Abstract
The present study scrutinizes lipases of different origins, immobilization methods, carriers, and reaction solvents to accelerate the octyl octanoate synthesis. The acylation reaction parameters including temperature, moisture level, shaking speed and enzyme dose were subsequently investigated and optimized following fully rotatable central composite design. The initial screening revealed that lipases of Rhizopus arrhizus, when applied as a biocatalyst (lipase bearing dead mycelia) furnished the highest acylation activity (147 μM L−1 min−1). Validation of reaction conditions disclosed that 250 I.U. of lipase based biocatalyst when incubated with 850 mM of acylating agent and 750 mM of the substrate at 35 °C, 3% moisture level and 150 RPM shaking speed produced 70% acylation yield with an acylation activity higher than 147 μM L−1 min−1. The observed results certify that lipase bearing dead mycelia of R. arrhizus might be an intelligent biocatalyst to manipulate the yield of acylation reactions encountered in the food industry.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Octanoic ester (n-Octyl octanoate); CAS 2306-88-9; a recruitment pheromone, is basically a fatty alcohol ester often used in food and pharmaceutical industries for its pleasant order of green tea and mildly sweet fruit taste (Burdock, 2016). Naturally, octyl octanoate occurs in apple, banana, grapes, strawberry, cranberry, ginger, goat-milk, butterfat, spearmint oil and salivary/labial gland extracts of certain social and forager insects (Schorkopf et al., 2009; Shigley et al., 1955). In order to meet its ever-increasing demands in food and pharmaceutical industries; octyl octanoate ester has been frequently synthesized by the esterification of octanoic acid with octyl alcohol in the presence of HCl or copper-chromium oxide catalyst at or above 320 °C (Yan et al., 2014). The above-mentioned reaction conditions not only economically cut the reaction down but also jeopardize product safety (Flashpoint 113 °C), stability (autoxidation) and selectivity (formation of di, and tri-esters due to acyl migration). These extreme reaction conditions can be avoided by applying enzyme-based biocatalysts. In this context, proteases (3.4.21) and Esterases (3.1.1) especially lipase (3.1.1.3) is an intelligent choice and has already been applied for the synthesis of long and short-chain fatty acid and amine esters. Lipase being very specific and regio, as well as enantioselective, catalyze the reactions in a particular direction (reduced or zero side products) (Buchholz et al., 2012; Gotor-Fernández et al., 2006; Mahmod et al., 2015). However, no single effort has been undertaken to synthesize octyl octanoate applying lipases as a practical biocatalyst.
Recently, enzyme immobilization has opened new cycles in biotechnological products, diagnostic applications, bio-affinity chromatography, biosensors, process development and automation while making the procedures exclusively achievable (Garcia-Galan et al., 2011; Mushtaq et al., 2017; Tran and Balkus, 2011). However, the immobilization methods and approaches unusually influence the reaction rates, biocatalyst life, and nature of side products depending upon the immobilization support, interaction involved, reaction solvents and conditions. The polarity of reaction media has been a bottleneck problem while applying lipases as biocatalysts for acylation reaction i.e. hydrophobic conditions favor acylation but the hydrophilic environment is crucial to keep the enzyme in its active configuration (Adlercreutz, 2013; Jesionowski et al., 2014). Similarly, unsaturated and saturated fats, alcohols and other antagonistic chemicals can denature the enzymes or may lead to the formation of various non-selective side-products (Barbosa et al., 2015). These problems can be avoided by making the reaction to take place at the interface of two phases (aqueous and non-aqueous) through enzyme immobilization (Cao et al., 2003). In this perspective, a wide range of immobilization approaches based on adsorption/deposition, embodiment/crosslinking, or covalent binding has been used to embrace the enzyme structure and activity (Adlercreutz, 2013). Similarly, the nature of supports, its chemistry and withholding time also affect the reaction rate and reproducibility. Finally, a thorough understanding of enzyme cocktail, immobilization approach, and behavior of biocatalyst towards various reaction conditions help us to figure out the reaction towards the selective and concurrent formation of the desired products. A suspicious survey of previously published literature accomplished that experimenters thus far have not addressed the bio-catalyzed esterification of octanoic acid into n-octyl octanoate. The key objectives of the present research were to provide scientific data regarding the selection of proteinaceous catalysts, immobilization support, and reaction solvent and optimize the reaction conditions to render the process monetarily achievable.
Materials and methods
The research work presented in this monograph was carried out in the Laboratories of Government College University, Lahore-Pakistan and Food and Biotechnology Research Centre PCSIR, Laboratories Complex Lahore Pakistan.
Enzymes, materials, and chemicals
Lipases of four different origins i.e. (i) Lipases isolated from Aspergillus niger, (ii) lipase bearing mycelia of Rhizopus arrhizus, (iii) Lipase of Candida rugosa type VII and (iv) Novozyme-435 were screened for their catalytic activity. The lipases of origin (i) and (ii) were indigenously produced in PD laboratory while lipases of origin (iii) and (iv) were purchased from Novozymes (Karachi, Pakistan). Standard n-octyl octanoate (CAS Number 2306-88-9) of purity ≥ 98% was supplied by Sigma-Aldrich (St. Louis, USA). All the solvents applied including n-hexane, n-octane, petroleum ether, diethyl ether, benzene, toluene, carbon tetrachloride, chloroform, acetone, tetrahydrofuran, and dimethylformamide were of HPLC grade (Merck Chemicals, GmbH Germany). Pure octanol and octanoic acid (purity ≥ 99) were provided by AppliChem (GmbH, Darmstadt Germany). All other chemicals used during the present research were of analytical grade (Merck Chemicals, GmbH Germany).
Experimental part A: initial screening
During the initial screening, lipases of four different origins (i–iv), immobilization techniques, nature of the carrier material, and reaction media were classically screened out for the optimum activity/immobilization efficiency.
Evaluation of lipase based biocatalysts
In order to evaluate immobilization efficiency, biocatalyst activity, and reusability, lipases from different sources (i–iv) immobilized on selected supports were investigated following the methods documented by Wohlgemuth (2010) and Bradford (1976). For immobilization efficiency, the crude protein (CP) and Immobilized protein (IP) were determined using bovine serum albumin as reference standard. The amount of protein in the enzyme preparation or those disappeared from the supernatant (mg/g of support) was followed to assess the immobilization efficiency. To assess the hydrolytic activities of soluble and immobilized lipases, hydrolysis was performed with 10% olive oil emulsion in gum acacia. The reaction mixture comprising 1.0 mL olive oil emulsion, 1.0 ml 100 mM phosphate buffer (pH 8.0) and 50 mg of immobilized/soluble lipases was incubated at 37 °C for 5 min. The enzyme cocktail was deactivated by adding 10 mL of commercial ethanol and the solution was subsequently titrated with 100 mM NaOH using Thymolphthalein as an indicator. One International Unit (I.U.) of lipase activity was defined as the amount of enzyme that releases unit μmol fatty acid emulsified fat per minute under the above conditions (Nisha et al., 2012).
Acylation activity
The acylation activities of soluble and immobilized enzymes were evaluated in terms of μmol of octyl octanoate produced per minutes using n-hexane as the acylation medium while using octanoic acid as acyl donor. Briefly, 1.0 g biocatalyst (free or immobilized lipase) was added to 60 mL of reaction mixture containing 1:1 (v/v) octanoic acid and octanol in Teflon stoppered 100 mL conical flask. The flasks were incubated at 30 °C with shaking speed of 120 rpm. The samples were drawn at regular intervals the product concentration was analyzed by HPLC (Agilent Technologies, 11,000 series) equipped with C-18 column (150 mm × 4.6 mm) an autosampler and Diode Array detector (DAD). The mobile phase consisting of 60:40 mixture of methanol: acetonitrile was passed at a flow rate of 1.0 mL min−1. The octyl octanoate eluted was quantified at λmax 210 nm following calibration curve of standard solution (2–200 ppm; R2 0.9967). One acylation unit of the enzyme was defined as the amount of enzyme that catalyzed the synthesis of one micromole of octyl octanoate per minute (μmol L−1 min−1) under given reaction conditions (Boyall et al., 2002).
Evaluation of immobilization supports/techniques
For the lipase immobilization, a variety of carriers and techniques listed in Table 1 were prepared and adopted following the methods described in our previous study (Rashid Choudhry et al., 2017).
Physical adsorption
Physical adsorption involves the binding of biological molecules (enzymes) to solid supports via van der Waals forces, short-range dispersion forces and sometimes hydrogen bonding. These interactions are rather weak and keep the integrative structure or conformation of the bound protein (Jesionowski et al., 2014). During physical adsorption, the lipases solution (10 mL of 2% lipase) in 0.1 m McIlvaine buffer was stirred with 2 g of an immobilization support for 120 min. All the immobilization supports except polyethylene were washed with 0.1 mol/L Mcilvaine buffer whereas the polyethylene based supports were first crushed and then washed with ethanol before activation.
Adsorption and cross-linking
To crosslink the immobilized lipases, accurately weighed 2 g of activated supports were mixed with 100 mL (1%) lipases in 0.1 M Mcilvaine buffer under moderate stirring for 60 min. The resultant mixture was passed through a sintered glass filter and the residues were further stirred with 30 mL of 2.5% solution glutaraldehyde in 20 mM phosphate buffer (pH 8.0) at 25 °C for 90 min.
Immobilization by precipitation and adsorption
Immobilization via precipitation provides a simpler strategy with enhanced protein loading capacities (Rashid Choudhry et al., 2017). In this strategy, 100 mL (1%) lipases in 0.1 M Mcilvaine buffer was stirred with 2 g zeolite, alumina or silica gel at 250 rpm for 5 min. The resultant mixture was cooled to 4 °C and the enzyme was allowed to precipitate at the solid support while adding 5 mL of chilled acetone.
Covalent attachment
In covalent attachment of lipases, glyoxal based supports i.e. Glyoxyl-agarose and Monoaminoethyl-N-ethyl-agarose (MANAE-agarose) were prepared while observing the conditions documented Fernandez-Lorente et al. (2008). In short, 200 mL of agarose in 1.7 N NaOH containing 2.85 g NaBH4 were mixed with 100 mL of glycidol under constant stirring; the resultant porous support was washed thoroughly with distilled water, filtered through a sintered glass filter, dried and finally soaked in 98% ethanol for 30 min. Likewise, MANAE-agarose support was prepared by mixing 60 g of Glyoxyl supports with 200 ml of 2 M ethylenediamine (EDA) under alkaline conditions (pH 10). The MANAE-agarose support was serially rinsed with acetate (pH 4) and borate (pH 9) buffers and finally washed with distilled water. The porous supports obtained thus were gently mixed with 100 mL of 1% lipase solution in 0.1 M Mcilvaine buffer (pH 10.0) for 60–90 min.
Gel entrapment
Lipase immobilization by entrapment in chemically inert gels was accomplished following the modified method of Reetz et al. (1996). In this assay, 100 mL of 1% lipase in 0.1 M Mcilvaine buffer of pH 8.5 was mixed with equal volume of 2% sodium alginate solution. The enzyme–alginate mixture was dropped into a solution of 0.1 M CaCl2 through a syringe. The gel beads were dried at 35 °C in a desiccator using CaCl2 as desiccant, ground and stored at 4 °C.
Cell immobilization
For whole cell immobilization, lipase bearing mycelia of R. arrhizus were sequentially washed with distilled water, chilled acetone and chilled ether to remove water and any lipids. The resultant mycelium was dried under vacuum to remove traces of solvents and used as during acylation reactions.
Selection of the reaction medium
A variety of non-aqueous solvents like n-hexane, n-octane, petroleum ether, diethyl ether, benzene, toluene, carbon tetrachloride, chloroform, acetone, tetrahydrofuran, and dimethylformamide) were evaluated for the biocatalysis of octanoic acid.
Experimental part B: optimization of acylation conditions
For lipase-catalyzed acylation of octanol with octanoic acid, the temperature (A), moisture level (B), shaking speed (C), and enzyme dose (D) were investigated over a range of 25–50 °C, 0.2–5.0%, 50–250 RPM, and 50–500 I.U., respectively following fully rotatable central composite design (Mushtaq et al., 2015). Table 2 provides the detail of various experiments conducted at the center (coded as zero), factorial (coded as + 1 and − 1) and the axial points (coded as + β and − β). The results observed under each experimental condition were processed for statistical analysis using Design Expert (version 11, Stat-Ease, Inc., Minneapolis, USA). Analysis of variance (ANOVA) was used to screen out statistically significant effects at 95 and 99.5% confidence level. A probability (p) < 0.05 was used to locate statistically significant terms at 95% confidence level while p < 0.001 designate the model terms significant at 99.5% confidence level. Experimental design adequacy was checked by lack of fit probability (p) and coefficient of variation (CV).
Results and discussion
Screening of biocatalyst
Acylation of octanol using octanoic acid as acylating agent (O-acylation) often follows an electrophilic substitution reaction in which octanoyl group of octanoic acid is transferred to the nucleophilic Oxygen atom of octanol. Lipases bind well with fatty acids or their esters to form an acyl-enzyme complex (Eq. 1) which in turns react with a non-aqueous nucleophile to transfer acyl group and reach original configuration (Eq. 2).


As mentioned above, application of proteinaceous catalyst has gained momentum quite rapidly to synthesize stereoselective products avoiding extreme reaction conditions and lipases of various origins have produced the most fruitful results. According to the current stage of our understanding, lipases of different origins must be dissimilar in nature and catalytic potential. Therefore, we screened lipases of four different origins (lipases isolated from Aspergillus niger, R. arrhizus, and Candida rugosa type VII and Novozyme-435) for their ability to catalyze the acylation reaction. Likewise, the enzyme cocktail applied the nature of immobilization support and approaches are too crucial towards product quality and quantity. In order to evaluate the effect of immobilization support and technique, variety of immobilization supports and techniques were applied (Table 1). Moreover, the intracellular lipase of Aspergillus niger and R. arrhizus were also immobilized by dead mycelia and commercially available immobilized lipases (Novozyme-435) were also investigated. The lipase of Candida rugosa type offered negligible intracellular activities so these were not immobilized within cell matrix.
It was observed that free lipases effectively catalyzed the hydrolysis of esters but all the enzyme formulations more or less denied catalyzing the acylation reaction (Table 1). This might be due to the fact that biocatalysts lose their catalytic activities in nonpolar solvents whereas under aqueous conditions these proteinaceous enzymes catalyze hydrolysis. This problem can be managed well while providing the enzymes an environment similar to natural one that keeps the enzyme in original conformation and retains its catalytic activity (Garcia-Galan et al., 2011). It was observed that lipases immobilized physically on porous supports offered better acylation activities as compared to covalent linking and entrapments. The highest acylation activity equal to 147 μM L−1 min−1 was offered by lipase bearing dead mycelia of R. arrhizus. However, the acylation activities of dead mycelium-based lipases decreased with the increase in the number of reaction cycles due to blockage of active sites. The biocatalyst reusability was evaluated after each reaction cycle and the lipase bearing dead mycelia efficiently catalyzed esterification 4–5 reaction cycles with only 5–10 total decrease acylation activity. The higher acylation activity and reusability of lipase bearing dead mycelia of R. arrhizus must be attributed to the presence of natural immobilization and reaction environment that could keep the biocatalyst in an active conformation. In opposition, the ionic and covalent immobilization may change the three-dimensional conformation of enzyme which may result in a decline in catalyst characteristics.
The data assembled in Table 1 provides an overview of claimed and observed activity of selected formulations and their ability to esterify octanoic acid. It was observed that lipase B from Candida rugosa type VII (the claimed activity of 11,600 I.U. g−1) offered an enzymatic activity approximately 9840 I.U. g−1 of solid in aqueous media but could support an acylation rate of 79 μM L−1 min−1. The same enzyme formulation, when immobilized on O-Propargyl dextrans (Degree of Substitution 0.42 and protein load 3.7 mg g−1), catalyzed 713 hydrolytic units g−1 but furnished an acylation activity equal to 98 μM L−1 min−1. The highest acylation activity of 147 μM L−1 min−1 was offered by lipase bearing dead mycelia for octyl octanoate synthesis as compared to all other biocatalysts available. Lipases of Candida rugosa type VII immobilized on Celite-535, O-Propargyl dextrans and Polyethylene offered an acylation activity equal to 79, 98 and 94 μM L−1 min−1, respectively. Lipases produced from Aspergillus niger when immobilized on O-Propargyl dextrans could offer only 35 μM L−1 min−1 acylation activity. Novozyme-435 physically adsorbed on an acrylic resin having a stretched biocatalyst history for the industrial scale production of biodiesel and fatty acid esters (Compton et al., 2000; Hernández-Martín and Otero, 2008) produced octyl octanoate equivalent to 47 μM L−1 min−1. A careful screening of previously published literature indicates no attempt has been made to synthesize octyl octanoate using free or immobilized lipases, however, these biocatalysts have been frequently applied for the esterification of octanoic acid into other esters like ethyl octanoate (Tomke and Rathod, 2016), geranyl octanoate (Tahir et al., 2009) and in all the cases acylation activities were found to be smaller than hydrolytic activities.
Selection of the reaction medium
The synthesis of octyl octanoate was investigated in various non-aqueous solvents because these solvents can reversibly or irreversibly denature the enzyme by extracting/dehydrating essential water. The presence of water continues the salt bridges, hydrogen bonding, and hydrophobic interactions live; the factors substantially responsible for active conformation of the enzymatic protein. Concurrently, the presence of water as reaction media favors the hydrolytic reaction rather than acylation. Although, lipase immobilization keeps the enzyme in its active configuration still selection of appropriate reaction media is crucial to make the acyl-enzyme complex move towards octyl octanoate while avoiding acyl migration and keeping the lipases in the native configuration.
Figure 1(A) elaborates the effectiveness of various investigated solvents towards acylation activity (μM L−1 min−1) while applying lipase bearing dead mycelia of R. arrhizus as a biocatalyst. Acetone and tetrahydrofuran absolutely failed to produce octyl octanoate while n-hexane and other non-polar solvents like cyclohexane, petroleum ether, and n-octane furnish comparable acylation activities. The highest acylation activity of about 70 μM L−1 min−1 was obtained for n-hexane based reaction media. Similar kind of trends was observed during our previous study regarding the synthesis of glucose monolaurate (Rashid Choudhry et al., 2017).
Effect of octanol and octanoic acid concentration
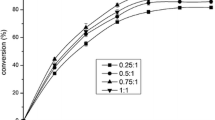
An increase in octanol concentration (nucleophile) can make the reaction to move in the forward direction but the presence of free octanol will not only reduce the reaction rate but also act like poison towards proteinaceous biocatalyst. Therefore, octanol concentration was carefully investigated over a wide range (50–1000 mM) while keeping the biocatalyst and acylating agent at most suitable level. Figure 1(B) indicates that acylation activity increases with the increase in octanol number of moles up to 850 mM and a further increase in octanol concentration inhibited the biocatalyst activity as previously reported by Reetz (2002). Actually, elevated concentration of octanol not only deactivate enzyme but also works as a nucleophile importing negative effect on acylation yield. Similarly, Fig. 1(C) explains the effect of octanoic acid concentration on acylation activity. It is evident from the plotted data that an increase in octanoic acid concentration up to 750 mM steadily improves the acylation yield but further increase in acylating agent merely impose economic losses. During the course of the reaction, octanoic acid forms acyl-lipase complex, so increase in octanoic acid concentration is only fruitful till saturation of active sites and additional octanoic acid may lead to side reactions.
Optimization of reaction conditions
After careful selection of reaction media, enzyme cocktail, substrate and acylating agent concentration, reaction conditions i.e. temperature, shaking speed, moisture level, and enzyme dose were investigated over a wide range to accelerates the formation of the selected product, avoid acyl migration and keep the biocatalyst in natural conformation. Table 2 provides detail of various reaction parameters investigated along with real and coded values. The acylation activity (μM L−1 min−1) observed against each experimental condition was processed for analysis of variance (ANOVA) and consequences have been assembled in Table 3. The larger “model” F-value of 26.91 indicates that chosen model is significant at 99.9% confidence limit while smaller “Lack Fit” probability indicates that selected experimental design fits well over given range of reaction parameters. The model fitness and suitability was further authenticated by Fig. 2(v), (vi) which displays an excellent agreement between observed and predicted values of acylation activity. The coefficient of variation (CV) value 7.47% indicates that results obtained during laboratory experiments are quite reliable (Mushtaq et al., 2015). Probability (p) value < 0.050 designates that model term significant while p > 0.050 points out the non-significant reactions parameters. In this context, reaction parameters including temperature (A), moisture level (B), enzyme dose (D), their interactions AB, AD, BD, and quadratic effects A2, B2 and D2 significantly (p < 0.05) influenced the response (acylation activity). Therefore, a second order polynomial equation (excluding non-significant terms) can be modeled in terms of coded factors to predict about the acylation yield
The linear effects of reaction parameters i.e. temperature, moisture level, shaking speed and enzyme dose can be better understood from Fig. 2(i–iv). According to Fig. 2(i), there is no indication of the thermal stability of the biocatalyst thus its efficiency is supposed to be higher under moderate reaction conditions (25–35 °C). The other possible reason for higher acylation activity within this temperature range might be the absence of acylation migration the phenomenon which takes place at elevated temperatures. The moisture level of 2–3% favors the acylation reaction and any change towards either side would adversely affect the acylation yield. The linear effect of shaking speed was found to be non-significant (p > 0.05) however, its interaction with other reaction parameters were cited worthy and will be discussed in the subsequent sections. Figure 2(iv) establishes that under above reaction conditions increase in lipase units up to 250 I.U. accelerate the acylation reaction but further increase in enzyme dose adversely affected the acylation reaction which might be due to the stabilization of the acyl-enzyme complex.
Figure 3(i–vi) provides a three-dimensional synergism of interactions between various reaction parameters investigated. The presence of curvatures in Fig. 3(i) infers that for optimum acylation yield the temperature and moisture level should rage 30–35 °C and 2.5–3.0% respectively. In contrast, the absence of any curvature in Fig. 3(ii) indicates that shaking speed does not influence the acylation rate, however; keeping the reaction at 150–200 RPM shaking speed and lower temperature has resulted out in elevated acylation rate. Similarly, smaller enzyme doses (I.U.) at lower temperature were found to be more effective than large enzyme dose at increased temperature. The justification that appears to be more reasonable for this kind of behavior might be the limiting factor i.e., an increase in enzyme dose beyond the limiting concentration would not accelerate the acylation yield (Mikhailova et al., 2008). The decrease in acylation activity with the increase in temperature might be linked to acylation migration or depletion of enzymatic activity. The acylation migration seems to be more responsible for the depletion in acylation activity at elevated temperature because lipases are fairly stable over a selected range of temperature (Poojari and Clarson, 2013).
Finally, a new set of experiments were conducted observing the most suitable reaction conditions (Table 3). It was interesting to note that lipases bearing dead Mycelia offered the acylation activity of 149.34 ± 1.15 μmol L−1 min−1 in the presence of 750 mM Octanoic Acid, 850 mM, and n-hexane as the reaction solvent. The incubation of above mentioned acylating agent and the substrate with biocatalyst bearing an activity equivalent to 250 I.U. at 35 °C, 3% moisture level and 150 RPM shaking speed furnished 70.82 ± 0.64% conversion rate at the end of 24 h.
The results of the present investigation disclosed that selection of enzyme cocktail, immobilization technique, nature of support applied, and reaction media equally influence the effectiveness of biocatalyst to catalyze acylation reaction. Although non-polar solvent improved the acylation rate but their presence adversely affected the lipolytic activities of the enzymes. Ideally, the acylation reaction should be carried out at the interface to avoid acyl migration and keep the enzyme cocktail in its natural configuration. Among the various biocatalyst investigated, lipase bearing dead mycelia of R. arrhizus offered the highest acylation yield at acceptable reaction rates. Lipases of Candida rugosa type VII immobilized on O-Propargyl dextrans and Polyethylene also furnished reasonable acylation activity while Lipase from Candida antarctica adsorbed on acrylic resin could only generate up to 47 μmol L−1 min−1. The careful optimization of reaction conditions enhanced the acylation activity and minimized the acylation migration. Overall, it was speculated that incorporation of lipase bearing dead mycelia of R. arrhizus as biocatalyst can overwhelm the acyl migration to make the synthesis of octyl octanoate monetarily achievable.
References
Adlercreutz P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 42: 6406–6436 (2013)
Barbosa O, Ortiz C, Berenguer-Murcia Á, Torres R, Rodrigues RC, Fernandez-Lafuente R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 33: 435–456 (2015)
Boyall D, Frantz DE, Carreira EM. Efficient enantioselective additions of terminal alkynes and aldehydes under operationally convenient conditions. Org. Lett. 4: 2605–2606 (2002)
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 (1976)
Buchholz K, Kasche V, Bornscheuer UT. Biocatalysts and Enzyme Technology, Wiley, London (2012)
Burdock GA. Fenaroli’s Handbook of Flavor Ingredients, CRC Press, Boca Raton (2016)
Cao L, van Langen L, Sheldon RA. Immobilised enzymes: carrier-bound or carrier-free? Curr. Opin. Biotechnol. 14: 387–394 (2003)
Compton DL, Laszlo JA, Berhow MA. Lipase-catalyzed synthesis of ferulate esters. J. Am. Oil. Chem. Soc. 77: 513–519 (2000)
Fernandez-Lorente G, Filice M, Terreni M, Guisan JM, Fernandez-Lafuente R, Palomo JM. Lecitase® ultra as regioselective biocatalyst in the hydrolysis of fully protected carbohydrates: Strong modulation by using different immobilization protocols. J. Mol. Catal. B 51: 110–117 (2008)
Garcia‐Galan C, Berenguer‐Murcia Á, Fernandez‐Lafuente R, Rodrigues RC. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 353: 2885–2904 (2011)
Gotor‐Fernández V, Busto E, Gotor V. Candida antarctica lipase B: an ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv. Synth. Catal. 348: 797–812 (2006)
Hernández-Martín E, Otero C. Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 99: 277–286 (2008)
Jesionowski T, Zdarta J, Krajewska B. Enzyme immobilization by adsorption: a review. Adsorption 20: 801–821 (2014)
Mahmod SS, Yusof F, Jami MS, Khanahmadi S, Shah H. Development of an immobilized biocatalyst with lipase and protease activities as a multipurpose cross-linked enzyme aggregate (multi-CLEA). Process Biochem. 50: 2144–2157 (2015)
Mikhailova AG, Likhareva VV, Rumsh LD. A rate-limiting step of enteropeptidase hydrolysis. Russ. J. Bioorg. Chem. 34: 186–191 (2008)
Mushtaq M, Sultana B, Bhatti HN, Asghar M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J. Food Sci. Technol. 52: 5048–5056 (2015)
Mushtaq M, Sultana B, Akram S, Anwar F, Adnan A, Rizvi SS. Enzyme-assisted supercritical fluid extraction: an alternative and green technology for non-extractable polyphenols. Anal. Bioanal. Chem. 409(14): 1–11 (2017)
Nisha S, Karthick SA, Gobi N. A review on methods, application and properties of immobilized enzyme. Chem. Sci. Rev. Lett. 1: 148–155 (2012)
Poojari Y, Clarson SJ. Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles in organic media. Biocatal. Agric. Biotechnol. 2: 7–11 (2013)
Rashid Choudhry A, Mushtaq M, Adnan A, Syed Q. Response surface methodology based optimization of glucose acylation bio-catalyzed by immobilized lipase. Biocatal. Biotransfor. 35(4): 238–248 (2017)
Reetz MT. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 6: 145–150 (2002)
Reetz MT, Zonta A, Simpelkamp J. Efficient immobilization of lipases by entrapment in hydrophobic sol-gel materials. Biotechnol. Bioeng. 49: 527–534 (1996)
Schorkopf DLP, Hrncir M, Mateus S, Zucchi R, Schmidt VM, Barth FG. Mandibular gland secretions of meliponine worker bees: further evidence for their role in interspecific and intraspecific defence and aggression and against their role in food source signalling. J. Exp. Biol. 212: 1153–1162 (2009)
Shigley JW, Bonhorst CW, Liang PM, Althouse PM, Triebold HO. Physical characterization of a) a series of ethyl esters and b) a series of ethanoate esters. J. Am. Oil Chem. Soc. 32: 213–215 (1955)
Tahir MN, Adnan A, Mischnick P. Lipase immobilization on O-propargyl and O-pentynyl dextrans and its application for the synthesis of click beetle pheromones. Process Biochem. 44: 1276–1283 (2009)
Tomke PD, Rathod VK. Enzyme as biocatalyst for synthesis of octyl ethanoate using acoustic cavitation: Optimization and kinetic study. Biocatal. Agric. Biotechnol. 7: 145–153 (2016)
Tran DN, Balkus KJ. Perspective of Recent Progress in Immobilization of Enzymes. ACS Catal. 1: 956–968 (2011)
Wohlgemuth R. Asymmetric biocatalysis with microbial enzymes and cells. Curr. Opin. Microbiol. 13: 283–292 (2010)
Yan K, Wu G, Lafleur T, Jarvis C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sust. Energy Rev. 38: 663–676 (2014)
Acknowledgements
The work present in this monograph was partially sponsored by Higher Education Commission of Pakistan. The authors are thankful to Mr. Michael Emil Wagner for assisting linguistic features.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors of present research declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rashid, A., Mushtaq, M., Syed, Q. et al. Application of lipase bearing dead mycelia as biocatalyst for octyl-octanoate synthesis. Food Sci Biotechnol 27, 1707–1718 (2018). https://doi.org/10.1007/s10068-018-0405-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0405-2