Abstract
Pediatric primary systemic vasculitides are a complex group of diseases. Vasculitis subgroups are mainly determined according to the size of the predominantly affected vessels. In patients with primary systemic vasculitis, the location of vascular involvement, the size of the vessels, the extent of vascular damage, and the underlying pathology determine the disease phenotype and severity. Cardiac involvement is rare in some pediatric vasculitis, such as IgA vasculitis and polyarteritis nodosa, while it is more common in some others like Kawasaki disease and Takayasu arteritis. On the other hand, chronic inflammation in the setting of systemic vasculitis forms a major cardiovascular risk factor. Accelerated atherosclerosis and the tendency to thrombosis are the main issues determining the cardiovascular risks in pediatric systemic vasculitis. Early diagnosis and treatment are essential in these patients to minimize morbidity and mortality. In this review, we aimed to raise physicians’ awareness of cardiac involvement and cardiovascular risks in pediatric patients with primary systemic vasculitis.
Key Points • In children, cardiac involvement is less common in some vasculitides, such as IgAV/HSP vasculitis and polyarteritis nodosa, while it is more common in Kawasaki disease and Takayasu arteritis. • The cardiovascular risk factors are primarily caused by chronic inflammation in pediatric primary systemic vasculitides. • Appropriate management of cardiac involvement and cardiovascular risk factors will improve cardiovascular outcomes in pediatric primary systemic vasculitides. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric primary systemic vasculitides are characterized by idiopathic inflammation of blood vessel walls [1]. Primary systemic vasculitides are mainly subgrouped according to the size of the predominantly affected vessels [1, 2] (Fig. 1). In the most recent nomenclature system, the relatively more common vasculitides are immunoglobulin A vasculitis/Henoch–Schonlein purpura (IgAV/HSP) and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) in small vessel vasculitis, Kawasaki disease (KD) and polyarteritis nodosa (PAN) in medium vessel vasculitis, Takayasu arteritis (TAK) in large vessel vasculitis, and Behçet’s disease (BD) in variable vessel vasculitis group (may affect all sizes of arteries/veins) in children [1].
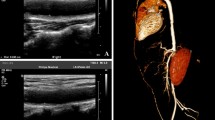
Cardiac involvement as a part of vasculature is not uncommon in systemic vasculitis (Fig. 2). It is more common in some pediatric systemic vasculitides, such as KD and TAK, compared to others. For instance, Kuo et al. [3] reported that 35% of KD patients (n = 341) had coronary artery dilatation during the acute presentation phase, and 4.1% had coronary artery lesions persisting for more than one year. Eleftheriou et al. [4] showed cardiac involvement in five of 11 children with TAK in their case series. On the other hand, there are only rare reports of children with cardiac involvement in IgAV/HSP and AAV [5, 6].
Summary of the cardiac involvement and cardiovascular risk factors in primary systemic vasculitis. AAV, antineutrophil cytoplasmic antibody-associated vasculitis; BD, Behçet’s disease; ECG, electrocardiography; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatous with polyangiitis; IgAV/HSP, immunoglobulin A vasculitis/Henoch–Schonlein purpura; KD, Kawasaki disease; MPA, microscopic polyangiitis; PAN, polyarteritis nodosa; TAK, Takayasu arteritis
Children with primary systemic vasculitis also have a significantly increased risk for cardiovascular events. Prolonged systemic inflammation, along with other risk factors associated with the specific subtype of vasculitis (such as damage to coronary arteries in KD), may lead to accelerated atherosclerosis and increased frequency of cardiovascular events [7].
Early diagnosis and effective management of inflammation and cardiovascular issues are required to improve outcomes in children with primary systemic vasculitis. This review will discuss the incidence/prevalence, pathogenesis, treatment, prevention, and outcomes of cardiac involvement and cardiovascular risk factors in pediatric patients (0–18 years) with primary systemic vasculitis. We will focus on the relatively more common vasculitides as IgAV/HSP, AAV, KD, PAN, TAK, and BD.
Search strategy
We reviewed the literature using Pubmed/MEDLINE, Scopus, and Web of Science databases until August 2022, using the following keywords; “immunoglobulin A vasculitis/Henoch-Schonlein purpura,” “antineutrophil cytoplasmic antibody-associated vasculitides,” “Kawasaki disease,” “polyarteritis nodosa,” “Takayasu arteritis,” “Behçet’s disease,” “cardiac involvement,” and “cardiovascular risk factors,” according to the published guideline [8]. Preference was frequently given to the sources published within the past 10 years. We searched the bibliographies of the retrieved articles written by experts in cardiac involvement and cardiovascular risk factors in pediatric primary systemic vasculitides. The research was limited to articles in English. The articles, which include data about cardiac involvement or cardiovascular risk factors in pediatric primary systemic vasculitides, have been evaluated meticulously.
Cardiac involvement and cardiovascular risk factors in small vessel vasculitides
IgA vasculitis/Henoch–Schonlein purpura (IgAV/HSP)
Immunoglobulin A vasculitis, previously known as HSP, is a non-granulomatous systemic vasculitis characterized by IgA-containing immune complex deposits of small vessel walls [1]. Approximately 90% of the patients are children under 10 years of age [9]. Its incidence is 3–55/100.000 children, and its prevalence is 6.1–20.4/100.000 in children [10]. The most common and characteristic symptoms of IgAV/HSP are non-thrombocytopenic petechiae and purpura (95%) [5]. Gastrointestinal and renal involvement are among the most common manifestations of this disease after skin lesions [5]. According to the European League Against Rheumatism/Pediatric Rheumatology International Trials Organization/Pediatric Rheumatology European Society (EULAR/PRINTO/PRES) (Ankara 2008) criteria, a patient is classified with IgAV/HSP in the presence of a vasculitic purpuric rash with lower limb predominance (mandatory) together with one of four criteria: (a) abdominal pain, (b) histopathology (IgA), (c) arthritis or arthralgia, (d) renal involvement [11]. The disease course is usually self-limited and benign in children [5]. Corticosteroids are mostly required in case of severe cutaneous lesions or gastrointestinal system involvement [5]. Other immunosuppressive drugs could be used in patients with renal or other major organ involvement.
Cardiac involvement
Cardiac involvement is rare in IgAV/HSP. Valvulitis, myocardial damage, myocardial infarction, atrial and ventricular dilation, arrhythmia, atrioventricular block, coronary artery aneurysm, pericardial effusion, and tamponade have been reported [5, 12, 13].
There are only case reports about cardiac involvement in the literature. A 6-year-old boy with rash, arthritis, proteinuria, hematuria, and chest pain had bilateral coronary artery dilatation, thickening of the ventricular wall, and mitral and tricuspid regurgitation [12]. The renal biopsy result of the patient was consistent with IgAV/HSP nephritis. Clinical findings of neither Kawasaki disease nor PAN were detected in this patient. Methylprednisolone, acetylsalicylic acid, and mycophenolate mofetil were initiated. Although nephritis improved with this treatment, coronary arterial findings progressed. Warfarin therapy was added to the treatment, and glucocorticoid therapy was gradually discontinued during the follow-up [12]. Another report revealed a coronary artery aneurysm in a 9-year-old boy who presented with fever, headache, abdominal pain, arthritis, and palpable purpura [14]. He was treated with intravenous immunoglobulin (IVIG), infliximab, and acetylsalicylic acid. Coronary arteries were normal three months later than this treatment [14]. In another child with IgAV/HSP, intracardiac thrombus and pericardial effusion were present in the right atrium [15]. A 17-year-old patient with fever, abdominal pain, nausea, vomiting, diarrhea, arthralgia, shortness of breath, rash, and chest pain diagnosed with IgAV/HSP also detected left ventricular dilatation in his echocardiography (ECHO) [16]. The diagnosis of IgAV/HSP was confirmed by skin biopsy. Pulse intravenous methylprednisolone followed by oral prednisone treatment was started. At the end of three months, renal findings improved, but there is no information about the cardiac outcome after this treatment [16].
Cardiovascular risk factors
Although the long-term risk of atherosclerosis of IgAV/HSP is not yet fully known, there may be no susceptibility to atherosclerosis in pediatric patients since the disease usually causes short-term inflammation. High expression of vascular endothelial growth factor (VEGF), a molecule with pro-atherogenic and pro-angiogenic properties, has been demonstrated in patients with vasculitis, especially in children with IgAV/HSP in the acute phase of the disease [17]. However, whether this may be a factor increasing the risk of atherosclerosis in IgAV/HSP patients remains unknown. Tracy et al. [18] reported a diagnosis of ischemic heart disease in 53 (2%) of 2689 adult-onset IgAV/HSP patients. However, they found no significant association between IgAV/HSP and ischemic heart disease. Canpolat et al. [19] reported that a pediatric patient previously diagnosed with IgAV/HSP developed myocardial infarction secondary to coronary artery thrombosis during the follow-up.
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAVs)
ANCA-associated vasculitides include granulomatous with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA), and microscopic polyangiitis (MPA). The annual incidence of AAV is ~ 0.5 per million children [20].
Although GPA is a common AAV subtype in adults, it is infrequent in children. According to the EULAR/PRINTO/PRES (Ankara 2008) criteria, three of the following six criteria are required for pediatric GPA classification: histopathology, upper airway involvement, laryngo-tracheo-bronchial stenoses, pulmonary involvement, ANCA positivity, and renal involvement [11]. Patients often present with findings secondary to upper respiratory tract involvement [21]. Common pulmonary manifestations include chronic cough, shortness of breath, hemoptysis, or alveolar hemorrhage. And the most frequent renal manifestation of GPA is rapidly progressive glomerulonephritis, leading to chronic kidney disease or end-stage renal disease [21]. PR3-ANCA (~ 80%) positivity is frequently present in GPA patients, and MPO-ANCA (10–15%) may also be detected in some patients [22]. In one of the largest cohorts to review characteristics of pediatric GPA patients, 183 GPA patients (62% female) were evaluated [23]. The mean age of the patients at the diagnosis was 13.4 ± 3.2 years. The involved systems were constitutional (88%), renal (83%), pulmonary (74%), upper airways (70%), and musculoskeletal (65%). Only 5% had cardiovascular system involvement.
Eosinophilic granulomatosis with polyangiitis is a small vessel vasculitis described especially in patients with asthma, ear, nose, and throat involvement, and blood and tissue eosinophilia [24]. In 1990, the American College of Rheumatology (ACR) defined the following six criteria for EGPA: asthma, eosinophilia > 10%, neuropathy, unfixed lung infiltrates, paranasal sinus abnormalities, and extravascular eosinophils on biopsy. In the presence of at least four of these criteria, EGPA can be diagnosed [24]. ANCA positivity (usually MPO-ANCA) is detected in ~ 40% of patients [25]. In the largest pediatric EGPA series (n = 14; F/M:1.3), the median age at diagnosis was 12.3 years [26]. All cases had respiratory involvement. The other organ systems most frequently involved were the upper airway (85%), skin (71%), digestive tract (64%), and heart (57%).
Microscopic polyangiitis is predominantly a disease of adults, and it is rarely seen in childhood. Rapidly progressive pauci-immune glomerulonephritis and alveolar hemorrhage are the most common clinical findings of MPA [27]. Also, the nervous system, musculoskeletal system, skin, cardiac system, gastrointestinal system, and eyes could be affected. ANCA is positive in 90% of the patients, and 60% of these have MPO-ANCA [27]. In the largest pediatric cohort of MPA (n = 48), the mean age at disease onset was 10.8 years, and girls outnumbered boys (F/M = 1.8) [23]. Constitutional findings and renal involvement were the most common clinical findings, and approximately half of the patients had gastrointestinal, musculoskeletal, and cutaneous involvement [23].
Corticosteroids and immunosuppressive drugs such as cyclophosphamide, mycophenolate mofetil, azathioprine, methotrexate, or rituximab are used in the treatment of AAV [28]. It is noteworthy that corticosteroids alone are usually sufficient in EGPA patients without severe organ involvement [28].
Cardiac involvement
Among AAV, cardiac involvement is relatively common in EGPA, while it is rare in GPA and MPA.
While cardiac involvement is present in 40–60% of adults with EGPA, it is less frequent among children [29]. In children with EGPA, pericardial effusion, cardiomyopathy, valvular insufficiency, myocardial infarction, coronary artery dilatation, pulmonary hypertension, cardiomyopathy, pericarditis, and pulmonary embolism can be seen [30]. ANCA negativity is correlated with a higher frequency of cardiac involvement [31].
Cardiovascular manifestations were observed in 5–6% of pediatric GPA patients [23, 32]. These were mainly venous thrombosis. In addition, pericarditis, myocardial infarction, arrhythmias, and heart valve involvement could be seen [33]. Varnier et al. [34] reported peripheral gangrene and sterile vegetation in the tricuspid valve of a 16-year-old boy with GPA. There are also a few reports of heart block and endocarditis in pediatric GPA [35].
Data regarding cardiac involvement in pediatric MPA is scarce [36]. Heart failure, pericarditis, valvular insufficiency, cardiomyopathy, heart block, myocardial infarction, and cardiac tamponade can be seen in adult patients [36]. In a study that included 48 pediatric MPA patients, cardiomyopathy was reported in two (4.1%) patients [23].
Cardiovascular risk factors
The chronic inflammatory nature of AAVs could contribute to the increased risk of atherosclerosis. In addition, atherosclerosis may occur due to endothelial dysfunction resulting from oxidative stress caused by ANCA-induced neutrophils. If vascular damage is not prevented by early immunosuppression in AAV, atherosclerosis can be an inevitable outcome [37]. Traditional cardiovascular risk factors (hypertension, diabetes mellitus, metabolic syndrome) are also more common in GPA patients [38]. Although there are no studies about the early occurrence of atherosclerosis in pediatric AAV, accelerated atherosclerosis has been demonstrated in adult studies, mainly in patients with GPA [39]. In a study conducted by Hajj-Ali et al. [39] on 46 adult patients with GPA, they evaluated the relationship between GPA and the development of subclinical atherosclerosis. The patients’ carotid artery intima-media thickness (IMT) was detected to be higher, especially in those with advanced age and high diastolic blood pressure. They also found that circulating microparticles activate vascular endothelial cells and platelets in vitro. Thus, they concluded that the cumulative burden of systemic inflammation in GPA correlated with the development of subclinical atherosclerosis [39].
Patients with AAV have also an increased risk of arterial thromboembolism [38]. This increase in thrombotic events is not only due to endothelial activation and inflammation but also to a higher prevalence of cardiovascular risk factors [38]. Myocardial infarction and ischemic stroke risk are also quite high in AAV patients due to accelerated atherosclerosis and thromboembolic events [40].
In addition, long-term use of corticosteroids in the treatment of AAV may also cause cardiovascular events. Pediatric patients with AAV usually require more than two years of treatment with glucocorticoids to achieve remission and prevent relapse [41]. Although the main side effects of glucocorticoids on the cardiovascular system are dyslipidemia and hypertension, corticosteroid use may predispose patients to coronary artery disease, especially in the long-term [42].
Cardiac involvement and cardiovascular risk factors in medium vessel vasculitides
Kawasaki disease (KD)
Kawasaki disease is a medium vessel vasculitis of early childhood [1]. The highest incidence is in Japan (330/100000), followed by Korea and Taiwan [43]. According to the 2017 American Heart Association (AHA) criteria, fever (lasting at least five days) and four of the following five criteria are required for diagnosing KD: polymorphous rash, lip, and oral mucosal changes, bilateral nonexudative conjunctivitis, unilateral cervical lymphadenopathy, and extremity changes such as edema or peeling [44]. IVIG and acetylsalicylic acid are used as initial therapy in KD [44]. Other drugs such as corticosteroids, cyclosporine, anakinra, and infliximab could be used in IVIG-resistant KD [44].
Cardiac involvement
Coronary artery involvement is the main factor affecting morbidity and mortality in KD. During the acute phase of the disease, dilatations and aneurysms may occur in the coronary arteries. It is critical to prevent coronary arterial involvement and damage by controlling inflammation at this phase [45]. Administration of IVIG within the first 10 days after the onset of KD reduces the prevalence of coronary artery aneurysms [45]. While coronary artery aneurysm develops in 25% of untreated patients, this rate decreases to 4–5% in patients who receive timely and appropriate treatment [45, 46].
Coronary artery involvement rates in KD differ between studies. In the Taiwan cohort [47], which included 13,290 patients, coronary aneurysm was seen in approximately 9% of the patients. In a Japanese cohort of 2628 patients [48], only IVIG treatment was given to 1094 patients, and IVIG and corticosteroid treatment was given to 724 of them. Coronary artery involvement was observed in 9% of the group receiving only IVIG and in 5.9% of the group receiving IVIG and corticosteroids.
Myo-pericarditis, congestive heart failure, valve insufficiency, and arrhythmia have also been reported in KD patients [49]. Hyperdynamic precordium and tachycardia may be seen during acute disease, small pericardial effusions, and rarely pericardial tamponade may occur [45]. Arrhythmia may be detected due to functional abnormalities of the sinus node and atrioventricular node (44). Long PR interval, nonspecific ST and T wave changes, and low voltage could be observed in the electrocardiography (ECG) if myocardial or pericardial involvement is present [49].
The prognosis of KD is determined by the presence and severity of cardiac involvement. The mortality rate is low (< 0.5%) in KD; however, the first year of the disease is quite risky due to acute myocardial infarction in patients with giant aneurysms [46]. Severe myocarditis causing hemodynamic deterioration and/or arrhythmias may also lead to death even in the first week of the disease [45].
Cardiovascular risk factors
Coronary aneurysms in KD usually regress to normal lumen diameter by the proliferation of myofibroblasts. However, endothelial dysfunction persists in these affected segments, even after regression [46]. Stenosis may develop over time at the proximal and distal ends of aneurysms, and the risk of myocardial ischemia may increase [44]. Myocardial ischemia in patients after KD may result from structural abnormalities, including coronary artery stenoses or obstructions, and extreme sluggishness of flow through aneurysms, particularly those in distal segments [44]. It may also arise from functional abnormalities, including vasospasm, endothelial dysfunction, and impaired myocardial flow reserve. Thromboprophylaxis is the most critical point in treating coronary artery stenosis and myocardial ischemia [45].
Coronary artery aneurysm was detected in 7.9% of 580 patients under the age of 40 who underwent angiogram due to myocardial ischemia [50]. In a review of 74 patients with a previous history of KD, the patients presented with cardiac symptoms at an average age of 24 years [51]. Myocardial infarction was detected in more than half while sudden death occurred in 16% of these patients. The diagnoses of patients were confirmed by autopsy findings [51]. In another study, myocardial infarction was seen in 16% of 76 patients diagnosed with KD in childhood, and 67% required a catheter and surgical intervention during the 25-year follow-up [52].
A study examining the long-term outcomes of 245 patients showed that the rates of no cardiac involvement and survival at 30th years after KD diagnosis were 36% and 90%, respectively [53]. Another study showed that 5% of young adults evaluated for myocardial ischemia might have a history of KD [54].
Atherosclerosis can occur in patients with KD as a result of several factors [55]. First, the arterial structure may change as a result of arterial damage caused by the disease process. This structural change may predispose to atherosclerosis. Second, possible ongoing inflammation could promote atherosclerosis and cause alterations in traditional and nontraditional atherosclerosis risk factors. Third, patients who had KD may be predisposed to having other types of atherosclerosis risk factors. Cho et al. [56] categorized the patients into three groups; group A, 19 KD patients with coronary arterial lesions that persisted or regressed; group B, 49 KD patients without coronary artery lesions; and group C, 30 healthy children. In groups A and B, the mean time after KD diagnosis was 68.95 ± 31 and 52.29 ± 26.51 months, respectively. The levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, and apolipoprotein B were significantly higher in group A than in group C. Furthermore, the levels of homocysteine and the aortic pulse wave velocity were significantly higher in groups A and B than in the group C. Their results suggest that high lipid profiles and arterial stiffness in children with a history of KD show an increased risk of early atherosclerosis [56]. In the study by Cheung et al. [57], apolipoprotein B levels and pulse wave velocity were higher in KD patients with and without coronary arterial lesions than in healthy controls. Thus, the authors concluded that an adverse cardiovascular risk profile, characterized by pro-atherosclerotic changes in lipid profile and increased arterial stiffness, occurs in children after KD [57].
Tsuda et al. evaluated 60 patients with KD-related myocardial infarction [58]. The median age at KD diagnosis was 1.4 (0.18–12) years, and the median age at the time of myocardial infarction was 2 (0.25–33) years. Giant aneurysms (≥ 8 mm) had been detected in 51 patients during the acute phase of KD; nine of these patients had experienced peripheral artery aneurysms and coronary aneurysms. Fifteen of 60 patients died during follow-up [58]. In a study that evaluated 1356 patients (median age at diagnosis was 3.1 years and median follow-up duration was 1.1 years), thrombosis, stenosis, intervention, myocardial infarction, and death were reported in 20%. Of note, this ratio was 18% for patients with a Z-score ≥ 10 [59]. In another study, Tsuda et al. [60] showed that 3% of 562 patients had newly developed dilated coronary artery lesions median 11 years later than the KD diagnosis. In summary, KD seems to cause an increased risk of early atherosclerosis and myocardial infarction.
Polyarteritis nodosa (PAN)
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically affects medium-sized muscular arteries [11]. It constitutes 9% of all childhood vasculitides and is the third most common primary systemic vasculitis after IgAV/HSP and KD [61]. To classify a child as having PAN according to the EULAR/PRINTO/PRES (Ankara 2008) criteria, a biopsy showing a small and mid-size artery necrotizing vasculitis or angiographic abnormalities is required in addition to the ≥ 2 of the following features: skin involvement, systemic hypertension, mononeuropathy or polyneuropathy, abnormal urine analysis or impaired renal function, testicular pain or tenderness, signs or symptoms suggesting vasculitis of any other major organ system such as gastrointestinal, cardiac, pulmonary, or central nervous systems [11]. PAN can affect almost any organ. In children, cardiac, respiratory, and neurological systems are less frequently affected, while skin, musculoskeletal, and gastrointestinal system involvements were relatively more common [2].
Cardiac involvement
In 5–20% of adult cases, PAN could cause cardiac involvement with ECG abnormalities, coronary arteritis, congestive heart failure, pericarditis, and myocarditis resulting in cardiomyopathy [62]. Myocardial ischemia or infarction, typically resulting from coronary artery aneurysms or stenosis, has also been reported [63]. It is noteworthy that cardiac involvement was present in 81.1% of adult patients in a PAN autopsy study published in 1985 [64].
In children, cardiac involvement is relatively rare. In our recent systematic review, cardiac involvement was present in 15 out of 164 reported pediatric PAN patients [65]. Cardiac involvements were pericarditis, valvular heart disease, and coronary artery aneurysm in these patients. In the largest study, including 110 children with PAN (63 with systemic PAN), 14% of patients exhibited cardiac involvement [66]. Unfortunately, the details of cardiac involvement were not mentioned in this report. In another pediatric study including 69 patients with systemic PAN, cardiac involvement was observed in three patients (4%) [67]. Two of these patients had valvular heart disease, and one had pericarditis.
Cardiovascular risk factors
Endothelial function is significantly impaired in patients with primary systemic necrotizing vasculitis such as PAN [68]. It has been suggested that diffuse endothelial dysfunction arises from a systemic response that may result from primary vasculitis but differs from the local inflammatory vasculitic process [68]. However, vasculitis involving the coronary arteries can also lead to atherosclerosis and myocardial infarction, besides vascular damage due to systemic inflammation. Secondary changes in involved arteries are common and include characteristic aneurysm formation, intravascular thrombosis, and segmental lumen narrowing [69]. In addition to all these, persistent systemic inflammation due to active vasculitis also contributes to accelerated atherosclerosis in refractory patients who are not treated timely or who do not respond to treatment [68].
There are limited data in the literature regarding accelerated atherosclerosis in PAN [70, 71]. Yanagawa et al. [70] reported premature atherosclerotic coronary disease, likely secondary to coronary arteritis in an adult PAN patient. Ribeiro et al. [71] found atherosclerotic lesions in medium-sized vessels in the histopathological examination of an adult PAN case. Early and effective treatment will allow normalization of endothelial function and thus, reduce vascular damage in the long-term in PAN.
It is noteworthy that prolonged corticosteroid treatment in PAN could also contribute to the risk of cardiovascular events, as discussed above in AAV.
Cardiac involvement and cardiovascular risk factors in large vessel vasculitis
Takayasu arteritis (TAK)
Takayasu arteritis is a large vessel vasculitis that mainly affects the aorta and its major branches [11]. The estimated incidence is 0.4–6.3 cases per million in childhood [72]. In the pediatric population, the mean age of disease onset is 12 years [72]. According to the EULAR/PRINTO/PRES (Ankara 2008) criteria, a pediatric patient is classified with TAK when she/he has angiographic aorta findings showing aneurysm/dilatation in its major branches and pulmonary arteries, narrowing, occlusion, or a thickened arterial wall (mandatory), and one of the following five features: pulse deficit or claudication, four-limb blood pressure discrepancy (> 10 mmHg), bruits, hypertension, and elevated acute-phase reactants [11]. Corticosteroids and immunosuppressive drugs, including methotrexate, cyclophosphamide, and biologic drugs, constitute TAK treatment’s mainstay [73, 74].
Cardiac involvement
Takayasu arteritis mainly affects large arteries, and the heart is the continuum of these large vessels. Thus, cardiac involvement is not uncommon in TAK. Aortic lesions, pulmonary artery involvement, coronary artery disease, and heart failure occur at a significantly high frequency in TAK patients [75].
Takayasu arteritis is one of the most common causes of aortitis [76]. Aortic dissection, aortic rupture, aortic thrombosis, acute coronary syndrome, and aortic regurgitation may also be seen secondary to aortitis in patients with TAK [76]. Pulmonary artery involvement in TAK manifests as pulmonary hypertension with a poor response to treatment [77]. In a study by Cakar et al. [78], including 19 pediatric TAK patients, one of the two cases with pulmonary artery involvement died.
Coronary artery disease in TAK has a poor prognosis [79]. About 10% of adult patients with TAK have coronary arterial involvement, but the prevalence in children is unknown [79]. Coronary artery involvement may cause myocardial infarction, angina, or heart failure. Hassan et al. [80] presented an 11-year-old boy with TAK who developed myocardial infarction and died. Wolf et al. [81] reported a pediatric TAK patient who had a left coronary artery aneurysm and died of acute myocardial infarction. There were diffuse vasculitis of the aorta, pulmonary arteries, and coronary vessels at the patient’s autopsy.
Heart failure from TAK is common in children and a significant cause of mortality [79]. Hypertension is the most common reason for heart failure. Myocarditis or involvement of coronary arteries, valves, or pulmonary arteries may also cause heart failure [79]. Das et al. [82] reported a 4-year-old girl who presented with features of heart failure and was diagnosed with TAK.
Half of the children with heart failure have mitral regurgitation, and aortic valve involvement can also be seen [83]. Aortic regurgitation is the most common valve lesion in adult TAK patients [83].
Cardiovascular risk factors
Accelerated atherosclerosis has also been demonstrated in TAK patients [84]. Recent data support the central role of inflammation in all stages of atherogenesis, from endothelial dysfunction to plaque rupture. In addition, both prolonged use of corticosteroids and traditional atherosclerotic risk factors may contribute to atherosclerosis [85]. Effective suppression of disease activity and effective control of traditional atherosclerotic risk factors are required to prevent atherosclerosis. However, since atherosclerosis does not usually cause symptoms during childhood, studies in the literature are restricted to adult patients. Seyahi et al. [84] reported atherosclerotic plaques in 27% of 30 adult TAK patients evaluated by carotid ultrasonography. There are also studies reporting atherosclerotic changes in autopsy reports of young patients with TAK [86]. In addition, an increased incidence of metabolic syndrome, hypertension, and hypertriglyceridemia was reported in TAK [85].
Cardiac involvement and cardiovascular issues in variable vessel vasculitis
Behçet’s disease (BD)
Behçet’s disease is a chronic inflammatory disease that can affect any type and size of the vessel, particularly the veins, and manifests with recurrent oral and genital ulcers, accompanied by the involvement of skin, eyes, joints, and gastrointestinal and central nervous systems [87]. In 4–26% of BD patients, the disease onset is during childhood [88]. There is no specific diagnostic test for BD [89]. According to pediatric BD criteria, patients with ≥ 3 of the following criteria are classified as having BD: recurrent oral aphthae (at least three times a year), genital ulceration or aphthae (with scarring), skin involvement (necrotic folliculitis, acne-like lesions, erythema nodosum), eye involvement (anterior uveitis, posterior uveitis, retinal vasculitis), neurological (except isolated headache), and vascular findings (venous thrombosis, arterial thrombosis, arterial aneurysm) [87]. In the largest pediatric BD cohort (n = 205), the age at symptom onset was around 11 years [90]. Oral (99.5%) and genital aphthosis (65.4%) and skin manifestations (48.8%) were the most common features [90].
In BD, venous lesions are more common than arterial occlusions and arterial aneurysms [91]. However, although rare, arterial complications significantly affect the course of the disease. Corticosteroids and immunosuppressive drugs constitute the mainstay of BD treatment in case of major organ involvement [92, 93]. The use of anticoagulants in the treatment of BD thromboses currently remains controversial.
Cardiac involvement
Cardiovascular involvement is present in less than 10% of pediatric patients [93]. The cardiac involvement manifests with pericarditis, endocarditis, intracardiac thrombosis, pulmonary artery involvement, myocardial infarction, endomyocardial fibrosis, or left ventricular aneurysm [91].
Pericarditis is a common cardiac finding in children, variably manifesting as recurrent, asymptomatic pericardial effusion [91]. Myocarditis and cardiomyopathy can also be seen rarely in children with BD.
Intracardiac thrombosis is one of the significant cardiac findings of BD [94]. And it is very often associated with other venous thromboses or pulmonary artery aneurysms [92]. Turkucar et al. [94] reported a pediatric BD patient who developed a thrombus in the right ventricular cavity and secondary pulmonary embolism. The patient responded well to early-onset immunosuppressive and corticosteroid therapy.
Coronary artery aneurysms can also be rarely seen in BD [92]. Cook et al. [95] reported an adolescent boy with BD who developed a large pseudoaneurysm of the left anterior descending coronary artery requiring a coronary artery bypass graft. In another case report [96], a teenage girl with a giant coronary aneurysm who developed life-threatening, persistent BD was presented. Thus, BD should be considered in the differential diagnosis of pediatric patients with a history of arterial, venous, and cardiac thrombosis or arterial aneurysms [86, 88].
Cardiovascular risk factors
In an inflammatory vascular disease such as BD, the endothelium may be affected during active or inactive disease, eventually contributing to atherosclerosis [97]. Disease duration, chronic corticosteroid use, and persistent inflammatory activity in BD can be counted among the causes of atherosclerosis [98]. In addition, classical risk factors such as hypertension and high lipid levels can contribute to the development of atherosclerosis. Despite all these reasons, atherosclerosis is extremely rare in BD among primary systemic vasculitides. In a study by Hassan et al. [99] in 30 BD patients, atherosclerotic plaques were found in five patients (16.7%). Also, carotid IMT measurements were higher in BD patients than in healthy controls, forming an independent risk factor for the development of atherosclerosis [97].
Thrombotic events are observed in BD [100]. Although BD more commonly causes venous thrombosis, an intracardiac thrombus is also one of the rare but severe complications of BD [94]. The underlying mechanism of thrombosis in these patients is unclear. Endothelial damage/activation is probably a significant factor affecting the increased incidence of thrombosis in BD [100]. In addition, abnormal fibrinolysis, protein C and S deficiencies, and altered platelet function may contribute to the development of thrombosis in BD [100].
There are also several reports of myocardial infarction in adults with BD, but causality remains unclear [101].
Cardiovascular issues associated with drugs used in vasculitis treatment
Treatment strategies vary according to the vasculitis subtype and the severity of vasculitis. For instance, IgAV/HSP patients with a mild disease course can be followed without treatment or with nonsteroidal anti-inflammatory drugs alone. On the other hand, IVIG and acetylsalicylic acid (and corticosteroids in high risk patients) are primary treatment in KD [44]. Corticosteroids and other immunosuppressive drugs including biologics could be used while treating IVIG-resistant KD cases. Patients with other vasculitis subtypes are usually treated with corticosteroids in addition to immunosuppressive drugs such as cyclophosphamide, azathioprine, mycophenolate mofetil, or methotrexate [102]. Less commonly, biologic drugs such as anti-tumor necrosis factor (TNF) agents, abatacept, rituximab, or tocilizumab are used in the treatment.
Corticosteroids are the mainstay for treatment of vasculitides. The usual practice is to taper the dose of corticosteroids after attaining remission; however, patients with systemic vasculitis are likely to be on low-dose corticosteroid therapy for several years. Chronic corticosteroid use is associated with hypertension, diabetes mellitus, congestive heart failure, and hyperlipidemia, all of which adversely influence cardiovascular outcomes [95]. The use of high-dose and long-term corticosteroids in the treatment of pediatric vasculitis also increases the risk for cardiovascular events [102]. Therefore, patients on corticosteroid therapy should be actively screened for cardiovascular risk factors and managed effectively. Furthermore, corticosteroids should be used wisely by fine tuning the balance between its benefits and complications.
The other immunosuppressive drugs used in treating primary systemic vasculitides such as cyclophosphamide and mycophenolate mofetil may also be related to cardiovascular events [102]. Some reports suggest a risk of irreversible cardiomyopathy and conduction block following cyclophosphamide therapy, but these side effects are extremely rare [103]. Mycophenolate mofetil may cause arrhythmia. This side effect is also very rare [104]. On the other hand, methotrexate and azathioprine have been shown to reduce cardiovascular risks and improve cardiac function [105, 106].
In biologic drugs, rituximab is frequently used in the management of AVV. There are some reports about the occurrence of ischemic coronary events and cardiac arrhythmias following rituximab infusion [107]. On the other hand, some studies have shown favorable alterations in the lipid profile with rituximab treatment [102]. Anti-TNF agents, which are rarely used as induction therapy in AAV and have a potential role in the treatment of IVIG-refractory KD, have been associated with decreased risk of cardiovascular events by virtue of a reduction in systemic inflammation [108]. Tocilizumab, the interleukin 6 receptor antagonist which may be used in TAK, also seems to favorably modulate overall cardiovascular risk in spite of proatherogenic alteration of the lipid profile [109]. On the other hand, modulation of cardiovascular risk with abatacept is not clear [102]. Being aware of the possible complications associated with treatment is mandatory to modify the therapeutic approaches effectively in vasculitis.
How to improve cardiac outcomes in pediatric primary systemic vasculitis
Primary systemic vasculitides are relatively rare conditions in children but carry a significant risk of morbidity and mortality despite novel therapeutic options [110]. Therefore, early recognition and treatment of these diseases are pretty important. With timely and appropriate treatment, disease complications and conditions that may develop secondary to chronic inflammation can be prevented. For instance, treatment with IVIG and acetylsalicylic acid in the early period of KD significantly reduces the risk of coronary artery aneurysms [110]. The diagnosis could be challenging, especially for patients who present with predominant cardiac features. Awareness of the specific cardiac involvements of different vasculitis subtypes is essential. This awareness secures early diagnosis, which leads to effective control of inflammation.
Corticosteroids should be tapered and discontinued as soon as possible in these patients. In addition, studies should increase for finding alternatives to corticosteroid treatment in vasculitis. Jayne et al. [111] reported that avacopan and corticosteroid treatments showed similar efficacy in the early period (at 26th week) in patients with AAV. At the same time, the remission rate was higher with avacopan than corticosteroids in the later period (52nd week).
Cardiovascular risk factors other than chronic inflammation, the leading cause of cardiovascular events in vasculitis, should also be managed well. Lifestyle changes, such as smoking cessation, prevention of obesity, and a healthy diet that eliminate the factors promoting atherosclerosis, are essential, especially in KD patients with coronary artery lesions [112]. In addition, implementing a screening and management program for improving modifiable cardiovascular risk factors like hypertension, sedentary lifestyle, and dyslipidemia may be beneficial for patients with vasculitis. In the long-term follow-up, blood pressure, body mass index, and waist circumference should be followed; diet, physical activity, smoking, and lipid profile should be checked at least once a year in all patients with or without coronary artery involvement. With the improvement of traditional cardiovascular risk factors and the close follow-up of the clinical and imaging findings of the patients, cardiovascular events will be significantly reduced.
Conclusion
This review provides a comprehensive view and extensive literature digest on the cardiac involvement and cardiovascular risk factors in pediatric primary systemic vasculitis. The rare occurrence of cardiac involvement in some pediatric vasculitis subtypes and restriction to only English articles in literature search were the main limitations. It is essential to be aware of the different presentations and cardiac diseases caused by systemic vasculitis to secure early diagnosis and effective treatment. Along with vascular lesions that usually change according to the vasculitis subtypes, chronic inflammation and treatment complications also contribute to the cardiovascular risk factors in these patients. Longitudinal and prospective studies analyzing the cardiac outcome in adult patients with vasculitis during childhood will provide further and valuable evidence.
References
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F et al (2013) 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65:1–11. https://doi.org/10.1002/art.37715
Eleftheriou D, Batu ED, Ozen S, Brogan PA (2015) Vasculitis in children. Nephrol Dial Transplant 30(Suppl 1):i94-103. https://doi.org/10.1093/ndt/gfu393
Kuo HC, Yu HR, Juo SH, Yang KD, Wang YS, Liang CD et al (2011) CASP3 gene single-nucleotide polymorphism (rs72689236) and Kawasaki disease in Taiwanese children. J Hum Genet 56:161–165. https://doi.org/10.1038/jhg.2010.154
Eleftheriou D, Varnier G, Dolezalova P, McMahon AM, Al-Obaidi M, Brogan PA (2015) Takayasu arteritis in childhood: retrospective experience from a tertiary referral centre in the United Kingdom. Arthritis Res Ther 17:36. https://doi.org/10.1186/s13075-015-0545-1
Du L, Wang P, Liu C, Li S, Yue S, Yang Y (2021) Multisystemic manifestations of IgA vasculitis. Clin Rheumatol 40:43–52. https://doi.org/10.1007/s10067-020-05166-5
Theisen A, Rodriguez M (2022) ANCA-associated vasculitis with cardiac valve vegetations in two teenage males: a case report and literature review. Pediatr Rheumatol 28:94. https://doi.org/10.21203/rs.3.rs-1178656/v1
Cohen TJ (2009) Translational mini-review series on immunology of vascular disease: accelerated atherosclerosis in vasculitis. Clin Exp Immunol 156:377–385. https://doi.org/10.1111/j.1365-2249.2009.03885.x
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Oni L, Sampath S (2019) Childhood IgA vasculitis (Henoch Schonlein Purpura)—advances and knowledge gaps. Front Pediatr 7:257. https://doi.org/10.3389/fped.2019.00257
Watts RA, Hatemi G, Burns JC, Mohammad AJ (2022) Global epidemiology of vasculitis. Nat Rev Rheumatol 18:22–34. https://doi.org/10.1038/s41584-021-00718-8
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R et al (2010) EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: final classification criteria. Ann Rheum Dis 69:798–806. https://doi.org/10.1136/ard.2009.116657
Kang Z, Wu W, Xun M, Ding Y, Li Z (2022) Henoch-Schönlein purpura/IgA vasculitis complicated by coronary artery aneurysm: a case report and literature review. Front Pediatr 9:781106. https://doi.org/10.3389/fped.2021.781106
Shah S, Hata J (2021) A rare and severe presentation of henoch-schönlein purpura in an adolescent with crescentic glomerulonephritis, arrhythmia, acute gastrointestinal bleed, and neurological complications. Cureus 13:e14169. https://doi.org/10.7759/cureus.14169
Bloom JL, Darst JR, Prok L, Soep JB (2018) A case of Henoch-Schonlein purpura with dilated coronary arteries. Pediatr Rheumatol Online J 16:54. https://doi.org/10.1186/s12969-018-0270-9
Yılmaz N, Yüksel S, Becerir T, Girişgen İ, Ufuk F, Gürses D et al (2021) Myocarditis and intracardiac thrombus due to Henoch-Schönlein purpura: case report and literature review. Clin Rheumatol 40:1635–1644. https://doi.org/10.1007/s10067-020-05317-8
Zaidi M, Singh N, Kamran M, Ansari N, Nasr S, Acharya A (2008) Acute onset of hematuria and proteinuria associated with multiorgan involvement of the heart, liver, pancreas, kidneys, and skin in a patient with Henoch-Schönlein purpura. Kidney Int 73:503–508. https://doi.org/10.1038/sj.ki.5002662
Topaloglu R, Sungur A, Baskin E, Besbas N, Saatci U, Bakkaloglu A (2001) Vascular endothelial growth factor in Henoch-Schonlein purpura. J Rheumatol 28:2269–2273
Tracy A, Subramanian A, Adderley NJ, Cockwell P, Ferro C, Ball S et al (2019) Cardiovascular, thromboembolic and renal outcomes in IgA vasculitis (Henoch-Schönlein purpura): a retrospective cohort study using routinely collected primary care data. Ann Rheum Dis 78:261–269. https://doi.org/10.1136/annrheumdis-2018-214142
Canpolat U, Yorgun H, Şahiner L, Kabakçi G (2012) Myocardial infarction due to coronary thrombosis in a patient with Henoch-Schönlein purpura. Herz 37:801–803. https://doi.org/10.1007/s00059-012-3597-x
Sacri A-S, Chambaraud T, Ranchin B, Florkin B, Sée H, Decramer S et al (2015) Clinical characteristics and outcomes of childhood-onset ANCA-associated vasculitis: a French nationwide study. Nephrol Dial Transplant 30(Suppl 1):i104-112. https://doi.org/10.1093/ndt/gfv011
Idolor ON, Guraya A, Muojieje CC, Kannayiram SS, Nair KM, Odion J et al (2021) Renal involvement in granulomatosis with polyangiitis increases economic health care burden: insights from the National Inpatient Sample Database. Cureus 13:e12515. https://doi.org/10.7759/cureus.12515
Moiseev S, Jw CT, Arimura Y, Bogdanos D, Csernok E, Damoiseaux J et al (2020) 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun Rev 19:102618. https://doi.org/10.1016/j.autrev.2020.102618
Cabral DA, Canter DL, Muscal E, Nanda K, Wahezi DM, Spalding SJ et al (2016) Comparing presenting clinical features in 48 children with microscopic polyangiitis to 183 children who have granulomatosis with polyangiitis (Wegener’s): an ARChiVe cohort study. Arthritis Rheumatol 68:2514–2526. https://doi.org/10.1002/art.39729
Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP et al (1990) The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 33:1094–1100. https://doi.org/10.1002/art.1780330806
Vaglio A, Buzio C, Zwerina J (2013) Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): state of the art. Allergy 68:261–273. https://doi.org/10.1111/all.12088
Fina A, Dubus JC, Tran A, Derelle J, Reix P, Fayon M et al (2018) Eosinophilic granulomatosis with polyangiitis in children: data from the French RespiRare® cohort. Pediatr Pulmonol 53:1640–1650. https://doi.org/10.1002/ppul.24089
Almaani S, Fussner LA, Brodsky S, Meara AS, Jayne D (2021) ANCA-associated vasculitis: an update. J Clin Med 10:1446. https://doi.org/10.3390/jcm10071446
Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T et al (2016) EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 75:1583–1594. https://doi.org/10.1136/annrheumdis-2016-209133
Mekic M, Begic E, Mutevelic S, Sinancevic A (2021) Churg-strauss syndrome along with cardiac complications. Int J Appl Basic Med Res 11:50–52. https://doi.org/10.4103/ijabmr.IJABMR_200_20
Gendelman S, Zeft A, Spalding SJ (2013) Childhood-onset eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome): a contemporary single-center cohort. J Rheumatol 40:929–935. https://doi.org/10.3899/jrheum.120808
Sartorelli S, Chassagnon G, Cohen P, Dunogué B, Puéchal X, Régent A et al (2022) Revisiting characteristics, treatment and outcome of cardiomyopathy in eosinophilic granulomatosis with polyangiitis (formerly Churg–Strauss). Rheumatology (Oxford) 61:1175–1184. https://doi.org/10.1093/rheumatology/keab514
Calatroni M, Oliva E, Gianfreda D, Gregorini G, Allinovi M, Ramirez GA et al (2017) ANCA-associated vasculitis in childhood: recent advances. Ital J Pediatr 43:46. https://doi.org/10.1186/s13052-017-0364-x
Życińska K, Borowiec A (2016) Cardiac manifestations in antineutrophil cytoplasmic autoantibody (ANCA) - associated vasculitides. Kardiol Pol 74:1470–1476. https://doi.org/10.5603/KP.a2016.0138
Varnier GC, Sebire N, Christov G, Eleftheriou D, Brogan PA (2016) Granulomatosis with polyangiitis mimicking infective endocarditis in an adolescent male. Clin Rheumatol 35:2369–2372. https://doi.org/10.1007/s10067-016-3337-3
Chirinos JA, Corrales-Medina VF, Garcia S, Lichtstein DM, Bisno AL, Chakko S (2007) Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol 26:590–595. https://doi.org/10.1007/s10067-005-0176-z
Knockaert DC (2007) Cardiac involvement in systemic inflammatory diseases. Eur Heart J 28:1797–1804. https://doi.org/10.1093/eurheartj/ehm193
Bello F, Bettiol A, Silvestri E, Mattioli I, Urban ML, Palermo A et al (2022) Evidence of subclinical atherosclerosis in eosinophilic granulomatosis with polyangiitis (EGPA). Rheumatology (Oxford) keac427. https://doi.org/10.1093/rheumatology/keac427
Mercuzot C, Letertre S, Daien CI, Zerkowski L, Guilpain P, Terrier B et al (2021) Comorbidities and health-related quality of life in patients with antineutrophil cytoplasmic antibody (ANCA) - associated vasculitis. Autoimmun Rev 20:102708. https://doi.org/10.1016/j.autrev.2020.102708
Hajj-Ali RA, Major J, Langford C, Hoffman GS, Clark T, Zhang L et al (2015) The interface of inflammation and subclinical atherosclerosis in granulomatosis with polyangiitis (Wegener’s): a preliminary study. Transl Res 166:366–374. https://doi.org/10.1016/j.trsl.2015.04.001
Mourguet M, Chauveau D, Faguer S, Ruidavets JB, Béjot Y, Ribes D et al (2019) Increased ischemic stroke, acute coronary artery disease and mortality in patients with granulomatosis with polyangiitis and microscopic polyangiitis. J Autoimmun 96:134–141. https://doi.org/10.1016/j.jaut.2018.09.004
James KE, Xiao R, Merkel PA, Weiss PF (2017) Variation in the treatment of children hospitalized with antineutrophil cytoplasmic antibody-associated vasculitis in the US. Arthritis Care Res (Hoboken) 69:1377–1383. https://doi.org/10.1002/acr.23142
Sholter DE, Armstrong PW (2000) Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol 16:505–511
Makino N, Nakamura Y, Yashiro M, Kosami K, Matsubara Y, Ae R et al (2019) Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr Int 61:397–403. https://doi.org/10.1111/ped.13809
McCrindle B, Rowley A, Newburger J, Burns J, Bolger A, Gewitz M et al (2017) American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135:e927–e999. https://doi.org/10.1161/CIR.0000000000000484
Khor CC, Davila S, Shimizu C, Sheng S, Matsubara T, Suzuki Y et al (2011) Genome-wide linkage and association mapping identify susceptibility alleles in ABCC4 for Kawasaki disease. J Med Genet 48:467–472. https://doi.org/10.1136/jmg.2010.086611
Son MBF, Newburger JW (2018) Kawasaki disease. Pediatr Rev 39:78–90. https://doi.org/10.1542/pir.2016-0182
Huang Y-H, Lin K-M, Ho S-C, Yan J-H, Lo M-H, Kuo H-C (2019) Increased incidence of Kawasaki disease in Taiwan in recent years: a 15 years nationwide population-based cohort study. Front Pediatr 7:121. https://doi.org/10.3389/fped.2019.00121
Miyata K, Miura M, Kaneko T, Morikawa Y, Matsushima T, Sakakibara H et al (2021) Evaluation of a Kawasaki disease risk model for predicting coronary artery aneurysms in a Japanese population: an analysis of post RAISE. J Pediatr 237:96-101.e3
Hedrich CM, Schnabel A, Hospach T (2018) Kawasaki disease. Front Pediatr 6:198. https://doi.org/10.3389/fped.2018.00198
Rizk SR, El Said G, Daniels LB, Burns JC, El Said H, Sorour KA et al (2015) Acute myocardial ischemia in adults secondary to missed Kawasaki disease in childhood. Am J Cardiol 115:423–427. https://doi.org/10.1016/j.amjcard.2014.11.024
Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T (1996) Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol 28:253–257. https://doi.org/10.1016/0735-1097(96)00099-x
Suda K, Iemura M, Nishiono H, Teramachi Y, Koteda Y, Kishimoto S et al (2011) Long-term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single-institution experience. Circulation 123:1836–1842. https://doi.org/10.1161/CIRCULATIONAHA.110.978213
Tsuda E, Hamaoka K, Suzuki H, Sakazaki H, Murakami Y, Nakagawa M et al (2014) A survey of the 3-decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J 167:249–258. https://doi.org/10.1016/j.ahj.2013.10.025
Daniels LB, Tjajadi MS, Walford HH, Jimenez-Fernandez S, Trofimenko V, Fick DB Jr et al (2012) Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation 125:2447–2453. https://doi.org/10.1161/CIRCULATIONAHA.111.082107
McCrindle BW, McIntyre S, Kim C, Lin T, Adeli K (2007) Are patients after Kawasaki disease at increased risk for accelerated atherosclerosis? J Pediatr 151(244–248):248.e1. https://doi.org/10.1016/j.jpeds.2007.03.056
Cho HJ, Yang SI, Kim KH, Kim JN, Kil HR (2014) Cardiovascular risk factors of early atherosclerosis in school-aged children after Kawasaki disease. Korean J Pediatr 57:217–221. https://doi.org/10.3345/kjp.2014.57.5.217
Cheung Y-f, Yung T-c, Tam SC, Ho MH, Chau AK (2004) Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J Am Coll Cardiol 43:120–124. https://doi.org/10.1016/j.jacc.2003.08.030
Tsuda E, Hirata T, Matsuo O, Abe T, Sugiyama H, Yamada O (2011) The 30-year outcome for patients after myocardial infarction due to coronary artery lesions caused by Kawasaki disease. Pediatr Cardiol 32:176–182. https://doi.org/10.1007/s00246-010-9838-y
Manlhiot C, Millar K, Golding F, McCrindle BW (2010) Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol 31:242–249. https://doi.org/10.1007/s00246-009-9599-7
Tsuda E, Kamiya T, Ono Y, Kimura K, Echigo S (2005) Dilated coronary arterial lesions in the late period after Kawasaki disease. Heart 91:177–182. https://doi.org/10.1136/hrt.2003.025338
Ozen S, Bakkaloglu A, Dusunsel R, Soylemezoglu O, Ozaltin F, Poyrazoglu H et al (2007) Childhood vasculitides in Turkey: a nationwide survey. Clin Rheumatol 26:196–200. https://doi.org/10.1007/s10067-006-0266-6
Hočevar A, Tomšič M, Perdan Pirkmajer K (2021) Clinical approach to diagnosis and therapy of polyarteritis nodosa. Curr Rheumatol Rep 23:14. https://doi.org/10.1007/s11926-021-00983-2
McWilliams E, Khonizy W, Jameel A (2013) Polyarteritis nodosa presenting as acute myocardial infarction in a young man: importance of invasive angiography. Heart 99:1219. https://doi.org/10.1136/heartjnl-2013-304022
Schrader ML, Hochman JS, Bulkley BH (1985) The heart in polyarteritis nodosa: a clinicopathologic study. Am Heart J 109:1353–1359. https://doi.org/10.1016/0002-8703(85)90365-5
Kasap Cuceoglu M, Sener S, Batu ED, Kaya Akca U, Demir S, Sag E et al (2021) Systematic review of childhood-onset polyarteritis nodosa and DADA2. Semin Arthritis Rheum 51:559–564. https://doi.org/10.1016/j.semarthrit.2021.04.009
Ozen S, Anton J, Arisoy N, Bakkaloglu A, Besbas N, Brogan P et al (2004) Juvenile polyarteritis: results of a multicenter survey of 110 children. J Pediatr 145:517–522. https://doi.org/10.1016/j.jpeds.2004.06.046
Eleftheriou D, Dillon MJ, Tullus K, Marks SD, Pilkington CA, Roebuck DJ et al (2013) Systemic polyarteritis nodosa in the young: a single-center experience over thirty-two years. Arthritis Rheum 65:2476–2485. https://doi.org/10.1002/art.38024
Hong Y, Eleftheriou D, Klein NJ, Brogan PA (2015) Impaired function of endothelial progenitor cells in children with primary systemic vasculitis. Arthritis Res Ther 17:292. https://doi.org/10.1186/s13075-015-0810-3
Wi J, Choi HH, Lee CJ, Kim T, Shin S, Ko YG et al (2010) Acute myocardial infarction due to polyarteritis nodosa in a young female patient. Korean Circ J 40:197–200. https://doi.org/10.4070/kcj.2010.40.4.197
Yanagawa B, Kumar P, Tsuneyoshi H, Kachel E, Massad E, Moussa F et al (2010) Coronary artery bypass in the context of polyarteritis nodosa. Ann Thorac Surg 89:623–625. https://doi.org/10.1016/j.athoracsur.2009.07.058
Ribeiro E, Cressend T, Duffau P, Grenouillet-Delacre M, Rouanet-Lariviere M, Vital A et al (2009) Rituximab efficacy during a refractory polyarteritis nodosa flare. Case Rep Med 2009:738293. https://doi.org/10.1155/2009/738293
Zucker EJ, Chan FP (2022) Pediatric cardiothoracic vasculitis: multimodality imaging review. Pediatr radiol 52:1895–1909. https://doi.org/10.1007/s00247-022-05431-6
Aeschlimann FA, Yeung RSM, Laxer RM (2022) An update on childhood-onset Takayasu arteritis. Front Pediatr 10:872313. https://doi.org/10.3389/fped.2022.872313
Batu ED, Sonmez HE, Hazirolan T, Ozaltin F, Bilginer Y, Ozen S (2017) Tocilizumab treatment in childhood Takayasu arteritis: case series of four patients and systematic review of the literature. Semin Arthritis Rheum 46:529–535. https://doi.org/10.1016/j.semarthrit.2016.07.012
Idhrees M, Thilagavathi N, Bashir M, Velayudhan BV (2020) Management of cardiac manifestations in Takayasu arteritis. Vessel Plus 4:23
Gornik HL, Creager MA (2008) Aortitis. Circulation 117:3039–3051. https://doi.org/10.1161/CIRCULATIONAHA.107.760686
Yang J, Peng M, Shi J, Zheng W, Yu X (2019) Pulmonary artery involvement in Takayasu’s arteritis: diagnosis before pulmonary hypertension. BMC Pulm Med 19:225. https://doi.org/10.1186/s12890-019-0983-7
Cakar N, Yalcinkaya F, Duzova A, Caliskan S, Sirin A, Oner A et al (2008) Takayasu arteritis in children. J Rheumatol 35:913–919
Kothari S (2001) Takayasu’s arteritis in children - a review. Images Paediatr Cardiol 3:4–23
Hassan O, Fereshtehnejad S-M, Hoseini R, Azita Z, Tafreshi R, Farideh H (2008) Extensive myocardial infarction in a child with Takayasu vasculitis: report of a case and literature review. Iranian J Pediatr 8:364–368
Wolf RL, Gould NS, Green CA (1987) Unusual arteritis causing myocardial infarction in a child. Arch Pathol Lab Med 111:968–971
Das A, Mundhra N, Patra C, Sarkar S, Ghosh N, Roy S (2014) Takayasu arteritis: a 4-year-old girl presenting with heart failure - case report and literature review. J Pediatr Sci 6:e219
Zhang Y, Yang K, Meng X, Tian T, Fan P, Zhang H et al (2018) Cardiac valve involvement in Takayasu arteritis is common: a retrospective study of 1,069 patients over 25 years. Am J Med Sci 56:357–364. https://doi.org/10.1016/j.amjms.2018.06.021
Seyahi E, Ugurlu S, Cumali R, Balci H, Seyahi N, Yurdakul S et al (2006) Atherosclerosis in Takayasu arteritis. Ann Rheum Dis 65:1202–1207. https://doi.org/10.1136/ard.2005.047498
Filer A, Nicholls D, Corston R, Carey P, Bacon P (2001) Takayasu arteritis and atherosclerosis: illustrating the consequences of endothelial damage. J Rheumatol 28:2752–2753
da Silva TF, Levy-Neto M, Bonfá E, Pereira RMR (2013) High prevalence of metabolic syndrome in Takayasu arteritis: increased cardiovascular risk and lower adiponectin serum levels. J Rheumatol 40:1897–1904. https://doi.org/10.3899/jrheum.130162
Koné-Paut I, Shahram F, Darce-Bello M, Cantarini L, Cimaz R, Gattorno M et al (2016) Consensus classification criteria for paediatric Behçet’s disease from a prospective observational cohort: PEDBD. Ann Rheum Dis 75:958–964. https://doi.org/10.1136/annrheumdis-2015-208491
Kone-Paut I (2016) Behcet’s disease in children, an overview. Pediatr Rheumatol Online J 14:10. https://doi.org/10.1186/s12969-016-0070-z
Batu ED (2019) Diagnostic/classification criteria in pediatric Behcet’s disease. Rheumatol Int 39:37–46. https://doi.org/10.1007/s00296-018-4208-9
Butbul Aviel Y, Batu ED, Sözeri B, Aktay Ayaz N, Baba L, Amarilyo G et al (2020) Characteristics of pediatric Behçet’s disease in Turkey and Israel: a cross-sectional cohort comparison. Semin Arthritis Rheum 50:515–520. https://doi.org/10.1016/j.semarthrit.2020.01.013
Boumaaz M, Asfalou I, El Kassimi I, Maha R, Benyass A, Zbir E (2021) Cardiovascular manifestations in juvenile-onset Behcet’s disease: unusual mode of revelation. Eur J Med Case Rep 5:85–88
Demirelli S, Degirmenci H, Inci S, Arisoy A (2015) Cardiac manifestations in Behcet’s disease. Intractable Rare Dis Res 4:70–75. https://doi.org/10.5582/irdr.2015.01007
Karincaoglu Y, Borlu M, Toker SC, Akman A, Onder M, Gunasti S et al (2008) Demographic and clinical properties of juvenile-onset Behçet’s disease: a controlled multicenter study. J Am Acad Dermatol 58:579–584. https://doi.org/10.1016/j.jaad.2007.10.452
Turkucar S, Dundar HA, Acari C, Unsal E (2019) Evaluation of cardiovascular involvement in pediatric Behcet’s disease: case report and literature review with intracardiac thrombus. Erciyes Med J 41:344–346
Cook AL, Rouster-Stevens K, Williams DA (2010) Hines MH. Giant aneurysm of the left anterior descending coronary artery in a pediatric patient with Behcet’s disease. Pediatr Cardiol 31:700–702. https://doi.org/10.1007/s00246-009-9636-6
Kahn PJ, Yazici Y, Argilla M, Srichai M, Levy DM (2012) Asymptomatic giant coronary aneurysm in an adolescent with Behcet’s syndrome. Pediatr Rheumatol Online J 10:2. https://doi.org/10.1186/1546-0096-10-2
Seyahi E, Ugurlu S, Cumali R, Balci H, Ozdemir O, Melikoglu M et al (2008) Atherosclerosis in Behçet’s syndrome. Semin Arthritis Rheum 38:1–12. https://doi.org/10.1016/j.semarthrit.2007.09.009
Seyahi E, Memisoglu E, Hamuryudan V, Tepe S, Aker UT, Balci H et al (2004) Coronary atherosclerosis in Behçet’s syndrome: a pilot study using electron-beam computed tomography. Rheumatology (Oxford) 43:1448–1450. https://doi.org/10.1093/rheumatology/keh359
Hassan S, Gheita T, Ghoneim S, Nasr L (2012) Subclinical atherosclerosis in Behçet’s disease. Arch Rheumatol 27:109–114
Wu X, Li G, Huang X, Wang L, Liu W, Zhao Y et al (2014) Behçet’s disease complicated with thrombosis: a report of 93 Chinese cases. Medicine (Baltimore) 93:e263. https://doi.org/10.1097/MD.0000000000000263
Kraiem S, Fennira S, Battikh K, Chehaibi N, Hmem M, Slimane ML (2004) [Behcet disease: uncommon cause of myocardial infarction]. Ann Cardiol Angeiol (Paris). 53:109–113. French. https://doi.org/10.1016/s0003-3928(03)00046-5
Misra DP, Shenoy SN (2017) Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol Int 37:151–167. https://doi.org/10.1007/s00296-016-3435-1
Dadfarmay S, Berkowitz R, Kim B (2012) Irreversible end-stage cardiomyopathy following a single dose of cyclophosphamide. Congest Heart Fail 18(4):234–237. https://doi.org/10.1111/j.1751-7133.2011.00279.x
Johal K, Ratuapli SK, Lam-Himlin DM, Gurudu SR (2014) Mycophenolate mofetil-induced segmental colitis mimicking ischemic colitis. Case Rep Gastroenterol 8:95–100. https://doi.org/10.1159/000360847
Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, Ridker PM et al (2011) Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 108:1362–1370. https://doi.org/10.1016/j.amjcard.2011.06.054
Zanoli L, Rastelli S, Inserra G, Lentini P, Valvo E, Calcagno E et al (2014) Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs. Atherosclerosis 234:346–351. https://doi.org/10.1016/j.atherosclerosis.2014.03.023
Cheungpasitporn W, Kopecky SL, Specks U, Bharucha K, Fervenza FC (2016) Non-ischemic cardiomyopathy after rituximab treatment for membranous nephropathy. J Renal Inj Prev 6:18–25. https://doi.org/10.15171/jrip.2017.04
Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, Quinn M et al (2011) Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 50:518–531. https://doi.org/10.1093/rheumatology/keq316
Strang AC, Bisoendial RJ, Kootte RS, Schulte DM, Dallinga-Thie GM, Levels JH et al (2013) Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 229:174–181. https://doi.org/10.1016/j.atherosclerosis.2013.04.031
Eleftheriou D, Brogan PA (2009) Vasculitis in children. Best Pract Res Clin Rheumatol 23:309–323. https://doi.org/10.1016/j.berh.2009.02.001
Jayne DR, Merkel PA, Schall TJ, Bekker P (2021) Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 384:599–609. https://doi.org/10.1056/NEJMoa2023386
Zeng YY, Zhang M, Ko S, Chen F (2021) An update on cardiovascular risk factors after Kawasaki disease. Front Cardiovasc Med 8:671198. https://doi.org/10.3389/fcvm.2021.671198
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of the Topical Collection entitled ‘Cardiovascular Issues in Rheumatic Diseases’
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sener, S., Arslanoglu Aydin, E. & Batu, E.D. Cardiac involvement and cardiovascular risk factors in pediatric primary systemic vasculitides. Clin Rheumatol 42, 673–686 (2023). https://doi.org/10.1007/s10067-022-06434-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06434-2