Abstract
Objectives
The aim was to comparatively assess the clinical and imaging features in patients with SAPHO syndrome.
Methods
The clinical data, laboratory results, imaging data of forty-six SAPHO patients were reviewed and the SAPHO patients were divided into spinal involvement group and non-spinal involvement group. Fifty patients with ankylosing spondylitis were recruited as control group. The clinical and radiological features of them were analyzed and compared.
Results
Thirty-four of 46 (73.9%) of all the SAPHO patients had spinal involvement. The lesions exhibited as abnormal hyper-intensity signal in vertebral bodies, vertebral body erosion or collapse, bone marrow edema, endplate inflammation, spondyldiscitis, paravertebral ossification, and facet joint involvement. Compared with patients in non-spinal involvement group, the age at disease onset was older (P = 0.033), the disease duration was longer (P = 0.048), and CRP level was elevated (P = 0.047) in patients in spinal involvement group. Compared with patients with ankylosing spondylitis, SAPHO patients were more likely to have cervical vertebra involvement (P = 0.024), endplate inflammation (P = 0.019), and spondyldiscitis (P = 0.001), but less multiple vertebral body and facet joint involvement (P = 0.002). Patients regularly received DMARDS or biologics treatment had symptoms relieved and lesions turned into chronic stage or better than before.
Conclusions
A total of 73.9% of the SAPHO patients had spinal involvement and the involvement could affect any part of the spine. Cervical vertebral involvement, endplate inflammation, and sponlypodiscitis were more common in SAPHO than in patients with ankylosing spondylitis. In SAPHO patients with spinal involvement, the disease duration was longer and the inflammatory reaction was more intensive. DMARDs and biologics may help to prevent the disease progress.
Key points: • To the best of our knowledge, this paper is the first one to comparatively study the clinical and radiological features of SAPHO syndrome, especially the characteristics of spinal involvement. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteomyelitis, SAPHO) syndrome is a chronic rare syndrome presented with inflammatory osteoarticular and cutaneous involvement. The concept of SAPHO syndrome was first introduced by Chamot et al. [1] in 1987. Khan [2] proposed the diagnostic criteria of SAPHO syndrome in 1994. Spinal involvement can be found in one-third of the patients [2,3,4] which presents as vertebral body osteosclerosis, hyperostosis, paravertebral ossification, or vertebral collapse [5].
The diagnosis of SAPHO syndrome is based on the typical dermatological and osteoarticular involvement. However, it is reported that skin manifestations are detected in 63.5% patients [6], which means at least 15% patients never experience skin involvement [7, 8]. In addition, the bone lesions of SAPHO syndrome share some overlapping features with some spondyloarthropathies such as reactive arthritis, psoriatic arthritis [9]. So the prevalence of SAPHO syndrome may be underestimated and a part of the patients might be misdiagnosed and received inappropriate treatment. Specific antibody or inflammatory factor has not been found to be helpful to diagnose the disease, so it is of vital importance for us to understand the specific osteoarticular syndrome and radiographic features of SAPHO syndrome. Although the conventional skeleton symptom and radiological findings have been detailed described in some literatures, the comparison of the skeleton symptom, radiological feature (including bone scanning, MR, and CT), and follow-up visits of SAPHO patients is limited. The purpose of our study is to summarize the above points of SAPHO syndrome.
Materials and methods
Study population and data collection
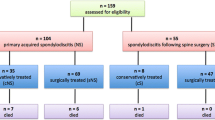
The ethic committees of Peking University Third Hospital did not require approval or informed patients consent for this retrospective study. The electronic medical data and radiological images of patients, who visited clinic or were hospitalized in Peking University Third Hospital from 2006 to 2018 and were diagnosed as “SAPHO syndrome” or “chronic osteomyelitis” were retrospectively reviewed. Patients whose symptoms lasting for at least 1 month and fulfilling the criteria of Chamot et al. [1] were included. The criteria were as follows: (a) osteoarticular manifestations of palmoplantar pustulosis or severe acne; (b) hyperostosis with or without dermatosis; and (c) chronic recurrent multifocal osteomyelitis involving axial or peripheral skeleton, with or without dermatosis. Exclusion criteria were septic osteomyelitis, infectious chest wall arthritis, bone tumor, and infectious palmoplantar pustulosis.
The electronic medical data and radiological images of patients, who visited clinic or were hospitalized in Peking University Third Hospital from 2006 to 2018 and were diagnosed as “AS (Ankylosing Spondylitis, AS)” were reviewed. Patients who met the criteria of New York classification revised in 1987 and were proved to have spinal involvement by radiological approach were chosen as control group.
All patients’ medical data were reviewed, including age, gender, age at SAPHO syndrome diagnosis, the onset and course of dermatological, and osteoarticular manifestations. Laboratory evaluation included the number of white blood cells, ESR (erythrocyte sedimentation rate), CRP (C-reactive protein), RF (rheumatoid factor) HLA-B27, TNF-α, IL-6, IL-17A, and pathological examination. Imaging data, including 99Tc bone scanning, PET-CT, CT, or MRI of the sites involved or whole-spine MRI were reviewed. Pathological results of the skeleton involved were also reviewed. All the imaging data and pathological data were analyzed by our cooperative group, which was made up of one rheumatologist, one radiologist, one expert on nuclear scintigraphy, and one pathologist. We followed up some of the SAPHO patients once 3 to 6 months to find changes in symptoms, and imaging were compared with the previous time.
Statistical analysis
Categorical variables are presented as numbers (percentage), and quantitative data are presented as mean value (SD). The Fisher test was used to compare categorical variables, and t test was used to compare quantitative variables. Statistical significance was assumed for P value less than 0.05. All analysis was computed using SPSS statistics V. 24.0.
Results
Clinical manifestations of patients
Forty-six patients diagnosed with SAPHO syndrome were included in our study. The mean age of the patients was 50.17 ± 10.93 years old, and the mean duration of disease was 5.43 ± 6.46 years. Twenty-nine patients had both osteoarticular and cutaneous involvement, while 17 patients presented with only osteoarticular symptoms. The cutaneous involvement presented as PPP (palmoplantar pustulosis) and SA (severe acne) (Fig. 1). Fourteen patients’ dermatological symptoms presented before osteoarticular symptoms, while fifteen patients had dermatological symptoms after or simultaneously with the osteoarticular symptoms. The most common osteoarticular symptoms were recurrent osteoarticular pain and swelling with or without morning stiffness which could occur at different sites of bone or joint. ESR and CRP were increased in 62.5% and 64.7% of all the SAPHO patients respectively. Serum TNF-α, IL-6, and IL-17A increased significantly. HLA-B27 was found positive in only one patient, and RF was negative in all the patients. In two patients, white blood cell number increased mildly. Details of the clinical manifestations of all the patients are shown in Table 1.
Imaging findings
According to the imaging findings, thirty-four patients were divided into spinal involvement and twelve patients were divided into non-spinal involvement group.
Anterior chest wall
The bone lesion region was presented as increased tracer uptake on the whole-body bone scanning. Totally, forty-four (95.7%) patients presented with anterior chest wall involvement, and the affected lesion included sternocostal joint (18/44), sternoclavicular joint (30/44), sternum body (12/44), sternum corner (4/44), manubrim (6/44), the first rib (13/44), and clavicle (8/44). We could observe the typical “bull head” change in the bone scanning image of six (13.6%) patients (Figs. 2, 5a).
Spine lesions
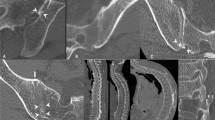
Among the thirty-four (73.9%) patients with spinal involvement, the thoracic spine was the most commonly involved (n = 22, 64.7%). The lumbar and the cervical spine were the next most commonly involved (both n = 17, 50.0%). Contiguous involvement of vertebral bodies was found in eighteen patients (52.9%). All the patients were accompanied with vertebral inflammatory changes, and variable manifestations could be found in their radiographic images including partial or diffuse abnormal hypertensive signal in vertebral bodies, vertebral body erosion or collapse, osteosclerosis, and bone marrow edema during the acute stage (Figs. 3, 4). Fat deposition was observed in the chronic stage. The acute and chronic change could be seen at the same time. The adjacent affected vertebral bodies of two patients presented as the “semicircular pattern” or “kissing syndrome.” Other radiological features included endplate inflammation (n = 8, 23.5%), spondylodiscitis (n = 16, 47.1%), paravertebral ossification (n = 4, 11.8%), facet joint involvement (n = 3, 8.9%) (Figs. 3, 4), and Schmorl nodes (n = 4, 11.8%).
CT and MR images in a 47-year-old woman diagnosed with SAPHO syndrome. a Sagittal CT image shows T7–8 vertebral bodies’ erosion, bone density increased, and irregular pattern of the endplate. T1-weighted (b) and T2-weighted (c) images demonstrate hypointensity signal in T7–8 vertebral bodies and slightly swelling of the paravertebral soft tissue. d STIR image shows a mixed hypo- and hyper-intensity signal in the affected vertebral bodies. e Sternoclavicular CT image depicts bone sclerosis and erosion with joint fusion. CT computed tomography. f 3DCT depict the sterno-costo-clavicular hyperostosis
CT and MR images in a 47-year-old man with back pain, limited motion of the shoulder joints, and dysphagia. d Sagittal CT image shows C3–7 vertebral bodies’ bone density increased, destruction in the anterior of vertebral bodies and ossification of the anterior longitudinal spinal ligament. T1-weighted (b) and T2-weighted (C) images demonstrate multiple hypointensity signal in C3–7 vertebral bodies, progressive anterior non-infectious vertebral fusion, and ankylosis along the cervical spine, swelling of the paravertebral soft tissue. d STIR image shows ossification of the anterior longitudinal ligaments along the cervical spine and eventually ended up with ankyloses. MR magnetic resonance
Sacroiliac joint
Eleven of the forty-six (23.9%) patients had their sacroiliac joint involved, six (13.0%) patients presented as bilateral joints affected and five presented as unilateral. The sacroiliac joint abnormalities presented as joint space narrowing and joint surface erosion or sclerosis (Fig. 5).
A 37-year-old man diagnosed with SAPHO syndrome. Technetium bone scintigraphy shows increased uptake in the sacroiliac joint and sternoclavicular joint (a). The right sacroiliac joint surface erosion on T1-weighted MR image (b) and joint undersurface bone erosion with bone marrow edema. d Enhance signal of the right sacroiliac joint and synovium can be seen in the enhanced MRI
Pathology
Five patients underwent biopsy, and all of them were single biopsy. Three patients had their biopsies taken from vertebral body; one patient’s biopsy was taken from clavicle and one from sternum. All five biopsies presented as fibrous tissue hyperplasia or bone metaplasia accompanied by lymphocyte or plasmocyte infiltration suggesting non-specific chronic inflammatory change. Coagulation necrosis was found in one lumbar vertebral biopsy. No evidence of microbial infections or malignancy was found.
Comparison
The comparison of clinical characteristics and osteoarticular involvement of the SAPHO patients with or without spinal involvement were summarized in Table 2. The patients with spinal involvement were older; their disease duration was longer, and the serum CRP level was significantly higher (4.59 ± 12.10 vs 3.04 ± 4.34, P = 0.047) than the patients without spinal involvement. The comparison of the osteoarticular involvement in the two groups is summarized in Table 3. The spinal involvement group were more likely to have sternum corner (P = 0.02) and sternocostal joint (P = 0.003) involvement.
The comparison of clinical characteristics and spinal involvement in SAPHO and AS patients was summarized in Table 4 and Table 5 respectively. The SAPHO syndrome was more likely to affect middle-aged women and the inflammatory back pain was not as typical as AS. With the respect of spinal lesion, SAPHO patients were more likely to suffer from cervical spine involvement (P = 0.024), endplate inflammation (P = 0.019), and spondyldiscitis (P = 0.001) when compared with AS patients. The percentage of contiguous or multiple vertebral body involvement in AS patients was significantly higher than in SAPHO patients (P = 0.002), and AS patients were more likely to suffer from facet joint involvement (P = 0.0032).
Follow-up information
Nineteen patients were regularly followed up every 3 to 6 months. In eight patients, remission of osteoarticular pain was achieved after taking NSAIDs plus DMARDs and/or TNF-α inhibitor. In seven patients, osteoarticular pain reappeared after they stopped medication voluntarily when remission was achieved. Imaging reexamination was performed in three patients and fat deposition could be found in the affected sites suggesting the lesion turned into chronic stage. Three patients took merely NSAIDs and the osteoarticular pain did not improved. One patient regularly received NSAIDs and TNF-αinhibitor, and the radiological imaging showed demission of bone marrow edema but the symptom did not become better.
Discussion
SAPHO syndrome is a rare syndrome presenting with inflammatory osteoarticular and cutaneous symptoms. The typical clinical symptoms include PPP, SA, and sternocostoclavicular hyperostosis. The diagnosis of SAPHO syndrome is not difficult among the patients with typical manifestations. But at least 15% of the patients never experience skin involvement, and in some patients, the cutaneous symptoms occur after the onset of the osteoarticular symptoms. In addition, some patients only have spinal involvement without the typical sternocostoclavicular involvement. For these difficult cases, understanding the specific osteoarticular syndrome and radiographic features of SAPHO syndrome is very important.
In our study, we retrospectively analyzed the clinical and imaging feature of forty-six patients suffered from SAPHO syndrome. Our epidemiological data suggested that SAPHO syndrome mainly affected middle-aged adults and this result was consistent with several previous studies suggested that this disease was predominantly found in patients with average age of 30–50 years old [10,11,12]. The age at diagnosis in SAPHO syndrome group was significantly older than AS group suggesting that SAPHO syndrome often occurs among middle-age people, while AS has a predominance in young people.
The pathogenesis of SAPHO syndrome is still unknown. There are two hypotheses explained the pathogenesis of the disease. One is that propionibacterium infection activates the innate immunity and T cell mediated immune process which leads to chronic inflammation presented as the typical symptoms of SAPHO syndrome [13, 14]. The other hypothesis is the genetic theory suggesting that SAPHO syndrome is possibly associated with HLA-B27 and presents as family tendency [13,14,15,16,17]. In our study, patients’ serum inflammatory markers including CRP, ESR, TNF, and IL-6 significantly elevated indicating that SAPHO syndrome is an inflammatory disease associated with immunological reaction. In addition, we found that serum CRP level was significantly elevated among patients with spinal involvement suggesting that spinal involvement represents a more intensive inflammatory response. The inflammatory markers elevated more evidently in SAPHO patients than in AS patients indicating that SAPHO syndrome tends to trigger a more intensive inflammatory reaction than AS. HLA-B27 was negative in the majority of our SAPHO patients and their first degree relatives did not suffer from the same disease suggesting that SAPHO syndrome may not be related to HLA-B27.
In our study, 63.0% (29/46) of the patients had both cutaneous and osteoarticular involvement and this result confirmed some currently published study that skin manifestation are detected in only 60–70% SAPHO syndrome patients, whereas at least 15% patients never experience skin manifestations [6,7,8]. Thus, it is of vital importance to find out the specific radiological features of SAPHO syndrome.
Osteoarticular involvement including anterior chest wall, axial skeleton, and sacroiliac joint is characteristic of SAPHO syndrome, and radiological examinations are necessary to detect those lesions. The osteoarticular manifestations include synovitis, hyperostosis, and osteitis. Some research found that inflammatory enthesopathy was also a feature of SAPHO syndrome [4, 18, 19]. In our study, forty-four patients accompanied with anterior chest wall involvement presented as local inflammatory pain and swelling. The whole-body bone scanning was of good sensitivity in identifying the lesion region and we found that sternoclavicle and sternum were most frequently involved presenting as increased uptake.
Spine is the second common location of SAPHO syndrome. Totally, 73.9% of all the patients in our study were accompanied with spinal involvement. Most of them presented as recurrent musculoskeletal pain or stiffness or limitation of motion. Our study found that the thoracic spine was the most frequently involved vertebral segment with a prevalence of 64.7% and this result was consistent with the finding by Takigawa et al. [20] or by Wenrui Xu et al. [21] but differed from the finding by Laredo et al. [5].
At active stage, the spinal involvement usually presents as vertebral inflammation including vertebral bodies collapse with osteolytic destruction, vertebral body corner lesions (most commonly involve one of the anterior corners), osteosclerosis or hyperostosis of the vertebral body, and bone marrow edema [22]. Previous research suggested that the affection was usually segmental, but two or more adjacent vertebral bodies could be affected [5]. Besides vertebral lesion, paravertebral soft tissue edema, spondylodiscitits, and endplate inflammation can also be observed. When it turns into chronic stage, fat deposition is the most common manifestation of the affected lesions. In our study, all the thirty-four patients presented as a mixture of spinal involvement features and the features above mentioned could be seen on CT or MR image. Among them, eighteen patients (52.9%) presented as multiple vertebral bodies involvement and the affected sites were adjacent or nonadjacent indicating that vertebral body involvement in SAPHO syndrome was multifocal. Thus, whole-spine CT or whole-body bone scanning is meaningful in evaluating axial skeleton lesions.
Vertebral body corner lesion was found in some patients with SAPHO syndrome in published research [9]. Wenrui Xu et al. [21] proposed two theories about the pathology: (1) inflammatory enthesis and (2) reactive osteitis elicited by slow microorganism infection which caused by a poor blood circulation in the special anatomical site. In our study, one patient had a biopsy of the affected lumbar spine and was found coagulation necrosis that might result from local asphyxia. This result suggested that the pathology might be related to microorganism infection. However, the pathogen culture of the biopsy was negative. Therefore, biopsy of multiple sites or different stages of the affected spinal segments should be performed if possible.
Laredo et al. [5] suggested that the corner erosion indicates enthesitis which was similar to the Romanus lesion found in AS. The Romanus lesion consists of erosion manifestation at the annulus fibrosis on the ring apophysis of the vertebral endplate and can be considered as an enthesis of the anterior or posterior longitudinal ligamentous complexes [22, 23]. The Romanus lesions undergo several radiographic stages: erosion of an anterior vertebral body corner, then reactive sclerosis of the adjacent cancellous bone, and finally ossification with formation of a syndesmophyte. However, unlike the typical Ramanous lesions in AS, the vertebral body involvement in SAPHO syndrome can progress to the adjacent vertebral endplate and/or the anterior cortex of the vertebral body [22] and this feature may help to distinguish the two different diseases in the aspect of radiology. Mcgauvran et al. [9] and Wenrui Xu [21] proposed a semicircular pattern of contiguous vertebral body involvement localized in either anterior or posterior vertebral body or a “kissing” appearance on both sides of a disc, which is suggestive of a local spread of the lesions and could be a specific feature of SAPHO syndrome. In our study, two patients were accompanied with this typical radiological feature at active stage. This semicircular or kissing appearance did not manifest among our AS patients.
As for the initial location in spine, Earwaker et al. [18] proposed that the non-specific spondylodiscitis might be the initial manifestation of SAPHO syndrome. Toussirot et al. [24] proposed that the lesion started from the vertebral body and then spread to the endplate or disc space. In our study, we found that the percentages of patients suffered from endplate and disc space involvement were significantly higher in SAPHO group than in AS group. All the SAPHO patients with spinal involvement had their vertebral body affected, and 23.5% and 47.1% of the patients suffered from disc and endplate involvement respectively. As a result, we propose that spondylodiscitis and endplate inflammation are two characteristics distinguishing SAPHO syndrome from AS in the radiological aspect. The result that all the patients were accompanied with vertebral body involvement coincided with the theory. Toussirot et al. brought up that the lesion started from the vertebral body and spread to other location. Since the pathology of spinal involvement is still ambiguous, further research and large sample investigations are still in need.
Some researchers proposed that SAPHO syndrome was one subtype of spondyloarthritis (SpA) [19, 25], and Takigawa et al. [20] supposed that SAPHO syndrome, especially presented as spinal involvement and PPP, should be recognized as a subtype of reactive spondyloarthroathy. In our study, only 11 patients (24.0%) had sacroiliac joint involvement, and none of them were HLA-B27 positive which is very important for the diagnosis of SpA in the classification criterion of ax-SpA ASAS proposed in 2009 [26]. What is more, the osteoarticular symptoms in our SAPHO patients did not present as inflammatory back pain. So we suppose that SAPHO syndrome is another type of chronic inflammatory disease that differs from SpA.
What is more, Schmorl nodes (SNs) are an associated vertebral anomaly occurs in SAPHO syndrome which were mentioned in published literatures. The SNs are protrusions of nucleus pulposus or intervertebral disc tissue through gap or weakening of the cartilaginous endplate and subchondral bone into the adjacent vertebral body. Common locations of SNs were lower thoracic and upper lumbar spine [27, 28]. Though SNs always present as an asymptomatic incidental finding in the general population, it can lead to severe back pain in some cases [29]. The hyposignal in T1WI and hypersignal in T2WI in the veterbral body adjacent indicate a presence of bone edema and inflammation which can be regarded as a key point to distinguish the symptomatic and asymptomatic patients [30, 31]. In our study, among the thirty-four SAPHO syndrome patients with spinal involvement, four patients were found SNs in MRI imaging, and three of them had back or shoulder pain symptom. Thus, we suppose that SNs might be a sensitive and identical presentation to help radiologists and rheumatologist diagnose the disease.
Currently, the aim of treating SAPHO syndrome is alleviating symptoms and alleviating the osteoarticular damage induced by inflammatory reaction. Non-steroidal anti-inflammatory drugs (NSAIDs) is thought to be the first-line treatment to relieve the osteoarticular symptom, but NSAIDs alone is not sufficient for those with severe symptoms. Previous study suggested that the use of corticosteroids could be effective in most of the patients, but the long term use would lead to serious complications [32, 33]. Some DMARDS and biphosphonates are regarded as effective treatment for SAPHO syndrome. TNF-α inhibitor is effective againist the cutaneous and osteoarticular involvement[34, 35].In our study, patients taking NSAIDs alone did not get remission. In contrast, some patients who regularly received bisphosphonate or TNF-α inhibitor achieved remission at different degree and the affected sites turned into chronic stage change or disappeared. However, SAPHO syndrome is a rare syndrome and the research about treatment is limited, so double-blind randomized controlled studies about the treatment seem to be necessary.
In conclusion, we found that SAPHO syndrome with spinal involvement experienced a more intensive inflammatory reaction than those without spinal involvement. When compared with AS patients, SAPHO syndrome patients suffered significantly more from cervical vertebral involvement and endplate inflammation which might be typical manifestations to distinguish AS from SAPHO syndrome. Large number of samples are still needed to verify the results in the future.
References
Chamot A M, Benhamou C L, Kahn M F, Beraneck, L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases. Rev Rhum Mal Osteoartic 1987;54:187–196
Kahn MF, Khan MA (1994) The SAPHO syndrome. Baillieres Clin Rheumatol 8:333–362
Cotten A, Flipo RM, Mentre A, Delaporte E, Duquesnoy B, Chastanet P (1995) SAPHO syn- drome. RadioGraphics 15:1147–1154
Boutin RD, Resnick D (1998) The SAPHO syndrome: an evolving concept for unifying several idiopathic disorders of bone and skin. AJR Am J Roentgenol 170:585–591
Laredo JD, Vuillemin-Bodaghi V, Boutry N, Cotten A, Parlier-Cuau C (2007) SAPHO syndrome: MR appearance of vertebral involvement. Radiology 242:825–831
Govoni M, Colina MA, Trotta F (2009) SAPHO syndrome and infections. Autoimmun Rev 8:256–259
Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME (2012) The SAPHO syndrome. Semin Arthritis Rheum 42:254–265
Na D, Xiao C, Liu Y, Wang J, Wang Z (2016) Multimodal imaging findings of SAPHO syndrome with no skin lesions: a report of three cases and review of the literature. Exp Ther Med 12:2665–2670
Mcgauvran AM, Kotsenas AL, Diehn FE, Wald JT, Carr CM, Morris JM (2016) SAPHO syndrome: imaging findings of vertebral involvement. AJNR Am J Neuroradiol 37:1567–1572
Kundu BK, Naik AK, Bhargava S, Srivastava D (2013) Diagnosing the SAPHO syndrome: a report of three cases and review of literature. Clin Rheumatol 32:1237–1243
Doornum SV, Barraclough D, Mccoll G, Wicks I (2000) SAPHO: rare or just not recognized? Semin Arthritis Rheum 30:70–77
Rukavina ISAPHO (2015) Syndrome: a review. J Child Orthop 9:19–27
Sapho HG (1999) Syndrome. A long-term follow-up study of 120 case. Semin Arthritis Rheum 29:159–171
Zimmermann P, Curtis N (2016) Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome-a challenging diagnosis not to be missed. J Inf Secur 5:106–114
Queiro R, Alonso S, Alperi M, Fernández M, Tejón P, Riestra JL et al (2012) Entheseal ultrasound abnormalities in patients with sapho syndrome. Clin Rheumatol 31:913–919
Hurtado-Nedelec M, Chollet-Martin S, Nicaise-Roland P, Grootenboer-Mignot S, Ruimy R, Meyer O et al (2008) Characterization of the immune response in the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Rheumatology (Oxford) 47:1160–1167
Ferguson PJ, Lokuta MA, El-Shanti HI, Muhle L, Bing X, Huttenlocher A (2010) Neutrophil dysfunction in a family with a SAPHO syndrome-like phenotype. Arthritis Rheum 58:3264–3269
Earwaker JWS, Cotten A (2003) SAPHO: syndrome or concept? Imaging findings. Skelet Radiol 32:311–327
Maugars Y, Berthelot JM, Ducloux JM, Prost A (1995) SAPHO syndrome: a followup study of 19 cases with special emphasis on enthesis involvement. J Rheumatol 22:2135–2141
Takigawa T, Tanaka M, Nakanishi K, Misawa H, Sugimoto Y, Takahata T, Nakahara H, Nakahara S, Ozaki T (2008) SAPHO syndrome associated spondylitis. Eur Spine J 17:1391–1397
Xu W, Li C, Zhao X, Lu J, Li L, Wu N, Zuo Y, Jing H, Dong Z, Zhang W, Zhang W (2017) Whole-spine computed tomography findings in SAPHO syndrome. J Rheumatol 44:648–654
Leone A, Cassar-Pullicino VN, Casale R, Magarelli N, Semprini A, Colosimo C (2015) The SAPHO syndrome revisited an emphasis on spinal manifestations. Skelet Radiol 44:9–24
Paparo F, Aleo E, Revelli M et al (2013) Spondyloarthropathies: what radiologists should know. ECR
Toussirot E, Dupond JL, Wendling D (1997) Spondylodiscitis in SAPHO syndrome. A series of eight cases. Ann Rheum Dis 56:52–58
Sonozaki H, Kawashima M, Hongo O, Yaoita H, Ikeno M, Okai K et al (1981) Incidence of arthro-osteitis in patients with pustulosis palmaris et plantaris. Ann Rheum Dis 40:554–557
Rudwaleit M, Landewe R, van der Heijde D, Listing J, Brandt J, Braun J, Burgos-Vargas R, Collantes-Estevez E, Davis J, Dijkmans B, Dougados M, Emery P, van der Horst-Bruinsma IE, Inman R, Khan MA, Leirisalo-Repo M, van der Linden S, Maksymowych WP, Mielants H, Olivieri I, Sturrock R, de Vlam K, Sieper J (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis: classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 68:770–776
Iampreechakul P, Lertbutsayanukul P, Suanprasert N (2019) Acute calcific discitis or symptomatic calcified Schmorl’s node of the upper thoracic spine in an adult: a case report and literature review. Asian J Neurosurg 14(3):1021–1029
Grivé E, Rovira A, Capellades J, Rivas A, Pedraza S (1999) Radiologic findings in two cases of acute Schmörl’s nodes. AJNR Am J Neuroradiol 20(9):1717–1721
Nicolas, Amoretti, Sylvain et al (2019) Symptomatic Schmorl’s nodes: role of percutaneous vertebroplasty. Open study on 52 patients. Neuroradiology 61(4):405–410
Takatalo J, Karppinen J, Niinimaki J, Taimela S, Mutanen P, Sequeiros RB, Nayha S, Jarvelin MR, Kyllonen E, Tervonen O (2012) Association of modic changes, Schmorl’s nodes, spondylolytic defects, highintensity zone lesions, disc herniations, and radial tears with low back symptom severity among young Finnish adults. Spine. 37:1231–1239
Williams FM, Manek NJ, Sambrook PN, Spector TD, Macgregor AJ. Schmorl’s nodes: common,highly heritable, and related to lumbardisc disease. Arthritis Rheum2007;57:855–860, Schmorl’s nodes: c
Assmann G, Kueck O, Kirchhoff T, Rosenthal H, Voswinkel J, Pfreundschuh M et al (2009) Efficacy of antibiotic therapy for SAPHO syndrome is lost after its discontinuation: an interventional study. Arthritis Res Ther 11:1–8
Firinu D, Garcialarsen V, Manconi PE, Del Giacco SR (2016) SAPHO syndrome: current developments and approaches to clinical treatment. Curr Rheumatol Rep 18:35
Burgemeister LT, Baeten DL, Tas SW (2012) Biologics for rare inflammatory diseases: TNF blockade in the SAPHO syndrome. Neth J Med 70:444–449
Ben Abdelghani K, Dran DG, Gottenberg JE, Morel J, Sibilia J, Combe B (2010) Tumor necrosis factor-alpha blockers in SAPHO syndrome. J Rheumatol 37:1699–1704
Funding
This study was supported by a Project of The National Natural Science Foundation of China (81501390).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, S., Deng, X., Zhang, L. et al. The comparison analysis of clinical and radiological features in SAPHO syndrome. Clin Rheumatol 40, 349–357 (2021). https://doi.org/10.1007/s10067-020-05187-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05187-0