Abstract
Purpose of the Review
Polyarteritis nodosa is a rare disease characterized by the necrotizing inflammation of medium-sized arteries. Different etiopathogenetic and clinical variants of the disease have been recognized over the past decades. In the present paper, we review the clinical features, diagnosis, and treatment of the different subtypes of the disease.
Recent Findings
The diagnosis of polyarteritis nodosa is primarily based on clinical findings, imaging, and histopathological investigations. Microbiological and genetic investigations complement the diagnostic work-up. Idiopathic and hereditary variants of polyarteritis nodosa are treated with immunomodulatory medications such as glucocorticoids, conventional immunomodulatory drugs (e.g., cyclophosphamide) and biologic agents (e.g., tumor necrosis factor inhibitors, interleukin 6 inhibitor), while hepatitis B virus-associated polyarteritis nodosa primarily requires antiviral therapy combined with plasma exchange.
Summary
PAN is a disease with heterogeneous presentations, severity, and therapeutic approaches. The overall prognosis of this disease is improving, mainly due to early diagnosis and more effective treatments. Treatment choices are guided mainly by the disease subtype and severity. In this review, we have presented the current knowledge on PAN clinical variants, their classification, diagnosis, and treatment approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1866, Kussmaul and Maier reported a case of an acutely and severely ill male with fever, myalgias, mononeuritis, abdominal pain, proteinuria, and subcutaneous skin nodules who succumbed to the disease. Autopsy revealed widespread nodular thickening of several arteries, histologically showing artery wall inflammation [1]. Their report constitutes the first description of classic polyarteritis nodosa (PAN), a rare systemic necrotizing vasculitis affecting medium and small arteries [2]. The term polyarteritis acuta nodosa was proposed in 1903 by Ferrari, who observed that vessel wall inflammation led to multiple aneurysms giving the affected arteries a “nodular” appearance [3]. The recognition of the systemic disease followed in 1931 with the first description of cutaneous PAN [4]. In 1970, the association between hepatitis B virus (HBV) infection and PAN was discovered [5]. Subsequently, hepatitis C virus (HCV) and human immunodeficiency virus (HIV) associated PAN cases have been identified [6]. PAN has also been reported in association with some other microorganisms (such as parvovirus B19, cytomegalovirus, Epstein-Barr virus, streptococcal infections); however, none of these microorganisms has been firmly confirmed to trigger the onset of PAN [7].
In recent years, the definition of PAN has evolved. The revised 2012 International Chapel Hill Consensus Conference stressed the involvement of medium sized vessels—the main visceral arteries and their branches, not affecting smaller vessels and glomeruli, as well as the absence of antineutrophil cytoplasmic antibodies (ANCA). PAN related to hepatitis B infection (HBV) could be classified as a form of vasculitis associated with a known etiology [8]. Moreover, the Dermatologic Addendum to the 2012 Revised International Chapel Hill Consensus Conference (CHCC) Nomenclature of Vasculitides defined cutaneous PAN as a chronic, relapsing vasculitis affecting small arteries and arterioles in the panniculus and dermal-subcutaneous junction [9]. Among drug-induced PAN cases, minocycline has been most frequently reported as a trigger of the disease [10, 11]. Infrequently, PAN lesions are incidentally discovered in surgical specimens of other organs, such as the appendix, gallbladder, or testis. After careful evaluation and exclusion of systemic disease, such cases are nowadays classified as single organ vasculitis [8, 12]. The spectrum of PAN has recently been expanded by the recognition of rare autosomal recessive mutations resulting in a deficiency of adenosine deaminase 2 (ADA2), which commonly presents with medium vessel vasculitis resembling PAN [13, 14]. PAN has also been reported in patients with Familial Mediterranean Fever, another autoinflammatory disease in which PAN was found in up to 1.5% of cases [15, 16].
Epidemiology
PAN is a rare disease, with an estimated prevalence around 31 cases/million, and an annual incidence from 0 to 9 cases/million people in European countries [17,18,19,20]. The disease is more frequent in men than in women (around 1.5:1 male preponderance). PAN may occur at any age; however, idiopathic systemic PAN occurs most frequently in patients in their 40s to 60s. With the decrease of transfusion-transmitted infections and HBV vaccination, the frequency of HBV-related PAN has significantly decreased in developed countries over recent decades, from 30 to 50% of all PAN cases to less than 5% [4, 21, 22]. The detailed prevalence and incidence of cPAN are not known; in the large PAN cohort from the French Vasculitis Study Group database, cutaneous PAN represented ~ 4% of PAN cases [23]. A greater proportion of cutaneous PAN is reported in children (up to 30% of juvenile PAN cases) [24]. Since the first reports of ADA2 deficiency-associated PAN cases, the reported number of patients with this mutation has been steadily increasing.
Clinical Characteristics
The stenosis/occlusion and the rupture of inflamed visceral arteries, with subsequent tissue/organ ischemia, damage, dysfunction, and/or bleeding, are the causes of the most profound PAN manifestations. Almost any organ/organ system can be affected by PAN, though pulmonary involvement is uncommon [23, 25]. The most frequently involved organs are the peripheral nervous system and the skin. Cutaneous features include livedo reticularis, subcutaneous nodules, skin infarcts and ulcers, as well as purpura [26]. Mononeuritis multiplex is the most common neurologic manifestation, followed by symmetric polyneuropathy. The involvement of the central nervous system is rare in idiopathic systemic PAN [27]. Gastrointestinal tract inflammation and ischemia often presenting as acute surgical abdomen is one of the most serious PAN manifestations, occurring in up to one-third of cases [28].
Kidneys are commonly affected, with vasculitis of the renal and interlobar arteries, less frequently of the smaller arcuate and interlobular arteries leading to tissue infarctions and microaneurysm rupture. Patients develop micro- or macro-hematuria, and often mild to moderate proteinuria. New onset hypertension is secondary to intrarenal artery involvement and occurs in up to 35% of PAN patients. Renal infarctions or uncontrolled hypertension may lead to chronic renal failure [29]. Clinically manifest heart involvement with coronary arteritis, pericarditis, and myocarditis resulting in cardiomyopathy is seen in 5–20% of cases [30]. Peripheral artery involvement can lead to Raynaud’s phenomenon or digital gangrene. Testicular pain and orchitis (usually unilateral due to testicular artery involvement) are very characteristic symptoms of PAN, present in around a quarter of male patients [31]. Eye involvement affects around 10% of PAN patients and ranges from mild conjunctivitis, episcleritis, keratitis, and uveitis to severe retinal vasculitis, the latter being one of the most frequent ophthalmologic manifestations in PAN [32]. In addition to organ-specific manifestations, patients commonly report nonspecific constitutional symptoms, such as fever, weight loss, intense myalgias, arthralgias, and malaise (recorded in over 90% of patients), which may precede specific organ manifestations by months. PAN patients also have an increased risk of venous thromboembolism as compared to the general population (RR 3.00, 95% CI 2.20–4.09), particularly in an active or recently active disease [33].
The natural history of untreated systemic PAN is rapidly progressive and can be fatal [34]. Pagnoux et al. reported a 20 % mortality rate in non-HBV-associated PAN patients who were followed on average 68 months [23]. Over the past few decades, along with better treatment options, the survival rate of patients with systemic necrotizing vasculitis, including PAN, has significantly improved: The mortality rate of patients diagnosed with systemic necrotizing vasculitis after 2010 is three times lower as compared to patients diagnosed before 1980 (the incidence of death decreased from 4.08 per 100 person-years in patients diagnosed before 1980 to 0.94 per 100 person-years after 2010) [35]. Large studies showed that the prognosis of systemic PAN depends on the severity of the disease [36, 37]. The French Vasculitis Study Group proposed the five factor score (FFS), a prognostic index consisting of five items, each graded with present = 1 or absent = 0: (1) presence of severe gastrointestinal tract disease (defined as bleeding, perforation, infarction, or pancreatitis), (2) serum creatinine > 140 μmol/l, (3) proteinuria (> 1 g/day), (4) cardiac disease (infarction or heart failure), and (5) central nervous system involvement. The 5-year mortality of patients with PAN with FFS = 0 was 12%, as compared to 26% mortality when FFS = 1, and 46% mortality when FFS was ≥ 2 (Table 1) [37].
PAN associated with HBV usually occurs early in the course of infection [38]. Though the clinical manifestations of HBV-associated PAN are largely similar to idiopathic PAN, the comparison of HBV-associated cases to non-HBV-associated PAN in a cohort from the French Vasculitis Study Group database revealed in the former a more severe disease, with more profound weight loss, more frequent peripheral nerve, cardiac (surgical), gastrointestinal tract, and testicular involvement, as well as development of (severe) hypertension. Mesenteric artery microaneurysms were also more prevalent in HBV-associated PAN [23]. As a result, a higher severity determined by the Birmingham vasculitis activity score (BVAS) and five factor score was found in HBV-associated PAN compared to the idiopathic disease form. Hepatitis, however, is frequently mild in these patients, with barely elevated transaminases and without overt jaundice [38]. Patients with HBV-associated PAN have a higher mortality but less frequent relapses than patients with idiopathic PAN [23, 38].
Cutaneous PAN is generally restricted to the extremities (legs are affected in over 95% of cases, followed by arms in one third of cases, and the trunk in less than 10%) [39]. It encompasses livedo reticularis, tender subcutaneous nodules, with or without ulceration, macules, purpura, extremity edema, and swelling. Constitutional symptoms may accompany also the clinical picture of cutaneous PAN [40]. Arteritis in adjacent skeletal muscle and the involvement of peripheral nerves have also been described. Myalgias are reported in around a third of cutaneous PAN cases, and a quarter of patients displays neuropathy on the electromyographic study [39, 41]. The clinical course is usually chronic with phases of remission and relapse. Patients with skin ulcers and increased inflammatory markers (high C-reactive protein, high blood neutrophil count, high neutrophil/lymphocyte ratio) have a higher risk of relapsing disease course [42, 43]. Cutaneous PAN does not commonly evolve into systemic PAN, and the prognosis is favorable [41, 44].
ADA2 deficiency has a variable clinical presentation. Frequently, but not always, the disease presents in youth. The clinical phenotype is variable, and vasculitis as the predominant clinical presentation was described first. Later, hematological and immunodeficiency phenotypes were recognized [45]. The skin and the central nervous system are the most frequently involved in ADA2 deficiency-associated PAN [46]. Cutaneous manifestations are reported in 75% and vary from livedo reticularis or livedo racemosa, to skin ulcers, Raynaud’s phenomenon, digital necrosis, purpura, skin nodules, and erythema nodosum-like lesions. Involvement of the central nervous system with recurrent strokes is reported in 50 to 77% of cases with ADA2 deficiency-associated PAN. The typical neurological event is the lacunar ischemic infarct in the deep-brain nuclei, midbrain, and/or brain stem involvement while sparing the subcortical white matter. Brain imaging may also reveal hemorrhagic infarcts, ventricular hemorrhage, and aneurysms. In addition, patients can develop constitutional symptoms, arthritis/arthralgia, as well as peripheral nerve, gastrointestinal tract and renal and testicular involvement. Lymphoproliferation with generalized lymphadenopathy and/or splenomegaly, cytopenia, and immunodeficiency of various degrees may accompany the clinical picture [47, 48].
The characteristics (clinical manifestations and therapy of choice) of different PAN variants are presented in Table 2.
Diagnosis
The diagnosis of PAN is based on a combination of clinical features, imaging (angiographic), and histopathological findings [49]. Clinically, PAN should be suspected in acutely ill patients with marked constitutional symptoms and multiorgan involvement, principally in cases with cutaneous, neurological, renal, and gastrointestinal involvement [23, 38, 50].
There are no laboratory findings specific for PAN. Inflammatory parameters (e.g., erythrocyte sedimentation rate, C-reactive protein) are, as well as other acute phase reactants, leukocytes, and platelets, generally elevated in generalized PAN. However, patients with cutaneous PAN and/ or minocycline-induced PAN could also have normal inflammatory parameters [10, 40]. Anemia develops as a result of chronic inflammation, but may result also from bleeding. Antibodies—including ANCA and cryoglobulins—are typically negative, and their presence should point towards other diseases/vasculitides [8, 51].
Screening for HBV, HCV, and HIV has important treatment consequences in the event of a positive result and should routinely be conducted.
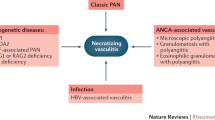
A simplified diagnostic algorithm for PAN variants is presented in Fig. 1.
Imaging
Microaneurysms involving medium-sized or small arteries that are often found together with stenotic/occlusive lesions are the hallmark of PAN. Microaneurysms are most frequently identified at renal and splanchnic artery branches (particularly hepatic artery and mesenteric artery). Nonetheless, they are commonly present also in skeletal muscle arteries. Typically, they are saccular or fusiform, and 1–5 mm in diameter (Fig. 2). In an angiographic study, Stanson et al. found aneurysms and segments of ectasia in 61% of PAN patients, and stenotic lesions in 98% of cases. Skeletal muscle arteries were affected in 18 out of 56 patients, nine in the extremities [52].
Conventional angiography is considered the optimal modality to identify vascular abnormalities, yielding a sensitivity and specificity of around 90% [53]. As standard angiography carries the risks associated with arterial cannulation (such as bleeding, embolization, and pseudoaneurysm formation), less invasive imaging techniques, such as computed tomography angiography (CTA) and magnetic resonance angiography, are also used nowadays to delineate inflammatory arterial lesions, though their sensitivity for the detection of microaneurysms may be lower compared to standard angiography (as very small microaneurysms are not clearly visible in computed tomography angiography or magnetic resonance angiography) [54]. In addition to vascular lesions, imaging (e.g., computer tomography, venous phase of CTA) could also reveal parenchymal changes (e.g., infarcts in kidneys, bowel wall thickening). The value/role of positron emission tomography has not been adequately assessed in PAN [55, 56]. There are only few reports of PAN-associated aneurysms detected by ultrasound (in renal and celiac arteries) [57, 58].
Imaging makes it possible to establish the extent of the disease in PAN but also to follow the disease course and treatment response (regression of microaneurysm in the case of effective treatment) [59].
In the presence of characteristic angiographic changes, PAN could be classified, even in the absence of histological confirmation [60]. Yet, image interpretation requires an experienced radiologist that adequately takes into consideration other structural vasculopathies (such as fibromuscular dysplasia or segmental arterial mediolysis) [61].
Histology
Histology represents the gold standard for diagnosing vasculitis. At the same time, it may help to exclude other diagnoses. Biopsies should be taken from symptomatic sites (e.g. skin, muscle, sural nerve). When different organs are involved, the less aggressive approach is favored (i.e., skin or muscle biopsy). Due to microaneurysm development and the potential for bleeding, ultrasound guided kidney and/or liver biopsies should be reserved for cases where the diagnosis cannot not be established otherwise [60].
In biopsy specimens from PAN patients, segmental necrotizing transmural inflammation of medium-sized or small arteries with the predilection for bifurcations and branching points can be demonstrated (Fig. 3). Small vessels, such as arterioles, capillaries, venules, and glomeruli (in kidney biopsies), are typically spared. In active lesions, mixed cell infiltrates are observed consisting of lymphocytes, macrophages, and variable numbers of neutrophils and eosinophils. Neutrophils are more frequently present in vessels with fibrinoid necrosis. Granulomas and giant cells are normally absent. At later stages, the inflammatory infiltrate is largely mononuclear, and neoangiogenesis becomes apparent. In advanced stages, vascular remodeling results in intimal hyperplasia and fibrosis of the vessel wall. Severe vessel wall injury may lead to formation of microaneurysms. In biopsy samples, different stages of the inflammatory processes often coexist—vessels with acute necrotizing lesions coincidence with vessels in the fibrotic, healing phase [62,63,64].
Polyarteritis nodosa: histology of muscle biopsy. Medium-vessel vasculitis. Inflammatory cell infiltrate (a) with associated fibrinoid necrosis (black arrow) (b) and elastic fibers destruction (asterisks) (c) of arterial wall inside the skeletal muscle. Stains H&E (a), Picro-Mallory (b), Van Gieson-Weigert (c), original magnification × 200
Specific Investigations
When genetic PAN is suspected, particularly in children with PAN, cases of familial PAN, early-onset strokes with systemic inflammation, or atypical immunodeficiency syndromes with autoimmunity and/or lymphoproliferation [45], a functional assay to measure ADA2 activity should be requested. In addition, genetic testing to determine mutations in the cat eye syndrome critical region candidate 1 (CECR1) gene located on chromosome 22q11.1 could be performed.
The mechanisms, how ADA2 deficiency results in clinical PAN, are not completely understood; however, immune dysfunction, growth factor dysfunction, and catalytic dysfunction might all play a role [45]. Patients with ADA2 deficiency have a skewed monocyte differentiation, with increased pro-inflammatory M1 and decreased anti-inflammatory M2 macrophage subsets in peripheral blood [65]. This may promote vasculopathy and inflammation. In addition, ADA2 has also been identified as a growth factor responsible for development and integrity of the endothelium [66, 67]. Chronically elevated adenosine levels may promote inflammation through tissue injury and fibrosis [68].
Classification Criteria and Diagnosis
In the absence of published diagnostic criteria to assist in making a diagnosis, clinicians in practice commonly (though inappropriately) use classification criteria for diagnostic purposes.
In 1990 the American College of Rheumatology (ACR) proposed sets of classification criteria for seven different types of vasculitis in adults, including PAN [69]. PAN criteria took into account clinical, laboratory, radiographic, and histological features, and the presence of 3 out of 10 items yielded a sensitivity of 82.2% and specificity of 86.6% for classifying the disease as PAN (Table 3) [70]. The most important limitation of the ACR criteria is that they do not separate microscopic polyangiitis (MPA) from PAN, nor do they consider ANCA testing as a relevant item for classifying vasculitis. The formal distinction between PAN and MPA was first made at the CHCC in 1994, defining MPA as a pauci-immune necrotizing vasculitis affecting small vessels, with or without involvement of medium-sized arteries [2]. The association of MPA with ANCA was added in the revised CHCC [8]. In 2007, a consensus stepwise algorithm was proposed, combining ACR and CHCC criteria, as well as surrogate clinical, imaging, or laboratory markers of vasculitis, including ANCA testing, to classify PAN- and ANCA-associated vasculitides [51]. The algorithm was developed for being used in epidemiologic studies and allowed patients to be classified into one category with a minimum of unclassified patients or overlapping diagnoses [51].
The imperfect performance of ACR 1990 classification criteria was stressed in a study of over 1500 patients with primary systemic vasculitis and comparators. The study demonstrated a lower sensitivity of the ACR 1990 criteria for the majority of vasculitides as compared to their historical performance. In PAN, the sensitivity of ACR 1990 classification criteria dropped from 82.2 to 40.6% [72].
Since the 1990 ACR classification criteria did not cover the pediatric population, EULAR published in cooperation with the Paediatric Rheumatology International Trials Organization (PRINTO) and the Paediatric Rheumatology European Society (PRES), the classification criteria for IgA vasculitis, childhood PAN, childhood granulomatosis with polyangitis, and childhood Takayasu arteritis in 2010 [73, 74]. Criteria of all types of childhood vasculitis yielded an exceptionally high classification performance, resulting in 89.6% sensitivity and 99.6% specificity for childhood PAN [74].
Based on the limitations of existing ACR classification criteria, a large international, multicenter study—the Diagnostic and Classification Criteria in Vasculitis (DCVAS) study—was conducted to develop new data-driven classification criteria, incorporating newer diagnostic tools such as different imaging techniques, and ANCA testing, as well as an expert consensus. Another aim of this project was to propose diagnostic criteria for different types of systemic vasculitides, which has never done before [75]. The draft classification criteria have already been presented, and the process of final endorsement by the ACR and the EULAR is in progress [76, 77].
The first attempt towards the development of PAN diagnostic criteria was published by Henegar et al. [78]. They analyzed the data of 949 patients with histologically proven systemic vasculitis from the French Vasculitis Study Group database (262 PAN patients and 687 patients with other types of vasculitis) and determined in a logistic regression model a set of nonredundant PAN-predictive criteria consisting of three positive predictive parameters, which are active HBV infection, arteriographic abnormalities, and peripheral nerve involvement, and five negative predictive parameters, which are asthma, ear-nose-throat involvement, glomerulopathy, ANCA, and cryoglobulinemia. They found that the combination of positive and negative criteria performed best in discriminating PAN from other forms of vasculitis. The new criteria also outperformed the ACR 1990 classification criteria [78].
Furthermore, a Japanese group recently proposed provisional diagnostic criteria for PAN consisting of seven items (fever ≥ 1 week or weight loss ≥ 4 kg, mononeuritis multiplex, gastrointestinal tract involvement, proteinuria < 2 +, negative ANCA testing (anti-MPO/pANCA), histological evidence of vasculitis in a medium or small artery, angiographic abnormality (finding aneurysms, stenoses, or occlusions of the visceral arteries, not due to other causes). Fulfilling ≥ 4 items resulted in 92.3% sensitivity and 91.7% specificity for PAN (Table 3) [71]. The proposed criteria need further validation in a larger cohort of patients.
Treatment
The number of new treatment studies in PAN is rare, which is mostly explained by the rarity and the heterogeneity of the disease. The EULAR recommendations for the management of medium vessel vasculitis were published in 2008 [79], while the provisional ACR recommendations for the management of PAN have been presented as an abstract at the ACR meeting 2019.
Treatment of Idiopathic Generalized PAN
The range of organ involvement and disease progression guides treatment in PAN. Mild forms of idiopathic PAN (with FFS = 0) are commonly treated with glucocorticoids alone. Prednisone is usually used at doses of 1 mg/kg/day with subsequent tapering [80, 81]. In glucocorticoid nonresponding or relapsing patients with non-severe PAN are 6 intravenous pulses of cyclophosphamide or 6-month azathioprine equally effective as a second-line treatment [81]. However, Puechal et al. did not report any significant benefit of an initial combination of glucocorticoids with azathioprine versus glucocorticoids alone for the induction of remission in necrotizing vasculitis (eosinophilic granulomatosis with polyangiitis, microscopic polyangiitis, PAN) without unfavorable prognostic factors [82]. One exception might be the presence of mononeuritis multiplex which is not associated with increased mortality (and does not lead to an increment of the FFS). These patients are more likely to fail monotherapy with glucocorticoids requiring additional immunosuppression, mostly cyclophosphamide [83].
In PAN with critical organ involvement, specified as an FFS ≥ 1, cyclophosphamide is generally administered together with glucocorticoids. Commonly prescribed cyclophosphamide doses are 2 mg/kg/day orally or a 600 mg/m2 monthly intravenous pulse therapy for 1 year. The cyclophosphamide dose should be adjusted in elderly patients and those with renal failure. Intermittent intravenous and daily oral cyclophosphamide administration were compared in a randomized clinical trial in patients with PAN or eosinophilic granulomatosis with polyangiitis [84]. No superiority in terms of efficacy was observed between the two regimens. A higher number of adverse effects led authors to recommend pulse over oral cyclophosphamide. The duration of treatment was assessed in a prospective study of PAN patients with FFS ≥ 1 [80]. The study compared the effectiveness of a 6-pulse cyclophosphamide treatment versus the standard 12-pulse regimen in combination with the same glucocorticoid treatment protocol (and without maintenance treatment after stopping cyclophosphamide). The authors of this trials found that the shorter 6-pulse regime was significantly less effective in preventing relapses and/or deaths as compared to the standard 12-pulse course in patients with severe PAN [80]. Oral cyclophosphamide still remains an option for refractory patients. As a remission-maintenance therapy, immunomodulators such as azathioprine (2 mg/kg per day) or methotrexate (up to 25 mg/week) can be considered [85]. The choice of medication depends on individual toxicity profiles. Methotrexate is commonly avoided in patients with renal or liver disease.
Due to the rarity of PAN, there are no clinical trials on the optimal therapy in refractory disease. Case reports and case series suggest that rituximab, anti-TNF agents, or tocilizumab may be considered a possible treatment option in patients with difficult to treat PAN [86,87,88,89,90,91]. Moreover, Krusche et al. reported the first case of tocilizumab used successfully as the primary therapy in PAN [91]. Akiyama et al. recently reviewed 11 PAN cases treated with tocilizumab and concluded that this agent has a glucocorticoid-sparing effect and might be an effective treatment option in refractory/relapsing disease. It could even achieve glucocorticoid-free remission in some cases [92]. The reduction of the signal transducer and activator of transcription (STAT) 3 activity through the IL-6 blockade has been speculated to be a potential mechanism of action [92]. More data is needed, but tocilizumab seems to be a valuable option, at least in difficult-to-treat cases.
A case of the successful use of tofacitinib in refractory PAN not responding to several biologics including TNF blockers, rituximab, and tocilizumab has been reported recently [93].
Cutaneous PAN
In mild cases of cutaneous PAN, treatment with nonsteroidal anti-inflammatory drugs, colchicine, or topical glucocorticoids could be tried. In more severe cases, systemic glucocorticoids are used. Moderate glucocorticoid doses, less than 30 mg qd are usually effective, though sometimes high doses of 1 mg/kg body weight are needed. In recurrent cases, azathioprine, or methotrexate can be used [40]. In addition, hydroxychloroquine, dapsone, or intravenous immunoglobulin has also been occasionally used as steroid sparring agents [40]. Patients with cutaneous PAN have a relatively good prognosis, without increased mortality, though the course is frequently chronic with recurrent relapses [43].
HBV-Associated PAN
Due to its rarity, randomized control trials have not been conducted in HBV-associated PAN. The therapeutic strategy is based mainly upon the understanding of disease pathogenesis (i.e., the deposition of HBsAg-anti-HBs immune complexes) [5, 94]. The combination of plasma exchange to clear immune complexes (scheduled initially four times per week for 3 to 4 weeks, followed by a gradual tapering over 6 weeks before being definitively stopped) with antiviral agents (such as interferon α or lamivudine) to suppress viral replication is the standard of care to treat this PAN subtype [95,96,97]. The newer antiviral agents (e.g., adefovir dipivoxil, entecavir, telbivudine, and tenofovir) are potentially also useful for the treatment of HBV-associated PAN, but evidence from clinical trials is lacking. The goal of antiviral treatment is to clear the viral trigger; once seroconversion from HBe antigen to HBe antibody is obtained, patients usually maintain long-term remission without relapse [95, 97]. Glucocorticoids should only be used initially and for a short period of time (e.g., 2 weeks) to rapidly control the severe organ- or life-threatening inflammatory process. Prolonged glucocorticoid use or the use of cytotoxic drugs is associated with an increased risk of HBV relapses and progression towards chronic hepatitis and liver cirrhosis [38].
ADA2 Deficiency-Associated PAN
Anti-tumor necrosis factor (anti-TNF) agents are the treatment of choice for PAN associated with the ADA2 deficiency [98, 99]. There are no preferences between different anti-TNF agents [99]. Anti-TNF agents control the widespread inflammation, with a major reduction of stroke risk [98]. Glucocorticoids and classic immunosuppressive agents (such as azathioprine, cyclosporin, cyclophosphamide, methotrexate, mycophenolate mofetil) are generally associated with a limited response [98]. A positive effect of thalidomide has been described, though adverse effects limit its use as first-line agent [98]. Due to concern of hemorrhagic stroke, antiplatelet and anticoagulation therapies are not recommended in these patients [14]. In case of an immunodeficiency phenotype or bone marrow dysfunction, a hematopoietic stem cell transplant might constitute another treatment option [100]. Caution is needed when a hematopoietic stem cell transplantation is considered in patients with the PAN phenotype due to the higher risk of complications (e.g., veno-oclusive disease) [101]. In addition, fresh frozen plasma has been proposed as a replacement therapy for the ADA2 protein, although a short half-life of ADA2 in plasma after administration is a limiting factor [99].
Conclusion
PAN is a systemic necrotizing vasculitis that typically affects medium-sized muscular arteries. The diagnosis of this rare disease is primarily based on clinical findings, imaging, and histopathological findings. Different PAN variants have been recognized, and they differ in their etiology as well as in the level of severity and prognosis. Cutaneous PAN usually has a favorable prognosis and requires a less aggressive therapeutic approach, based on nonsteroidal anti-inflammatory drugs to glucocorticoids; the focus of HBV-associated PAN is to control of the viral infection and remove immune complexes. TNF alpha inhibitors are the drugs of choice for PAN associated with ADA2 deficiency. Treatment of idiopathic generalized PAN is guided by disease severity and is in severe cases based on the combination of glucocorticoids and cyclophosphamide. Anti-TNF agents, tocilizumab, or rituximab may be a treatment option in refractory PAN.
References
Kussmaul A, Maier R. Über eine bisher nicht beschriebene eigentümliche Arterienerkrankung (Periarteritis nodosa), die mit Morbus Brightii und rapid fortschreitender allgemeiner Muskellähmung einhergeht. Dtsch Arch Klin Med. 1866;1:484–518.
Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92.
Ferrari E. Über Polyarteritis acuta nodosa (sogenannte Periarteritis nodosa), und ihre Beziehungen zur Polymyositis und Polyneuritis acuta. Beitr Pathol Anat. 1903;34:350–86.
Lindberg K. Ein beitrag zur kenntnis der periarteritis nodosa. Acta Med Scand. 1931;76:183.
Trepo C, Thivolet J. Hepatitis associated antigen and periarteritis nodosa (PAN). Vox Sang. 1970;19:410–1.
Patel N, Patel N, Khan T, Patel N, Espinoza LR. HIV infection and clinical spectrum of associated vasculitides. Curr Rheumatol Rep. 2011;13:506–12. https://doi.org/10.1007/s11926-011-0214-6.
Teng GG, Chatham WW. Vasculitis related to viral and other microbial agents. Best Pract Res Clin Rheumatol. 2015;29(2):226–43. https://doi.org/10.1016/j.berh.2015.05.007.
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. https://doi.org/10.1002/art.37715.
Sunderkötter CH, Zelger B, Chen KR, Requena L, Piette W, Carlson JA, et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol. 2018;70:171–84. https://doi.org/10.1002/art.40375.
Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum. 2012;42(2):213–21. https://doi.org/10.1016/j.semarthrit.2012.03.006.
Parker M, McGill NW. Minocycline-associated polyarteritis nodosa and self-limiting hepatitis: a reminder of a potentially re-emerging culprit in drug-induced autoimmune syndromes. Scand J Rheumatol. 2018;47(6):507–8. https://doi.org/10.1080/03009742.2017.1416669.
Hernández-Rodríguez J, Gary S, Hoffman GS. Updating single-organ vasculitis. Curr Opin Rheumatol. 2012;24(1):38–45. https://doi.org/10.1097/BOR.0b013e32834d8482.
Navon Elkan EP, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370:921–31. https://doi.org/10.1056/NEJMoa1307362.
Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370:911–20. https://doi.org/10.1056/NEJMoa1307361.
Özçakar ZB, Çakar N, Uncu N, Çelikel BA, Yalçinkaya F. Familial Mediterranean fever-associated diseases in children. QJM. 2017;110:287–90. https://doi.org/10.1093/qjmed/hcw230.
Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine. 2005;84:1–11. https://doi.org/10.1097/01.md.0000152370.84628.0c.
Mohammad AJ, Jacobsson LT, Mahr AD, Sturfelt G, Segelmark M. Prevalence of Wegener’s granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in Southern Sweden. Rheumatology (Oxford). 2007;46:1329–37. https://doi.org/10.1093/rheumatology/kem107.
Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J, Rodriguez-Ledo P, Llorca J. The epidemiology of the primary systemic vasculitides in Northwest Spain: implications of the Chapel Hill consensus conference definitions. Arthritis Rheum. 2003;49(3):388–93. https://doi.org/10.1002/art.11115.
Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum. 2000;43(2):414–9. https://doi.org/10.1002/1529-0131(200002)43:2<414::AID-ANR23>3.0.CO;2-0.
Watts RA, Lane SE, Scott DG, Koldingsnes W, Nossent H, Gonzalez-Gay MA, et al. Epidemiology of vasculitis in Europe. Ann Rheum Dis. 2001;60:1156e7–1157. https://doi.org/10.1136/ard.60.12.1156a.
Trepo C, Guillevin L. Polyarteritis nodosa and extrahepatic manifestations of HBV infection: the case against autoimmune intervention in pathogenesis. J Autoimmun. 2001;16:269–74. https://doi.org/10.1006/jaut.2000.0502.
Karadag O, Erden A, Bilginer Y, Gopaluni S, Sari A, Armagan B. A retrospective study comparing the phenotype and outcomes of patients with polyarteritis nodosa between UK and Turkish cohorts. Rheumatol Int. 2018;38:1833–40. https://doi.org/10.1007/s00296-018-4122-1.
Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis study group database. Arthritis Rheum. 2010;62:616–26. https://doi.org/10.1002/art.27240.
Ozen S, Anton J, Arisoy N, Bakkaloglu A, Besbas N, Brogan P, et al. Juvenile polyarteritis: results of a multicenter survey of 110 children. J Pediatr. 2004;145:517–22. https://doi.org/10.1016/j.jpeds.2004.06.046.
Alibaz-Oner F, Koster MJ, Crowson CS, Makol A, Steven R, Ytterberg SR, et al. Clinical spectrum of medium-sized vessel vasculitis. Arthritis Care Res (Hoboken). 2017;69(6):884–91. https://doi.org/10.1002/acr.23007.
Chasset F, Francès C. Cutaneous manifestations of medium- and large-vessel vasculitis. Clin Rev Allergy Immunol. 2017;53(3):452–68. https://doi.org/10.1007/s12016-017-8612-9.
de Boysson H, Guillevin L. Polyarteritis nodosa neurologic manifestations. Neurol Clin. 2019;37(2):345–57. https://doi.org/10.1016/j.ncl.2019.01.007.
Ebert EC, Hagspiel KD, Nagar M, Schlesinger N. Gastrointestinal involvement in polyarteritis nodosa. Clin Gastroenterol Hepatol. 2008;6:960–6. https://doi.org/10.1016/j.cgh.2008.04.004.
Maritati F, Iannuzzella F, Pavia MP, Pasquali S, Vaglio A. Kidney involvement in medium- and large-vessel vasculitis. J Nephrol. 2016;29:495–505. https://doi.org/10.1007/s40620-016-0303-8.
Miloslavsky E, Unizony S. The heart in vasculitis. Rheum Dis Clin N Am. 2014;40:11–26. https://doi.org/10.1016/j.rdc.2013.10.006.
Bae YD, Choi HJ, Lee JC, Park JJ, Lee YJ, Lee EB, et al. Clinical features of polyarteritis nodosa in Korea. J Korean Med Sci. 2006;21:591–5. https://doi.org/10.3346/jkms.2006.21.4.591.
Rothschild PR, Pagnoux C, Seror R, Brézin AP, Delair E, Guillevin L. Ophthalmologic manifestations of systemic necrotizing vasculitides at diagnosis: a retrospective study of 1286 patients and review of the literature. Semin Arthritis Rheum. 2013;42:507–14. https://doi.org/10.1016/j.semarthrit.2012.08.003.
Ungprasert P, Koster MJ, Thongprayoon C, Warrington KJ. Risk of venous thromboembolism among patients with vasculitis: a systematic review and meta-analysis. Clin Rheumatol. 2016;35:2741–7. https://doi.org/10.1007/s10067-016-3394-7.
Frohent PP, Sheps SG. Long-term follow-up study of periarteritis nodosa. Am J Med. 1967;43:8–14. https://doi.org/10.1016/0002-9343(67)90144-1.
Jardel S, Puéchal X, Le Quellec A, Pagnoux C, Hamidou M, Maurier F, et al. Mortality in systemic necrotizing vasculitides: a retrospective analysis of the French vasculitis study group registry. Autoimmun Rev. 2018;17:653–9. https://doi.org/10.1016/j.autrev.2018.01.022.
Gayraud M, Guillevin L, Toumelin PL, Cohen P, Lhote F, Casassus P, et al. Long-term follow up of polyarteritis nodosa, microscopic polyangiitis and Churg Strauss syndrome: analysis of four prospective trials including 278 patients. Arthritis Rheum. 2001;44:666–75. https://doi.org/10.1002/1529-0131(200103)44:3<666::AID-ANR116>3.0.CO;2-A.
Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996;75:17–28. https://doi.org/10.1097/00005792-199601000-00003.
Guillevin L, Mahr A, Callard P, Godmer P, Pagnoux C, Leray E, et al. Hepatitis B virus-associated polyarteritis nodosa. Clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine. 2005;84:313–22. https://doi.org/10.1097/01.md.0000180792.80212.5e.
Criado PR, Marques GF, Morita TC, de Carvalho JF. Epidemiological, clinical and laboratory profiles of cutaneous polyarteritis nodosa patients: report of 22 cases and literature review. Autoimmun Rev. 2016;15:558–63. https://doi.org/10.1016/j.autrev.2016.02.010.
Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750–6. https://doi.org/10.1111/j.1365-4632.2010.04522.x.
Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathological study of 79 cases. Br J Dermatol. 1997;136:706–13.
Kato A, Hamada T, Miyake T, Morizane S, Hirai Y, Yamasaki O, et al. Clinical and laboratory markers associated with relapse in cutaneous polyarteritis nodosa. JAMA Dermatol. 2018;154(8):922–6. https://doi.org/10.1001/jamadermatol.2018.1601.
Munera-Campos M, Bielsa I, Martínez-Morillo M, Aparicio G, Olivé A, Ferrándiz C. Manifestations, clinical course and prognostic markers in cutaneous polyarteritis nodosa. J Dtsch Dermatol Ges. 2020;18:1250–9. https://doi.org/10.1111/ddg.14271.
Chen KR. Cutaneous polyarteritis nodosa: a clinical and histopathological study of 20 cases. J Dermatol. 1989;16:429–42.
Kendall JL, Michael J, Springer JM. The many faces of a monogenic autoinflammatory disease: adenosine deaminase 2 deficiency. Current Rheumatology Reports. 2020;22:64. https://doi.org/10.1007/s11926-020-00944-1.
Human A, Pagnoux C. Diagnosis and management of ADA2 deficient polyarteritis nodosa. Int J Rheum Dis. 2019;22(Suppl 1):69–77. https://doi.org/10.1111/1756-185X.13283.
Moens L, Hershfield M, Arts K, Aksentijevich I, Meyts I. Human adenosine deaminase 2 deficiency: a multi-faceted inborn error of immunity Immunol Rev. Jan. 2019;287:62–72. https://doi.org/10.1111/imr.12722.
Sahin S, Adrovic A, Kasapcopur O. A monogenic autoinflammatory disease with fatal vasculitis: deficiency of adenosine deaminase 2. Curr Opin Rheumatol. 2020;32:3–14. https://doi.org/10.1097/BOR.0000000000000669.
Jayne D. The diagnosis of vasculitis. Best Pract Res Clin Rheumatol. 2009;23:445–53. https://doi.org/10.1016/j.berh.2009.03.001.
Sönmez HE, Armağan B, Ayan G, Barut K, Batu ED, Erden A, et al. Polyarteritis nodosa: lessons from 25 years of experience. Clin Exp Rheumatol. 2019;37(Suppl 117):52–6.
Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222e7–27. https://doi.org/10.1136/ard.2006.054593.
Stanson AW, Friese JL, Johnson CM, McKusick MA, Breen JF, Sabater EA, et al. Polyarteritis nodosa: spectrum of angiographic findings. Radiographics. 2001;21:151–9. https://doi.org/10.1148/radiographics.21.1.g01ja16151.
Hekali P, Kajander H, Pajari R, Stenman S, Somer T. Diagnostic significance of angiographically observed visceral aneurysms with regard to polyarteritis nodosa. Acta Radiol. 1991;32:143–8.
Ozaki K, Miyayama S, Ushiogi Y, Matsui O. Renal involvement of polyarteritis nodosa: CT and MR findings. Abdom Imaging. 2009;34:265–70. https://doi.org/10.1007/s00261-008-9377-7.
Bleeker-Rovers CP, Bredie SJ, van der Meer JW, Corstens FH, Oyen WJ. F-18-fluorodeoxyglucose positron emission tomography in diagnosis and follow-up of patients with different types of vasculitis. Neth J Med. 2003;61:323–9.
Watanabe TT, Shiojiri T. PET-CT and polyarteritis nodosa-associated artery aneurysms. QJM. 2019;1(112):219–20. https://doi.org/10.1093/qjmed/hcy308.
Wang H, Li J, Jiang Y, et al. Polyarteritis nodosa with multiple aneurysms and renal arteriovenous fistula successfully diagnosed by colour Doppler sonography. Clin Rheumatol. 2013;32(Suppl. 1):S89e92. https://doi.org/10.1007/s10067-010-1519-y.
Soudack M, Gaitini D, Ofer A. Celiac artery aneurysm: diagnosis by color doppler sonography and three-dimensional CT angiography. J Clin Ultrasound. 1999;27:49–51. https://doi.org/10.1002/(sici)1097-0096(199901)27:1<49::aid-jcu9>3.0.co;2-z.
Darras-Joly C, Lortholary O, Cohen P, Brauner M, Guillevin L. Regressing microaneurysms in 5 cases of hepatitis B virus related polyarteritis nodosa. J Rheumatol. 1995;22:876–80.
Albert DA, Rimon D, Silverstein MD. The diagnosis of polyarteritis nodosa. I A literature-based decision analysis approach. Arthritis Rheum. 1988;31:1117e27. https://doi.org/10.1002/art.1780310906.
Weinrich JM, Lenz A, Adam G, François CJ, Bannas P. Radiologic imaging in large and medium vessel vasculitis. Radiol Clin N Am. 2020;58(4):765–79. https://doi.org/10.1016/j.rcl.2020.02.001.
Lie JT. Systemic and isolated vasculitis. A rational approach to classification and pathologic diagnosis. Pathol Annu. 1989;24:25–114.
Cid MC, Grau JM, Casademont J, Campo E, Coll-Vinent B, López-Soto A, et al. Immunohistochemical characterization of inflammatory cells and immunology activation markers in muscle and nerve biopsy specimens from patients with systemic polyarteritis nodosa. Arthritis Rheum. 1994;37:1055–61. https://doi.org/10.1002/art.1780370711.
Holl-Ulrich K, Noack F, Feller AC. Vasculitis: histopathology and differential diagnosis. Z Rheumatol. 2009;68:320–8. https://doi.org/10.1007/s00393-008-0402-6.
Zavialov AV, Garcia E, Glaichenhaus N, Franco R, Zavialov AV, Lauvau G. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol. 2010;88:279–90. https://doi.org/10.1189/jlb.1109764.
Zavialov AV, Engstrom A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J. 2005;391:51–7. https://doi.org/10.1042/BJ20050683.
Zavialov AV, Yu X, Spillmann D, Lauvau G, Zavialov AV. Structural basis for the growth factor activity of human adenosine deaminase ADA2. J Biol Chem. 2010;285:12367–77. https://doi.org/10.1074/jbc.M109.083527.
Karmouty-Quintana H, Xia Y, Blackburn MR. Adenosine signaling during acute and chronic disease states. J Mol Med (Berl). 2013;91:173–81. https://doi.org/10.1007/s00109-013-0997-1.
Fries JF, Hunder GG, Bloch DA, Michel BA, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary Arthritis Rheum. 1990 Aug;33(8):1135e6–1136. https://doi.org/10.1002/art.1780330812.
Lightfoot RW Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, Mc Shane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33(8):1088e93.
Yamamoto S, Oiwa H. Provisional seven-item criteria for the diagnosis of polyarteritis nodosa. Rheumatol Int. 2020;40(8):1223–7. https://doi.org/10.1007/s00296-020-04535-2.
Seeliger B, Sznajd J, Robson JC, Judge A, Craven A, Grayson PC, et al. Are the 1990 American College of Rheumatology vasculitis classification criteria still valid? Rheumatology (Oxford). 2017;56(7):1154–61. https://doi.org/10.1093/rheumatology/kex075.
Bloch DA, Michel BA, Hunder GG, McShane DJ, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Patients and methods. Arthritis Rheum. 1990;33(8):1068e73–1073. https://doi.org/10.1002/art.1780330803.
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798e806. https://doi.org/10.1136/ard.2009.116657.
Luqmani RA, Suppiah R, Grayson PC, Merkel PA, Watts R. Nomenclature and classification of vasculitis– update on the ACR/EULAR diagnosis and classification of vasculitis study (DCVAS). Clin Exp Immunol. 2011;164(Suppl 1):11–3. https://doi.org/10.1111/j.1365-2249.2011.04358.x.
Robson JC, Grayson PC, Ponte C, Suppiah R, Craven A, Khalid S, et al. OP0021 draft classification criteria for the anca associated vasculitides. Ann Rheum Dis. 2018;77:60–1. https://doi.org/10.1136/annrheumdis-2018-eular.2892.
Ponte C, Grayson P, Suppiah R, Robson J, Gribbons K, Craven A, et al. 077. Classification criteria for large-vessel vasculitis, Rheumatology, Volume 58, Supplement 2, March 2019, kez058.017, https://doi.org/10.1093/rheumatology/kez058.017
Henegar C, Pagnoux C, Puéchal X, Zucker JD, Bar-Hen A, Le Guern V, et al. A paradigm of diagnostic criteria for polyarteritis nodosa: analysis of a series of 949 patients with vasculitides. Arthritis Rheum. 2008;58(5):1528–38. https://doi.org/10.1002/art.23470.
Mukhtyar C, Guillevin L, Cid MC, , Dasgupta D, de Groot K, Gross W, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009;68(3):310–317. https://doi.org/10.1136/ard.2008.088096.
Guillevin L, Cohen P, Mahr A, Arène JP, Mouthon L, Puéchal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis with poor prognosis factors: a prospective trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in sixty-five patients. Arthritis Rheum. 2003;49(1):93–100. https://doi.org/10.1002/art.10922.
Ribi C, Cohen P, Pagnoux C, Mahr A, Arène JP, Puéchal X, et al. French Vasculitis study group. Treatment of polyarteritis nodosa and microscopic polyangiitis without poor-prognosis factors: a prospective randomized study of one hundred twenty-four patients. Arthritis Rheum. 2010;62(4):1186–97. https://doi.org/10.1002/art.27340.
Puechal X, Pagnoux C, Baron G, Quemeneur T, Neel A, Agard C. Adding azathioprine to remission-induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors. Arthritis Rheumatol. 2017;69(11):2175–86. https://doi.org/10.1002/art.40205.
Samson M, Puéchal X, Devilliers H, Ribi C, Cohen P, Bienvenu B, et al. Mononeuritis multiplex predicts the need for immunosuppressive or immunomodulatory drugs for EGPA, PAN and MPA patients without poor prognosis factors. Autoimmun Rev. 2014;13(9):945–53. https://doi.org/10.1016/j.autrev.2014.08.002.
Gayraud M, Guillevin L, Cohen P, Lhote F, Cacoub P, Deblois P, et al. Treatment of good-prognosis polyarteritis nodosa and Churg–Strauss syndrome: comparison of steroids and oral or pulse cyclophosphamide in 25 patients. French cooperative study group for vasculitides. Br J Rheumatol. 1997;36(12):1290–7. https://doi.org/10.1093/rheumatology/36.12.1290.
de Menthon M, Mahr A. Treating polyarteritis nodosa: current state of the art. Clin Exp Rheumatol. 2011;29(1 Suppl 64):S110–6.
Muratore F, Pazzola G, Soriano A, Pipitone N, Croci S, Bonacini M, et al. Unmet needs in the pathogenesis and treatment of vasculitides. Clin Rev Allergy Immunol. 2018;54:244–60. https://doi.org/10.1007/s12016-017-8643-2.
Ginsberg S, Rosner I, Slobodin G, Rozenbaum M, Kaly L, Jiries N, et al. Infliximab for the treatment of refractory polyarteritis nodosa. Clin Rheumatol. 2019;38(10):2825–33. https://doi.org/10.1007/s10067-019-04474-9.
Seri Y, Shoda H, Hanata N, Nagafuchi Y, Sumitomo S, Fujio K, et al. A case of refractory polyarteritis nodosa successfully treated with rituximab. Mod Rheumatol. 2017;27(4):696–8. https://doi.org/10.3109/14397595.2015.1014153.
Ostrovršnik J, Hočevar A, Lestan B, Sodin Semrl S, Lakota K, Tomsic M. Long-term follow-up on tocilizumab treatment of AA amyloidosis secondary to polyarteritis nodosa. Amyloid. 2016;23:260–1. https://doi.org/10.1080/13506129.2016.1232648.
Krusche M, Ruffer N, Kötter I. Tocilizumab treatment in refractory polyarteritis nodosa: a case report and review of the literature. Rheumatol Int. 2019;39(2):337–44. https://doi.org/10.1007/s00296-018-4210-2.
Krusche M, Ruffer N, Schneider U, Meyer M, Burmester G, Kötter I. Tocilizumab treatment for polyarteritis nodosa. Rheumatology (Oxford). 2020;59(10):e63–5. https://doi.org/10.1093/rheumatology/keaa079.
Akiyama M, Kaneko Y, Takeuchi T. Tocilizumab for the treatment of polyarteritis nodosa: a systematic literature review. Ann Rheum Dis. 2020;9:annrheumdis-2020-218710. https://doi.org/10.1136/annrheumdis-2020-218710.
Rimar D, Alpert A, Starosvetsky E, Rosner I, Slobodin G, Rozenbaum M, et al. Tofacitinib for polyarteritis nodosa: a tailored therapy. Ann Rheum Dis. 2016;75(12):2214–6. https://doi.org/10.1136/annrheumdis-2016-209330.
Michalak T. Immune complexes of hepatitis B surface antigen in the pathogenesis of periarteritis nodosa. Am J Pathol. 1978;90:619–32.
Guillevin L, Lhote F, Leon A, Fauvelle F, Vivitski L, Trepo C. Treatment of polyarteritis nodosa related to hepatitis B virus with short term steroid therapy associated with antiviral agents and plasma exchanges. A prospective trial in 33 patients. J Rheumatol. 1993;20:289–98.
Guillevin L, Lhote F, Sauvaget F, Deblois P, Rossi F, Levallois D, et al. Treatment of polyarteritis nodosa related to hepatitis B virus with interferon-alpha and plasma exchanges. Ann Rheum Dis. 1994;53:334–7. https://doi.org/10.1136/ard.53.5.334.
Guillevin L, Mahr A, Cohen P, Larroche C, Queyrel V, Loustaud-Ratti V, et al. Short-term corticosteroids then lamivudine and plasma exchanges to treat hepatitis B virus-related polyarteritis nodosa. Arthritis Rheum. 2004;51(3):482–7. https://doi.org/10.1002/art.20401.
Caorsi R, Penco F, Grossi A, Insalaco A, Omenetti A, Alessio M, et al. ADA2 deficiency (DADA2) as an unrecognised cause of early onset polyarteritis nodosa and stroke: a multicentre national study. Ann Rheum Dis. 2017;76(10):1648–56. https://doi.org/10.1136/annrheumdis-2016-210802.
Ombrello AK, Qin J, Hoffmann PM, Kumar P, Stone D, Jones A, et al. Treatment strategies for deficiency of adenosine deaminase 2. N Engl J Med. 2019;380(16):1582–4. https://doi.org/10.1056/NEJMc1801927.
Hashem H, Kumar AR, Müller I, Babor F, Bredius R, Dalal J, et al. Hematopoietic stem cell transplantation rescues the hematological, immunological and vascular phenotype in DADA2. Blood. 2017;130:2682–8. https://doi.org/10.1182/blood-2017-07-798660.
Van Eyck L Jr, Hershfield MS, Pombal D, Kelly SJ, Ganson NJ, Moens L, et al. Hematopoietic stem cell transplantation rescues the immunologic phenotype and prevents vasculopathy in patients with adenosine deaminase deficiency. J Allergy Clin Immunol. 2015;135(1):283–7. e5. https://doi.org/10.1016/j.jaci.2014.10.010.
Acknowledgments
The authors would like to thank Assoc. Prof. Vesna Jurčić, MD PhD and Assoc. Prof. Miroslav Mayer, MD PhD who kindly provided imaging material for the manuscript.
Funding
Alojzija Hočevar, Matija Tomšič and Katja Perdan Pirkmajer are supported by Slovenian Research Agency Grants P3-0314. Katja Perdan Pirkmajer is also supported by Slovenian Research Agency Grant J7-8276.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethics Approval
Ethics approval was not needed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vasculitis
Rights and permissions
About this article
Cite this article
Hočevar, A., Tomšič, M. & Perdan Pirkmajer, K. Clinical Approach to Diagnosis and Therapy of Polyarteritis Nodosa. Curr Rheumatol Rep 23, 14 (2021). https://doi.org/10.1007/s11926-021-00983-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-00983-2