparticles. However, the total phenolic content and concentration of active agent released increased, probably due to the oxidation and degradation of the encapsulating agents during the storage.
So, to conclude, the procedure followed leads to a very successful elderberry extract encapsulation methodology, allowing a complete entrapment of the extract inside the microparticles of modified chitosan, sodium alginate, and gum arabic. However, to ensure an efficient incorporation of the microparticles into food, pharmaceutical, or cosmetic products, further tests can be required.
References
Ağalar, H. G., Demirci, B., Demirci, F., & Kırımer, N. (2017). The volatile compounds of the elderflowers extract and the essential oil. Records of Natural Products, 11(5), 491–496. https://doi.org/10.25135/rnp.63.16.08.058.
Aguiar, J., Estevinho, B. N., & Santos, L. (2016). Microencapsulation of natural antioxidants for food application - the specific case of coffee antioxidants - a review. Trends in Food Science and Technology, 58, 21–39. https://doi.org/10.1016/j.tifs.2016.10.012.
Aliakbarian, B., Paini, M., & Alberto, A. (2015). Effect of encapsulating agent on physical-chemical characteristics of olive pomace polyphenols-rich extracts. 43, 97–102. https://doi.org/10.3303/CET1543017.
Bakowska-Barczak, A. M., & Kolodziejczyk, P. P. (2011). Black currant polyphenols: their storage stability and microencapsulation. Industrial Crops and Products, 34(2), 1301–1309. https://doi.org/10.1016/j.indcrop.2010.10.002.
Balanc, B., & Trifkovic, K. (2015). Trends in encapsulation technologies for delivery of food bioactive compounds. https://doi.org/10.1007/s12393-014-9106-7.
Bashash, M., Zamindar, N., & Bolandi, M. (2014). Evaluation of antioxidant activities of Iranian sumac (R. coriaria L.) fruit and spice extracts with different solvents. Journal of Food Measurement and Characterization, 8(3), 213–217. https://doi.org/10.1007/s11694-014-9182-7.
Boonchu, T., & Utama-ang, N. (2015). Optimization of extraction and microencapsulation of bioactive compounds from red grape (Vitis vinifera L.) pomace. 52(February), 783–792. https://doi.org/10.1007/s13197-013-1079-7.
Bryła, A., Lewandowicz, G., & Juzwa, W. (2015). Encapsulation of elderberry extract into phospholipid nanoparticles. Journal of Food Engineering, 167, 189–195.
Carlan, I. C., Estevinho, B. N., & Rocha, F. (2017). Study of microencapsulation and controlled release of modified chitosan microparticles containing vitamin B12. Powder Technology, 318, 162–169. https://doi.org/10.1016/j.powtec.2017.05.041.
Carlan, I. C., Estevinho, B. N., & Rocha, F. (2018). Study of different encapsulating agents for the microencapsulation of Vitamin B12. 17(4), 855–864.
Carvalho, I. T., Estevinho, B. N., & Santos, L. (2016). Application of microencapsulated essential oils in cosmetic and personal healthcare products – a review. International Journal of Cosmetic Industry, 38(2), 109–119.
Casanova, F., Estevinho, B. N., & Santos, L. (2016). Preliminary studies of rosmarinic acid microencapsulation with chitosan and modified chitosan for topical delivery. Powder Technology, 297, 44–49. https://doi.org/10.1016/j.powtec.2016.04.014.
Comunian, T. A., Ravanfar, R., Alcaine, S. D., & Abbaspourrad, A. (2018). Water-in-oil-in-water emulsion obtained by glass microfluidic device for protection and heat-triggered release of natural pigments. Food Research International, 106(September 2017), 945–951. https://doi.org/10.1016/j.foodres.2018.02.008.
Consoli, L., Grimaldi, R., Sartori, T., & Menegalli, F. C. (2016). Gallic acid microparticles produced by spray chilling technique : production and characterization. LWT - Food Science and Technology, 65, 79–87.
Costa, D. C., Costa, H. S., Albuquerque, T. G., Ramos, F., Castilho, M. C., & Sanches-silva, A. (2015). Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends in Food Science & Technology, 45(2), 336–354.
Davidov-pardo, G., & Mcclements, D. J. (2014). Resveratrol encapsulation: designing delivery systems to overcome solubility , stability and bioavailability issues. Trends in Food Science & Technology, 38(2), 88–103.
Dawidowicz, A. L., Wianowska, D., & Baraniak, B. (2006). The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). 39, 308–315. https://doi.org/10.1016/j.lwt.2005.01.005.
Dias, M. L., Agüero, L., Zaldivar-silva, D., & Pe, L. (2017). Alginate microparticles as oral colon drug delivery device : a review, 168, 32–43. https://doi.org/10.1016/j.carbpol.2017.03.033
Dong, Y., Martin, K., He, S., & Balling, S. (2017). Gum arabic authentication and mixture quantification by near infrared spectroscopy. Food Control, 78, 144–149.
Đorđević, V., Balanč, B., Belščak-Cvitanović, A., Lević, S., Trifković, K., Kalušević, A., Kostić, I., Komes, D., Bugarski, B., & Nedović, V. (2014). Trends in encapsulation technologies for delivery of food bioactive compounds. Food Engineering Reviews, 7(4), 452–490. https://doi.org/10.1007/s12393-014-9106-7.
Estevinho, B. N., & Rocha, F. (2017). A Key for the Future of the Flavors in Food Industry: Nanoencapsulation and Microencapsulation. In A. E. Oprea & A. M. Grumezescu (Eds.), Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety (pp. 1–16). Oxford: Elsevier Inc.. https://doi.org/10.1016/B978-0-12-811942-6.00001-7.
Estevinho, B. N., Damas, A. M., Martins, P., & Rocha, F. (2012). Study of the inhibition effect on the microencapsulated enzyme ttps://doi.org/. Environmental Engineering and Management Journal, 11(11), 1923–1930.
Estevinho, B. N., Rocha, F., Santos, L., & Alves, A. (2013a). Microencapsulation with chitosan by spray drying for industry applications – a review. Trends in Food Science & Technology, 31(2), 138–155. https://doi.org/10.1016/j.tifs.2013.04.001.
Estevinho, B. N., Rocha, F., Santos, L., & Alves, A. (2013b). Using water soluble chitosan for flavour microencapsulation in food industry. Journal of Microencapsulation, 30(6), 571–579. https://doi.org/10.3109/02652048.2013.764939.
Estevinho, B. N., Damas, A. M., Martins, P., & Rocha, F. (2014a). Microencapsulation of 764939nfor flavo with different biopolymers by a spray-drying process. FRIN, 64, 134–140. https://doi.org/10.1016/j.foodres.2014.05.057.
Estevinho, B. N., Damas, A. M., Martins, P., & Rocha, F. (2014b). The influence of microencapsulation with a modified chitosan (water soluble) on b -galactosidase activity. 1575–1586. https://doi.org/10.1080/07373937.2014.909843.
Estevinho, B. N., Ramos, I., & Rocha, F. (2015). Effect of the pH in the formation of galactosidase microparticles produced by a spray-drying process. International Journal of Biological Macromolecules, 78, 238–242. https://doi.org/10.1016/j.ijbiomac.2015.03.049.
Estevinho, B. N., Carlan, I., Blaga, A., & Rocha, F. (2016). Soluble vitamins (vitamin B12 and vitamin C) microencapsulated with different biopolymers by a spray drying process. Powder Technology, 289, 71–78. https://doi.org/10.1016/j.powtec.2015.11.019.
Farooq, M., Sagbas, S., Sahiner, M., Siddiq, M., Turk, M., Aktas, N., & Sahiner, N. (2017). Synthesis , characterization and modification of gum arabic microgels for hemocompatibility and antimicrobial studies. Carbohydrate Polymers, 156, 380–389. https://doi.org/10.1016/j.carbpol.2016.09.052.
Gao, X., Ohlander, M., Jeppsson, N., Bjo, L., & Trajkovski, V. (2000). Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. 1485–1490.
Gashua, I. B., Williams, P. A., & Baldwin, T. C. (2016). Food hydrocolloids molecular characteristics , association and interfacial properties of gum arabic harvested from both Acacia senegal and Acacia seyal. Food hydrocolloids, 61, 514–522. https://doi.org/10.1016/j.foodhyd.2016.06.005.
Gómez-Mascaraque, L. G., Casagrande Sipoli, C., de La Torre, L. G., & López-Rubio, A. (2017). Microencapsulation structures based on protein-coated liposomes obtained through electrospraying for the stabilization and improved bioaccessibility of curcumin. Food Chemistry, 233, 343–350. https://doi.org/10.1016/j.foodchem.2017.04.133.
Gonçalves, A., Estevinho, B. N., & Rocha, F. (2016). Microencapsulation of vitamin A : a review. Trends in Food Science & Technology, 51, 76–87. https://doi.org/10.1016/j.tifs.2016.03.001.
Gonçalves, A., Estevinho, B. N., & Rocha, F. (2017a). Design and characterization of controlled-release vitamin A microparticles prepared by a spray-drying process. Powder Technology, 305, 411–417. https://doi.org/10.1016/j.powtec.2016.10.010.
Gonçalves, B., Moeenfard, M., Rocha, F., Alves, A., Estevinho, B. N., Santos, L., et al. (2017b). Microencapsulation of a natural antioxidant from coffee - chlorogenic acid (3-caffeoylquinic acid). Food and Bioprocess Technology, 10(8), 1521–1530. https://doi.org/10.1007/s11947-017-1919-y.
Hohnová, B., Šalplachta, J., & Karásek, P. (2017). Pressurized hot water extraction followed by high-performance liquid chromatography for determination of polyphenols in Sambucus nigra L. branches in dependence on vegetative period of the plant. Journal of Food and Nutrition Research, 56(3), 299–303.
Mahdavee Khazaei, K., Jafari, S. M., Ghorbani, M., & Kakhki, H. (2014). Application of maltodextrin and gum arabic in microencapsulation of saffron petalhy for determination of polyphenols in Sambucus nigra L. branche. Carbohydrate Polymers, 105(1), 57–62. https://doi.org/10.1016/j.carbpol.2014.01.042.
Mahdavi, S. A., Jafari, S. M., Ghorbani, M., & Assadpoor, E. (2014). Spray-drying microencapsulation of anthocyanins by natural biopolymers: a review. Drying Technology, 32(5), 509–518. https://doi.org/10.1080/07373937.2013.839562.
Mahdavi, S., Jafari, S., Assadpoor, E., & Dehnad, D. (2016a). Microencapsulation optimization of natural anthocyanins with maltodextrin, gum arabic and gelatin. International Journal of Biological Macromolecules, 85, 379–385. https://doi.org/10.1016/j.ijbiomac.2016.01.011.
Mahdavi, S., Jafari, S., Assadpour, E., & Ghorbani, M. (2016b). Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. Journal of Food Engineering, 181, 59–66. https://doi.org/10.1016/j.jfoodeng.2016.03.003.
Murugesan, R., & Orsat, V. (2011). Spray drying of elderberry (Sambucus nigra L.) juice to maintain its phenolic content. Drying Technology, 29(14), 1729–1740. https://doi.org/10.1080/07373937.2011.602485.
Stănciuc, N., Oancea, A. M., Aprodu, I., Turturică, M., Barbu, V., Ionita, E., et al. (2018). Investigations on binding mechanism of bioactives from elderberry (Sambucus nigra L.) by whey proteins for efficient microencapsulation. Journal of Food Engineering, 223, 197–207. https://doi.org/10.1016/j.jfoodeng.2017.10.019.
Strugała, P., Loi, S., Ba˙zanów, B., Kuropka, P., Kucharska, A. Z., Włoch, A., & Gabrielska, J. (2018). A comprehensive study on the biological activity of elderberry extract and cyanidin 3-O-glucoside and their interactions with membranes and human serum albumin. molecules Article, 23(2566), 1–22. https://doi.org/10.3390/molecules23102566.
Tan, C., Selig, M. J., & Abbaspourrad, A. (2018). Anthocyanin stabilization by chitosan-chondroitin sulfate polyelectrolyte complexation integrating catechin co-pigmentation. Carbohydrate Polymers, 181(August 2017), 124–131. https://doi.org/10.1016/j.carbpol.2017.10.034.
Veberic, R., Jakopic, J., Stampar, F., & Schmitzer, V. (2009). European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chemistry, 114(2), 511–515. https://doi.org/10.1016/j.foodchem.2008.09.080.
Vrchotová, N., Dadáková, E., Matějíček, A., Tříska, J., & Kaplan, J. (2017). Effect of variety on content of bioactive phenolic compounds in common elder (Sambucus nigra L.). Natural Product Research, 31(6), 700–703. https://doi.org/10.1080/14786419.2016.1214826.
Woranuch, S., & Yoksan, R. (2013). Eugenol-loaded chitosan nanoparticles : I . Thermal stability improvement of eugenol through encapsulation. Carbohydrate Polymers, 96(2), 578–585. https://doi.org/10.1016/j.carbpol.2012.08.117.
Funding
This work was financially supported by project UID/EQU/00511/2019 - Laboratory for Process Engineering, Environment, Biotechnology and Energy – LEPABE funded by national funds through FCT/MCTES (PIDDAC); Project POCI-01-0145-FEDER-028715 (MicroDelivery - Development of controlled delivery functional systems by microencapsulation of natural and active compounds with therapeutic, nutritional and technological interest), funded by FEDER funds through COMPETE2020 – Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES; Project “LEPABE-2-ECO-INNOVATION” – NORTE-01-0145-FEDER-000005, funded by Norte Portugal Regional Operational Programme (NORTE 2020), under PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). Berta Estevinho acknowledges FCT for the contract based on the “Lei do Emprego Científico” (DL 57/2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ribeiro, A.M., Estevinho, B.N. & Rocha, F. Spray Drying Encapsulation of Elderberry Extract and Evaluating the Release and Stability of Phenolic Compounds in Encapsulated Powders. Food Bioprocess Technol 12, 1381–1394 (2019). https://doi.org/10.1007/s11947-019-02304-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02304-z