Abstract
This contribution proposes an enzyme-assisted eco-friendly process for the extraction of non-extractable polyphenols (NEPPs) from black tea leftover (BTLO), an underutilized tea waste. BTLO hydrolyzed with various enzyme formulations was extracted using supercritical carbon dioxide and ethanol as co-solvent (SC-CO2 + EtOH). A conventional solvent extraction (CSE) was performed using EtOH + H2O (80:20, v/v) for comparison purposes. The results revealed that hydrolysis of BTLO with 2.9% (w/w) kemzyme at 45 °C and pH 5.4 for 98 min improved the liberation of NEPPs offering 5-fold higher extract yield (g/100 g) as compared with non-treated BTLO. In vitro antioxidant evaluation and LC-MS characterization of extracts revealed the presence of phenolic acids (mainly caffeic and para-coumaric acid) of high antioxidant value. Scanning electron micrograph of the hydrolyzed BTLO samples indicated noteworthy changes in the ultrastructure of BTLO. Moreover, polyphenol extracts obtained by SC-CO2 + EtOH extraction were found to be cleaner and richer in polyphenols as compared to CSE. The devised enzyme-assisted SC-CO2 + EtOH extraction process in the present work can be explored as an effective biotechnological mean for the optimal recovery of antioxidant polyphenols.

Enzymatic pretreatment can effectively liberate non-extractable polyphenols (NEPPs) while hydrolyzing the cellulosic and hemicellulosic framework of black tea left overs (BTLO)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant-based polyphenols are being increasingly focused due to their established health benefits and protective effects against various coronary and stress-initiated diseases [1, 2]. Black tea, an exceptional source of polyphenols, constitutes more than 90% of tea beverages consumed in the western world. Sometimes, misnomer “fermentation” is used to describe the tea manufacturing, but actually it involves polyphenol oxidase that catalyzes the oxidation of withered, crushed, and evenly censored leaves of Camellia sinensis shrubs [3]. Ready-to-drink tea infusion is usually prepared by brewing 1–2 g of black tea per 100 mL of boiling water (90–95 °C) for 40–80 s [4], and finally, black tea residues, which are a potential source of polyphenols, are rejected as an underutilized leftover [5, 6]. According to estimation, 3–3.5 million tons of black tea is produced annually and thus generates an almost comparable fraction of black tea leftover (BTLO) [7].
A thorough study of previously published data about plant phenolics revealed that a predominant portion of plant phenolic compounds, so-called non-extractable polyphenols (NEPPs), are glycosidically linked with cellulosic and hemicellulosic microfibrils and do not leach during simple solvent extraction or in boiling water [8–10]. Several kinds of pre-treatments like ultrasound [11, 12], microwave [13], ohmic heating [14], and acid or alkaline hydrolysis [15, 16] have been suggested to set free these bound phenolics. Some of these exhibited appreciable recovery rates, but concerns have been conveyed regarding the antioxidant and nutritional quality of leached polyphenols [16, 17].
Supercritical carbon dioxide (SC-CO2) has turned out to be an alternative, green, eco-friendly solvent. Moreover, SC-CO2-based technology has attracted researchers for its ability to extract the compounds prone to thermal deterioration and oxidation due to its mild operational temperature [18–20]. However, the polarity of SC-CO2 has been a barrier for the versatility of this technique to extract a diversity of phytochemicals, but this problem has been overwhelmed by slotting in co-solvents such as methanol, ethanol, and water. To date, supercritical fluid-based extraction techniques are considered expensive because of low extraction yield when applied to the extraction of polyphenols from the complex biological matrix. Enzymatic hydrolysis before SC-CO2 extraction is going to compensate for the capital cost because it can improve mass transfer, reduce the particle size, increase the contact area, enhance solvent distribution [21, 22], and above all may facilitate the liberation of NEPPs [23].
According to reports, most of the data available in the literature on “total polyphenol content” refers only to extractable polyphenols (EPPs) and neglect a significant share of NEPPs. In fact, the “total polyphenol content” in a typical food is essentially composed of EPP plus NEPPs [2, 24]. Conventionally practiced “hot water tea boiling” extracts mainly free-form polyphenols (EPP), so BTLO is expected to be a potential source of NEPPs. Therefore, the present study was designed to screen out various enzymatic formulations for hydrolysis of BTLO and subsequent extraction of polyphenols (NEPPs) by a modified SC-CO2 extraction process.
Materials and methods
Black tea leftover (BTLO) was generated by boiling a sufficient amount (100 g/L) of international brand Lipton (Unilever, Karachi, Pakistan) in water using a glass kettle. The tea fusion was filtered through a strainer to separate out the residues (BTLO), washed thoroughly with distilled water, dried under ambient conditions, and stored in airtight bags for further processing. The enzymatic hydrolysis and SCF-based extraction were executed at the Rizvi Laboratory, Department of Food Science, Cornell University, NY, USA. The SEM-based morphological and LC-MS-based polyphenol characterization was performed at the Cornell Center for Materials Research and Department of Ecology and Evolutionary Biology, both at Cornell University. The Bioanalytical Laboratory, Department of Chemistry, University of Agriculture Faisalabad, Pakistan, had provided a facility for antioxidant evaluation of polyphenols extracted.
Enzymes, standards, and reagents
Table 1 provides details of various enzyme formulations used, their active ingredients, and their origin. The reagents and standards including 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), Folin-Ciocalteu reagent, 2,2-azinobis (3-ethylbenzothiazoline-6-fulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, 3,4-dihydroxy benzoic, p-hydroxy benzoic, gallic, linoleic, vanillic, caffeic, p-coumaric, ferulic, syringic, and sinapic acids, and butylated hydroxytoluene (BHT) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All the other chemicals such as potassium persulfate, ammonium thiocyanate, potassium ferrocyanide, Na2CO3, and acetic acid and solvents including methanol, ethanol, and water used were of analytical and/or HPLC grade (Merck, Germany).
Enzymatic hydrolysis and SCFE
For enzymatic hydrolysis, accurately weighed 5 g of BTLO was mixed with 10 mL phosphate buffer and blended with different concentrations of selected enzyme formulations [please see Electronic Supplementary Material (ESM) Table S1] observing various reaction conditions (temperature, pH, and withholding time). At the end of incubation, the enzyme formulation was deactivated by heating the slurry at 90 °C for 5 min and the contents were degassed in an ultrasonic reactor (UTECH, Albany, NY, USA). The enzymatically hydrolyzed BTLO was inserted to an extraction vessel of SFT-250 (SFE/SFR System; Supercritical Fluid Technologies, Newark, DE, USA) and polyphenols were extracted using SC-CO2 with ethanol as co-solvent using SFT-250 Supercritical Fluid Extractor/Reactor System (Supercritical Fluid Technologies, Newark, DE, USA) equipped with a 100-mL extraction vessel (Fig. 1). Polyphenols were extracted at 55 °C and 300 bar pressure keeping the SC-CO2 and ethanol flow rates at 2 and 0.2 g/min for 30–120 min, respectively.
Morphological characterization of enzymatically hydrolyzed BTLO
A small portion of BTLO hydrolyzed under the optimum conditions of various enzyme formulations was dried under liquid nitrogen, mounted on a specimen hold with magnet tape, coated with palladium using a sputter coater, and visualized in a field emission scanning electron microscope MIRA3 (TESCAN Technologies, Brno, Czech Republic).
Determination of polyphenols
The polyphenol content of extracts obtained by conventional solvent (EtOH + H2O) extraction and SC-CO2 + EtOH was determined by modifying a frequently used Folin-Ciocalteu reagent-based colorimetric assay [25]. In this experiment, an accurately weighed 0.5 mg of each extract was mixed with 0.5 mL of 10% Folin-Ciocalteu reagent and diluted with 7.5 mL deionized water. The solution was maintained at 40 °C for 10 min and neutralized with 1.5 mL of 20% sodium carbonate (w/v). The final aliquot was kept at 40 °C for a further 20 min and then chilled in an ice bath. Different concentrations of gallic acid (10–200 ppm) were also processed under the same conditions to plot the calibration curve (R 2 = 0.9978). Two hundred fifty microliters of all the samples and standard solutions were transferred to 96-well plate and measured read at λ max 755 nm (BioTek, High Land Park, USA).
UHPLC-DAD-ESI-TQTOF-MS characterization of phenolic acids
For individual phenolics, the extracts obtained were further hydrolyzed by treating with 1% acidified methanol in the presence of BHT as a preservative antioxidant. The upper layer (250 μL), after passing through a 0.45-μm syringe filter (Millipore), was delivered in an autosampler (Finnigan Surveyor) of ultra-high-performance chromatography (UHPLC) system equipped with a Phenomenex Gemini-NX column (150 × 2 mm; 3 μm internal diameter). The separation was executed using gradient mode elution system made up of 0.1% formic acid in water (A) and acetonitrile (B) and programmed at a flow rate of 0.2 mL/min. The optimum separation was observed when ratios of A and B components were 95A/5B, 35A/65B, and 25A/75B for 0–40, 40–45, and 45–52 min, respectively. The identification and quantification of eluted phenolics were accomplished by comparing the retention time and mass spectrum with external phenolic acid standards (see ESM Fig. S1). A diode array detector (DAD) was set at 280 nm whereas Quantum access triple quadrupole mass spectrometer (MS) was scanned over 100.00–1200.00 MS range under positive mode electrospray ionization (ESI). The other mass spectrometer parameters were tuned as spray voltage 5 kV, ion tube temperature 316 °C, sheath gas (N2) flow 15 Arb, and auxiliary gas (N2) flow 20 Arb. The chromatographic data obtained was processed by Dionex Chromeleon System.
Antioxidant quality characterization of BTLO extracts
The antioxidant characteristics of extracts obtained by conventional solvent and SC-CO2 + EtOH were evaluated for their radical scavenging capacity (RSC), Trolox equivalent antioxidant capacity (TEAC), ferric reducing power (FRP), and linoleic acid peroxidation inhibition capacity (LPIC) following various in vitro assays.
RSC
For RSC, a previously described procedure [26] was modified to micro levels and performed in a 96-well plate. Briefly, 100 μL of freshly prepared DPPH (1 mg/mL) was pipetted out to the microwell of the plate and added up with 110 μL of extracts containing 1000, 100, 10, and 1 μg/mL. The plates were incubated at 30° for 15 min and measured at λ max 517 nm (BioTek, High Land Park, USA).
The maximal inhibitory concentration (IC50) was appraised from the plot of RSC (%) versus extract concentration (μg/mL).
TEAC
To evaluate the antioxidant content of BTLO extracts obtained against observed conditions, 10 μL of each extract (50 mg/mL) was incubated at 30 °C with 190 μL of diluted ABTS radical cations (ABTS•+ ) for 6 min [27]. The ABTS•+ were generated by mixing 0.5/1 (v/v) solutions of 7 mM ABTS and 2.45 mM potassium persulfate. This mixture was incubated in the dark for 8 h and diluted with 80% ethanol till an absorbance of 0.700 ± 0.050 at λ max 734 nm. The decrease in absorbance at 734 nm caused by BTLO extract was compared with Trolox (known antioxidant), and results were expressed as milligrams TE per gram of extract.
LPIC
The ability of extract to shield peroxidation in linoleic acid (C18:2) system was assessed following the protocol described previously [28]. In this assay, 5 mg of BTLO extract was mixed with 130 μL linoleic acid (10%), 1 mL of ethanol (99.8%), and 1 mL of 0.2 M sodium phosphate buffer of pH 7. The aliquot was incubated at 40 °C for 72 h and the extent of linoleic acid oxidation assessed by the thiocyanate method of Beker and Bakir [29]. Briefly, 200 μL of the sample solution, 1 mL of 75% ethanol, 1 mL of 30% ammonium thiocyanate, and 200 μL of 20 mM FeCl2 solution in 3.5% HCl were mixed with constant stirring. After 3 min of incubation at 40 °C, absorbance was measured at λ max 500 nm that represents peroxide content formed.
FRP
For ferric reducing power, different amounts of BTLO extracts (2.5–10.0 mg) were separately dissolved in 5.0 mL of phosphate buffer (0.2 M sodium phosphate) of pH 6.6 and incubated with 5.0 mL of 1.0% potassium ferricyanide at 50 °C for 20 min. Now, 5 mL of 10% trichloroacetic acid was added and the reaction mixture was centrifuged at 980 rpm in a refrigerated centrifuge (5 °C) for 10 min. Finally, 100 μL of the upper layer was transferred to a well of the 96-well plate, added up with an equal volume of distilled water, 50 μL of 0.1% ferric chloride (FeCl3), and absorbance measured at 700 nm.
Statistical analysis
Triplicate experiments were conducted for enzymatic hydrolysis and in vitro antioxidant capacities to report data as mean ± SD at 95% confidence interval. The results were further analyzed by Minitab Software Package version 16.0 (Minitab, Inc., State College, PA, USA) to check the statistical variation between means. A probability (p) value <0.05 indicated statistically significant differences.
Results and discussion
Enzymatic hydrolysis
Extraction has been considered one of the key steps in the preparation of analytical samples and phytochemical extracts as well as for the related characterization of chemical compounds. The complexity and importance of this process have recently been realized when analytical laboratories were equipped with state-of-the-art spectroscopic and chromatographic instruments. Moreover, an increased understanding of the health and therapeutic benefits of phytochemicals has prompted the researchers to innovate intelligent extraction and isolation techniques. Supercritical carbon dioxide (SC-CO2) has been known as a reliable, alternative, green, and eco-friendly solvent among analytical and organic chemists. However, limited recovery rates are main restrictions against the versatility of these techniques. Enzymatic hydrolysis prior to SC-CO2 can liberate covalently bound plant polyphenols and again go greener as compared to ultrasound [11, 12], microwave [13], ohmic heating, and acid/alkaline hydrolysis [14].
Meanwhile, the use of enzymes owing to their selective hydrolytic abilities is becoming popular in the food and beverages sectors. Nevertheless, the effectiveness of enzymes as valuable biocatalysts is decidedly influenced by the nature of the substrate and process parameters. In the present study, various enzyme formulations were tested and optimized for the maximum liberation of polyphenols. The extraction of polyphenols from hydrolyzed black tea leftover (BTLO) was subsequently accomplished by SC-CO2 + EtOH maintaining an extraction time of 120 min, CO2 flow rate 2 g/min, ethanol injection 0.2 g/min, pressure 300 bar, and temperature 50 °C [30] as well as by conventional solvent (EtOH + water). Enzymatic hydrolysis parameters such as enzyme concentration (%), withholding time (min), temperature (°C), and pH were optimized to gain effective breakdown of BTLO material.
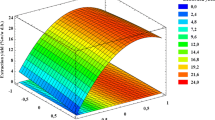
Figures 2 and 3 provide a comparison of the quantities of crude extracts obtained from enzymatically hydrolyzed BTLO during supercritical solvent (SC-CO2 + EtOH) and conventional solvent (EtOH + water), respectively. It is obvious from these figures that quantities of crude extracts obtained during enzyme-assisted solvent extraction were higher as compared to enzyme-assisted supercritical fluid extraction. On the whole, kemzyme formulation produced larger amounts of crude extracts rich in polyphenols. The quantities of the extracts obtained by SC-CO2 + EtOH were significantly lesser than conventional solvent, but HPLC-DAD-ESI-MS and antioxidant quality characterization revealed that extracts produced by the former method (SC-CO2 + EtOH) contained higher amounts of high-value polyphenols. It is obvious from Figs. 2a and 3a that acidic pH (4–6) is more favorable for kemzyme while cocktail enzyme was effective over pH 5.5–6.5. A small shift in pH beyond the optimum caused a sharp decrease in the amount of extract obtained which can be linked to the fact that activities of the enzyme are pH dependent. Similarly, Figs. 2b and 3b indicate that kemzyme concentration up to 4% satisfactorily hydrolyzed the substrate as a further increase in enzyme concentration reduces its efficiency (as happened in the case of alcalases). Next to kemzyme, cocktail and alcalase formulations were found to be effective when applied in the range of 2–3% (by weight of BTLO). The optimum values of temperature and incubation time were found to be 35 °C and 60 min for kemzyme while alcalase was noted to be more effective at a higher temperature with longer withholding time. Prolonged incubation may deteriorate the antioxidant quality of extract in addition to economic losses. A summary of the optimum hydrolysis conditions against each enzyme formulation is provided in Table 1. The results observed during the present attempt agreed with a previous study conducted by Li and Smith [31], who observed that extract yield of polyphenols in citrus peel obtained from five different species varied with nature of enzyme applied, pH, incubation time, and temperature.
The plot of SC-CO2 + EtOH consumed with extraction time (Fig. 4) revealed that enzymatic hydrolysis not only enhanced the extract yield but also reduced extraction time (consumption of SC-CO2, energy, and labor) that may outweigh the cost of enzymes. Moreover, it is obvious from the data plotted in Fig. 4 that the maximum amount of total crude extract (20.74 g) was extracted from 100 g of BTLO hydrolyzed with enzyme formulation within 45 min while untreated BTLO offered only 2.97 g of total crude extract/100 g at the end of 60 min.
The subsequent LC-MS analysis and in vitro antioxidant testing authenticated that extracts produced via SC-CO2 were rich in polyphenols of good antioxidant powers. Among the enzyme formulations applied, alcalases were second to cause a rapid liberation of bound polyphenols from BTLO, but the extract yields were significantly (p < 0.05) lower than kemzymes. It is clear from the figure that the incorporation of enzymatic hydrolysis during conventional and supercritical solvent extraction not only enhanced the production of crude extract but also attenuated the extraction time.
Total polyphenols (TPs)
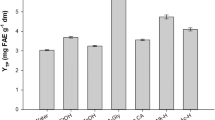
It would be of practical interest to know what kinds of bioactives are present in BTLO extracts obtained by SCFE and CSE and what would be the extent of the antioxidant character of these polyphenols. Therefore, the extracts obtained from enzymatically hydrolyzed BTLO were processed for total and individual polyphenol profile and antioxidant capacities. Table 2 provides a summary of optimum enzymatic hydrolysis conditions for each enzymatic treatment, the quantity of crude extract produced (extract yield), and total polyphenols (mg GAE/g of BTLO extracts). It was interesting to note that quantities of crude extracts produced were higher for CSE, but the levels of polyphenols were larger in the extracts obtained by SC-CO2. Furthermore, the mean values of polyphenols varied significantly in response to enzymatic formulation applied as indicated by various superscripted letters. In all the cases, the amounts of crude extracts and their polyphenol contents were higher than the conventional CSE extracts obtained by soaking BTLO in a buffer of pH 7 and extracting under the same conditions of both techniques.
Overall, kemzyme comprising mainly α-amylase and endoglycosidases (endo-1,3(4)-beta-glucanase, endo-1,4-beta-xylanase) was found to be more efficient to liberate bound polyphenols from BTLO. The hydrolysis of BTLO with 2.9% (w/w) of said enzyme formulation at 45 °C, pH 5.6, and 90 min produced 29.15 ± 0.81 and 21.44 ± 0.60 (g/100 g of BTLO) crude extracts containing 283.45 ± 6.19 and 521.44 ± 16.23 mg (GAE/g of extract) during conventional and supercritical fluid-based extraction, respectively. Alcalase enzyme formulation was found to be second to produce amounts of crude extracts rich in polyphenols. The higher extract yield and subsequent total polyphenol liberation can be ascribed to hydrolytic activity of enzymes [8, 32]. Enzymatic pretreatment is reported to swiftly cleave the cellulosic network, improve mass transfer, reduce the particle size, and allow the liberation of bioactives that were not accessible during conventional solvent extraction [33, 34].
In fact, enzymatic hydrolysis facilitated the liberation of non-extractable polyphenols (that do not leach during simple organic or aqueous extraction), and this can be evident by the scanning electron micrographs of BTLO taken after enzymatic hydrolysis. It is obvious from Fig. 5 that various enzyme formulations macerated the BTLO microfibrils up to varying extents. The comparison of enzymatically hydrolyzed BTLO (Fig. 5b–d) with control (5a) indicates that the kemzyme thoroughly digested the BTLO microfibrils and caused cleavage of glycosidic linkages that eventually eased the liberation of polyphenols. Other enzyme formulation also cleaved the cellulosic network, reduced the particle size, and improved the mass transfer, but their effectiveness was found too limited as compared to kemzyme, particularly in the case of BTLO polyphenols.
HPLC-DAD-ESI-MS analysis of BTLO extracts
The conventional and SC-CO2 extracts from BTLO obtained under optimum conditions of enzymatic hydrolysis were further hydrolyzed to characterize phenolic acids by refluxing with dilute HCl, which were then separated, authenticated, and quantified by UHPLC-DAD-QA-ESI-TQTOF-MS set in positive ion mode. In this mode, species are detected as protonated molecules (M + 1) instead of true molecular mass. Each polyphenol standard was scanned for 100–1200.00 m/z and stored in UHPLC-mass spectral data. Table 2 provides the detail of major phenolic acids and their corresponding M + 1 values, along with the concentration determined. It was interesting to note that p-coumaric acid (208.33 ± 8.35–237.44 ± 4.51 μg/mL) was the major phenolic acid in BTLO extracts obtained by SC-CO2 and CSE, followed by caffeic, ferulic, and syringic acids. The phenolic acid derivatives in SC-CO2 extracts were generally higher than those of CSE. It might be hypothesized that the phenolic compounds linked glycosidically to cellulosic and hemicellulosic moieties do not leach during conventional aqueous or organic solvents. Moreover, a thorough review of previously documented research showed that none of the analytical chemists attempted to extract NEPPs from BTLO. However, plenty of data is available to authenticate the presence of polyphenols in tea infusions [35].
Antioxidant activities of BTLO extracts
The reason for the ever-increasing interest in plant phenolics, so far considered, is their ability to provide first-line protection against coronary and stress-initiated health disorders. Therefore, the efficiency of an extraction technique should be measured in terms of antioxidant quality of the extracts: their ability to retard or regulate the process of oxidation [36]. A number of methods have been used to evaluate the antioxidant potential of different plant foods and other medicinal and functional food products. In order to provide reliable data on antioxidant quality of extract, the researchers mostly assessed free radical scavenging capacity (RSC), reducing power (RP), total antioxidant equivalents (comparative to standard antioxidant Trolox or ascorbic acid), and inhibition of peroxidation in the linoleic acid system [37, 38]. A single assay could not ensure the complete antioxidant behavior of the extracts obtained; therefore, it is recommended to evaluate the antioxidant potential of plant foods using multiple antioxidant assays [39]. Table 3 provides an overview of the observed antioxidant activities of SC-CO2 + EtOH and EtOH + H2O extracts from enzymatically hydrolyzed BTLO. It is obvious from the observed data that enzymatically hydrolyzed BTLO has liberated a greater amount of ABTS radical cations comparative to Trolox. BTLO, when hydrolyzed applying kemzyme formulation under optimized reaction conditions and extracted via SC-CO2 + EtOH, offered 1156.56 ± 46.88 μM TE/g TEAC. Alcalase formulation also produced extracts of BTLO with a good quantity of retained antioxidants (936.47 ± 31.26 μM TE/g) as compared to untreated BTLO (577.15 ± 18.63 μM TE/g). Similarly, extracts obtained by SC-CO2 + EtOH exhibited higher Trolox equivalent antioxidant capacities as compared to conventional solvent extracts (EtOH + H2O). The variation in antioxidant activity in response to hydrolytic enzyme formulation might be linked to the liberation of NEPPs while the difference in antioxidant behavior of extracts obtained by CSE and SCFE supports the higher selectivity of SC-CO2 + EtOH. The variations in antioxidant quality of the extract produced by various enzymes can be linked to variable hydrolytic activities and substrate-enzyme specificity of the enzyme formulations employed. Overall, the tested enzyme formulations having endoglycosidases (endo-1,3(4)-beta-glucanase, endo-1,4-beta-xylanase) produced extracts with higher antioxidant activities. Henceforth, it can be claimed that incorporation of enzymatic maceration has improved the antioxidant quality of black tea by increasing the liberation of NEPPs. Moreover, observed trends in Trolox equivalent antioxidant capacities were strongly correlated (r 2 > 90%) with a concentration of phenolic constituents of the related extracts.
A comparison of the observed TEAC values with previously reported data revealed that antioxidant quality of NEPPs was comparable with certain standard antioxidants such as ascorbic acid (161 ± 4.8 M TE/g), gallic acid (90.5 ± 2.7 M TE/g), β-carotene (240 ± 21.2 M TE/g), and higher than the frequently investigated fruit residues, i.e., grape seeds, kinnow peel and seeds, and litchi [40, 41].
The findings of the present work disclosed that BTLO obtained by boiling black tea in water for sufficient time still contains a substantial level of phenolics with higher radical scavenging capacity (0.21 ± 0.01 μg/mL DPPH IC50). However, the quantity and quality of polyphenols liberated varied significantly (p < 0.0) within the employed enzyme formulation that can be linked to their hydrolyzing potential (see ESM Table S1). It was further observed that extracts obtained by SCFE were good scavengers of DPPH free radicals (smaller IC50) as compared to those produced through CSE. The results observed regarding DPPH free radical scavenging of BTLO extracts encouraged the utilization of enzyme complexes for the valorization of such underutilized materials into potent phenolic antioxidants. Other pre-treatments, which can compete for enzymatic maceration, include the application of ultrasound frequency, exposure to microwave, and acid and alkali hydrolysis. Researchers have observed that acid hydrolysis produced a good quantity of extract with higher levels of total phenolics, but their ability to scavenge DPPH free radical declined that might be due to protonation (acidic hydrolysis) or deprotonation of phenolics [15, 16]. Meanwhile, the extracts obtained from enzymatically hydrolyzed materials have exhibited an appreciable level of anti-radical activities [9, 42, 43].
Lipid peroxidation, a process responsible for many chronic diseases, is mainly initiated by some changes in the cell membrane of mammals and plants. Mechanistically, these changes in cell membrane activate lipase enzymes, which in turn liberate polyunsaturated fatty acids (PUFAs) mainly linoleic acid. The presence of lipoxygenases converts these PUFAs into free radicals and finally results in aging, cell injury, or cell proliferation. Antioxidants have been successfully used to inhibit the formation of peroxides [44, 45]. The potential of enzymatically hydrolyzed and untreated BTLO extracts obtained by SC-CO2 + EtOH and EtOH + H2O is depicted in Table 3. It is clear from the results obtained that BTLO when hydrolyzed with kemzyme, cocktail, and alcalase formulation and extracted using SCFE offered relatively higher protection (95.05 ± 2.01%) against the formation of lipid peroxidation in the linoleic acid system. Overall, extracts obtained by SCFE from hydrolyzed BTLO were found to be stronger preservatives and protectors toward linoleic acid peroxidation. Nevertheless, conventional solvent extracts (CSE) obtained from untreated (control) BTLO were observed least effective (76.25 ± 2.93) against linoleic acid peroxidation.
The previously cited literature indicates that ferric ions are involved in the physiology of free radicals. Therefore, the metal ion concentration above approved level may produce free radicals above the threshold limit. Hence, material either chelating with a metal ion or reducing oxidants may be equally beneficial for a biological system. The RP of a typical compound can be linked to its ability to transfer electrons and is therefore taken as a good indicator of the antioxidant activity/potential. The presence of reductones is detected by the breakdown of free-radical chain caused by hydrogen atoms donated. In this assay, the presence of reductones in the antioxidant sample reduces Fe3+/ferricyanide complex to Fe2+/ferrous ions with a change in absorbance. The observed reducing power results were in accordance with their phenolic contents and antioxidant activities. On the whole, in the present experiment, the reducing power increased linearly with the increase in extract concentration (2.5–10 μg/mL) as shown in Fig. 6. Secondly, the extract obtained by SCF (SC-CO2 + EtOH) exhibited a higher reducing power as compared to those obtained by CSE (EtOH + H2O). Hydrolysis of BTLO with various enzyme formulation improved the reducing power of BTLO extracts following an overall order as kemzyme > alcalase = pectinex = cocktail > control > acid cellulase > viscozyme. The observed trend for reducing power revealed that the entire enzyme formulations did not produce the extracts of equivalent antioxidant quality. Therefore, more comprehensive studies are required to understand the mechanisms of action of hydrolyzing enzyme formulations and establish a structure-activity relationship to support and elucidate a superior antioxidant quality of the polyphenols extracted.
Conclusion
Hydrolysis of black tea leftover (BTLO) under the optimum conditions using different enzyme formulations caused effective liberation of non-extractable polyphenols (NEPPs). However, the antioxidant ability of extracted polyphenols varied significantly depending upon active constituents of enzyme formulation and extraction solvent applied. Supercritical carbon dioxide with ethanol as co-solvent (SC-CO2 + EtOH) produced extracts rich in polyphenols of potent antioxidant attributes. It can be concluded from the findings of the present contribution that enzymatic hydrolysis of black tea can be a viable biotechnological route to improving its antioxidant quality and maximize potential health benefits. Moreover, hydrolytic activities of appropriate enzyme formulations might render supercritical fluid extraction as an alternative, intelligent, and green technology for food, pharmaceutical, and cosmetic industries.
References
Cheng A, Chen X, Wang W, Gong Z, Liu L. Contents of extractable and non-extractable polyphenols in the leaves of blueberry. Czech J Food Sci. 2013;31(3):275–82.
Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–52.
Bertsch W. The use of tea bricks as currency among the Tibetans. Tibet J. 2009;34(2):35–81.
Kim Y, Goodner KL, Park J-D, Choi J, Talcott ST. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011;129(4):1331–42.
Spiro M, Jaganyi D. Kinetics and equilibria of tea infusion. Part 11—the kinetics of the formation of tea scum. Food Chem. 1994;49(4):359–65.
Kinugasa H, Takeo T. Deterioration mechanism for tea infusion aroma by retort pasteurization. Agric Biol Chem. 1990;54(10):2537–42.
Traoré D. Cocoa and coffee value chains in West and Central Africa: constraints and options for revenue-raising diversification. Food Agric Organ. 2009;3:1–78.
Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldívar SO. Bound phenolics in foods, a review. Food Chem. 2014;152:46–55.
Soto ML, Moure A, Domínguez H, Parajó JC. Recovery, concentration and purification of phenolic compounds by adsorption: a review. J Food Eng. 2011;105(1):1–27.
Martins S, Mussatto SI, Martinez-Avila G, Montanez-Saenz J, Aguilar CN, Teixeira JA. Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review Biotechnol Adv. 2011;29(3):365–73.
Bimakr M, Rahman RA, Taip FS, Adzahan NM, Sarker MZ, Ganjloo A. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules. 2012;17(10):11748–62.
Carrera C, Ruiz-Rodriguez A, Palma M, Barroso CG. Ultrasound assisted extraction of phenolic compounds from grapes. Anal Chim Acta. 2012;732:100–4.
Beejmohun V, Fliniaux O, Grand E, Lamblin F, Bensaddek L, Christen P, et al. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem Anal. 2007;18(4):275–82.
Pataro G, Donsì G, Ferrari G. Aseptic processing of apricots in syrup by means of a continuous pilot scale ohmic unit. LWT-Food Sci Technol. 2011;44(6):1546–54.
Yaqoob S, Sultana B, Mushtaq M. In vitro antioxidant activities of Trianthema portulacastrum L. hydrolysates. Prev Nutri Food Sci. 2014;19(1):1–27.
Bener M, Shen Y, Apak R, Finley JW, Xu Z. Release and degradation of anthocyanins and phenolics from blueberry pomace during thermal acid hydrolysis and dry heating. J Agric Food Chemi. 2013;61(27):6643–9.
Komes D, Belscak-Cvitanovic A, Horzic D, Rusak G, Likic S, Berendika M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem Anal. 2011;22(2):172–80.
Wai CM, Laintz K inventors. Idaho Research Foundation, Inc., assignee. Supercritical fluid extraction. United States patent US 5,356,538. 1994.
Herrero M, Cifuentes A, Ibanez E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae: a review. Food Chem. 2006;98(1):136–48.
Lang Q, Wai CM. Supercritical fluid extraction in herbal and natural product studies—a practical review. Talanta. 2001;53(4):771–82.
Kong Y, Fu Y-J, Zu Y-G, Liu W, Wang W, Hua X, et al. Ethanol modified supercritical fluid extraction and antioxidant activity of cajaninstilbene acid and pinostrobin from pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Food Chem. 2009;117(1):152–9.
Fathordoobady F, Mirhosseini H, Selamat J, Manap MYA. Effect of solvent type and ratio on betacyanins and antioxidant activity of extracts from Hylocereus polyrhizus flesh and peel by supercritical fluid extraction and solvent extraction. Food Chem. 2016;202:70–80.
Tow WW, Premier R, Jing H, Ajlouni S. Antioxidant and antiproliferation effects of extractable and nonextractable polyphenols isolated from apple waste using different extraction methods. J Food Sci. 2011;76(7):T163–72.
Saura-Calixto F, Serrano J, Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007;101(2):492–501.
Chaovanalikit A, Wrolstad R. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J Food Sci. 2004;69(1):67–72.
Chen Z, Bertin R, Froldi G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013;138(1):414–20.
Arts MJ, Haenen GR, Voss HP, Bast A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem Toxicol. 2004;42(1):45–9.
Gülçin Ì, Güngör Şat İ, Beydemir Ş, Elmastaş M, İrfan KÖ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004;87(3):393–400.
Beker BY, Bakir T, Sonmezoglu I, Imer F, Apak R. Antioxidant protective effect of flavonoids on linoleic acid peroxidation induced by copper(II)/ascorbic acid system. Chem Phys Lipids. 2011;164(8):732–9.
Agostini F, Bertussi RA, Agostini G, Atti Dos Santos AC, Rossato M, Vanderlinde R. Supercritical extraction from vinification residues: fatty acids, alpha-tocopherol, and phenolic compounds in the oil seeds from different varieties of grape. Scientific World J. 2012;2012:1–9.
Li B, Smith B, Hossain MM. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep Purif Technol. 2006;48(2):189–96.
Zou Y, Chang SK, Gu Y, Qian SY. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J Agric Food Chem. 2011;59(6):2268–76.
Bonoli M, Marconi E, Caboni MF. Free and bound phenolic compounds in barley (Hordeum vulgare L.) flours. Evaluation of the extraction capability of different solvent mixtures and pressurized liquid methods by micellar electrokinetic chromatography and spectrophotometry. J Chromatogr A. 2004;1057(1–2):1–12.
Soylu S. Accumulation of cell-wall bound phenolic compounds and phytoalexin in Arabidopsis thaliana leaves following inoculation with pathovars of Pseudomonas syringae. Plant Sci. 2006;170(5):942–52.
Walch SG, Tinzoh LN, Zimmermann BF, Stuhlinger W, Lachenmeier DW. Antioxidant capacity and polyphenolic composition as quality indicators for aqueous infusions of Salvia officinalis L. (sage tea). Front Pharmacol. 2011;2:79.
Ercisli S, Tosun M, Karlidag H, Dzubur A, Hadziabulic S, Aliman Y. Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from northeastern Turkey. Plant Food Hum Nutr. 2012;67(3):271–6.
Zhu F, Cai YZ, Sun M, Ke J, Lu D, Corke H. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse Kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum. J Agric Food Chem. 2009;57(14):6082–9.
Zhang Y, Seeram NP, Lee R, Feng L, Heber D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J Agric Food Chem. 2008;56(3):670–5.
Pohanka M, Bandouchova H, Sobotka J, Sedlackova J, Soukupova I, Pikula J. Ferric reducing antioxidant power and square wave voltammetry for assay of low molecular weight antioxidants in blood plasma: performance and comparison of methods. Sensors. 2009;9(11):9094–103.
Wang Y, Yang M, Lee SG, Davis CG, Kenny A, Koo SI, et al. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J Nutri Biochem. 2012;23(12):1725–31.
Zulueta A, Esteve MJ, Frígola A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009;114(1):310–6.
Fu Y-J, Liu W, Zu Y-G, Tong M-H, Li S-M, Yan M-M, et al. Enzyme assisted extraction of luteolin and apigenin from pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Food Chem. 2008;111(2):508–12.
Wu X, Yu X, Jing H. Optimization of phenolic antioxidant extraction from Wuweizi (Schisandra chinensis) pulp using random-centroid optimization methodology. Int J Mol Sci. 2011;12(9):6255–66.
Rene A, Abasq ML, Hauchard D, Hapiot P. How do phenolic compounds react toward superoxide ion? A simple electrochemical method for evaluating antioxidant capacity. Anal Chem. 2010;82(20):8703–10.
Palmer DM, Kitchin JS. A double-blind, randomized, controlled clinical trial evaluating the efficacy and tolerance of a novel phenolic antioxidant skin care system containing Coffea arabica and concentrated fruit and vegetable extracts. J Drugs Dermatol. 2010;9(12):1480–7.
Acknowledgments
The work presentment in this paper was financially sponsored by Higher Education Commission (HEC), Pakistan, under the International Research Support Initiative Program (IRSIP); PIN: IRSIP 24 PS 17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 396 kb)
Rights and permissions
About this article
Cite this article
Mushtaq, M., Sultana, B., Akram, S. et al. Enzyme-assisted supercritical fluid extraction: an alternative and green technology for non-extractable polyphenols. Anal Bioanal Chem 409, 3645–3655 (2017). https://doi.org/10.1007/s00216-017-0309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0309-7