Abstract
SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis) is a rare autoimmune disease which, due to its clinical presentation and symptoms, is often misdiagnosed and unrecognized. Its main features are prominent inflammatory cutaneous and articular manifestations. Treatments with immunosuppressive drugs have been used for the management of SAPHO with variable results. To date, the use of anti-TNF-α agents has proved to be an effective alternative to conventional treatment for unresponsive or refractory SAPHO cases. TNF-α is a pro-inflammatory cytokine and pivotal regulator of other cytokines, including IL-1 β, IL-6, and IL-8, involved in inflammation, acute-phase response induction, and chemotaxis. IL-1 inhibition strategies with anakinra have shown efficacy as first and second lines of treatment. In this review, we will describe the main characteristics of biological drugs currently used for SAPHO syndrome. We also describe some of the promising therapeutic effects of ustekinumab, an antibody against the p40 subunit of IL-12 and IL-23, after failure of multiple drugs including anti-TNF-α and anakinra. We discuss the use and impact of the new anti-IL-1 antagonists involved in the IL-17 blockade, in particular for the most difficult-to-treat SAPHO cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SAPHO syndrome is a chronic immune-mediated condition that compromises the skin, joints, and bones. The syndrome’s acronym reflects the variable combination of synovitis, acne, pustulosis, hyperostosis, and osteitis. The disease is rare, frequently unrecognized (and misdiagnosed), and characterized by prominent inflammatory cutaneous and articular manifestations [1]. SAPHO syndrome had been initially classified among the spondyloarthropathies (SpA). Recent evidence suggests that SAPHO syndrome fits better as a primitive inflammatory osteitis, in the spectrum of autoinflammatory diseases (AIDs) [2–4]. A related bone AID with a similar clinical manifestation is known as chronic recurrent multifocal osteomyelitis (CRMO), which has a pediatric onset [5, 6] and has been used to investigate the pathogenesis of this type of immune condition.
In this literature review, we describe the main existing as well as new therapeutic approaches to treating SAPHO syndrome. In order to identify all relevant literature published to date, we searched EMBASE and Ovid Medline through May 26, 2015. The following search terms were used: SAPHO, synovitis, acne, pustulosis, hyperostosis, osteitis (combined). Terms for drug therapy included: anti-TNF agents, infliximab, adalimumab, etanercept, golimumab, certolizumab, anti-IL-1 agents, anakinra, canakinumab, rilonacept, ustekinumab, rituximab, anti-il-6 receptor antibody, tocilizumab, costimulation modulator, and abatacept. Although this is not a systematic review, we also included appropriate suffixes in the search to capture relevant papers regardless of whether the syndrome was mentioned in the abstract or the title.
Clinical Picture
SAPHO syndrome is often misdiagnosed or diagnosed late, because it is a rare disease that shares clinical features with several other disorders, such as infectious discitis, seronegative SpA, and psoriatic arthritis (PsA) [7]. The clinical presentation of SAPHO syndrome is heterogeneous and insidious. It is estimated that about 50–70 % of patients who might have SAPHO also suffer from anterior chest wall (ACW) syndrome, which commonly involves the sternum, clavicles, and/or sternoclavicular joints in different combinations [8•]. These characteristics are related to an underlying chronic inflammatory sterile osteitis resulting in swelling, tenderness, and pain in bone structures and adjacent tissues. The inflammatory involvement of the axial skeleton may result in single or multiple spondylodiscitis and enthesophytes formation. Inflammatory enthesopathy, sacroiliitis, mandibular osteitis, and peripheral arthritis occur frequently [9, 10].
Skin involvement usually precedes the onset of articular symptoms, but it may occur at any time during the course of the disease [11]. Palmoplantar pustulosis (PPP), pustular psoriasis, psoriasis vulgaris, severe acne, or hidradenitis suppurativa are the main skin abnormalities in patients with SAPHO syndrome [10].
Understanding of SAPHO’s natural course is limited, but so far two possible natural courses have been identified: a self-limited one, restricted to a time span of 1 year with little or no flares after remission, and a chronic one, characterized by exacerbations alternating with remissions or by persistent disease activity [10].
Pathogenesis
Due to the presentation of multiple symptoms and clinical signs, SAPHO syndrome could be considered an umbrella term for several disorders that share sterile, inflammatory lesions of the bone and involve an osteitic/hyperostotic process [12].
Still, our understanding of SAPHO syndrome comes from recent investigations on human osteoinflammatory syndromes, such as those caused by recessively inherited mutations in LPIN2 and IL1RN [13•, 14], of which we have limited knowledge, due to their rare incidence.
Experimental evidence using murine models of CRMO have shown that chronic non-bacterial osteitis such as CRMO and SAPHO might be caused by mutations of PSTPIP2; however, no causative mutation of PSTPIP2 has been identified in human disease to date [15, 16••]. Such assumptions on the inherited mutations are difficult to confirm due to the scant epidemiological evidence available. We are aware of two cohort studies that examined genes PSTPIP2, LPIN2, NOD2, PSTPIP1, and PTPN22, but did not show SNPs or causal mutations in the two, which might indicate that SAPHO is a polygenic disease [17, 18].
The role of inflammation in the expression of the syndrome has been better documented. Studies on a murine chronic multifocal osteomyelitis (CMO) model have shown that neutrophils produce excessive amounts of IL-1β and that its production is inflammasome-independent [16••]. Inflammatory bone and skin manifestations have shown to be TH17-dependent in mice deficient for Interleukin-1 receptor antagonist (IL-1RA) [19, 20]. It is worth noting that children with genetic deficiency of IL-1RA (DIRA) show a CRMO-like phenotype and skin pustulosis (similar to SAPHO), which is linked to an expansion of IL-17-producing cells [21].
Recent human immunologic studies have identified increased Th17 cells in the peripheral blood of SAPHO patients [22], reduced IL-10 production by stimulated monocytes fromCRMO patients [23], or IL-10 undetectable plasma levels [24].
The role of IL-1β enhanced release in many AIDs has been established, and it has been implicated in leading toward a severe systemic inflammatory syndrome, possibly mediated by a TH17 skewed phenotype [25, 26]. The TH17 increase in peripheral blood of SAPHO subjects resembles the one recently found in patients with other AIDs.
A single study explored the ex-vivo neutrophils responses in SAPHO patients, showing dysregulation of plasmatic IL-8 and IL-18 and altered neutrophil responses to functional stimuli [24].
A central feature in the dermatologic manifestations of SAPHO is neutrophilic pustular dermatoses [27]. Palmoplantar pustulosis is most common, affecting up to 60 % of patients who develop dermatologic manifestations [9]. Acne conglobata and acne fulminans occur in up to 25 % of patients. Rarely, pyoderma gangrenosum and Sweet’s syndrome have been reported [10].
Neutrophilic dermatoses represent a clinically heterogeneous group of disorders hallmarked by an accumulation of neutrophils in the skin and rarely at the level of internal organs [28]. Pustular psoriasis and other neutrophilic dermatoses, such as Pyoderma gangrenosum and Sweet’s syndrome, are nowadays considered autoinflammatory conditions, which are characterized by recurrent episodes of sterile inflammation, without circulating autoantibodies and autoreactive T-cells [29, 30]. The role exerted by overexpression of the pro-inflammatory cytokines TNF-α, IL-8, and IL-17 in the pathophysiology of the whole spectrum of neutrophilic dermatoses associated to SAPHO has been demonstrated [29, 31, 32], similar to psoriasis and other autoimmune diseases [33–35].
Interleukin-17 amplifies the recruitment of neutrophils and monocytes by increasing the local production of chemokines [36], most notably IL-8, synergizing with various other cytokines [37], in particular with TNF-α, to induce a distinct pattern of endothelial activation that sustains and enhances neutrophil influx to sites of inflammation [38].
Diagnosis
One of the main challenges in the diagnosis of SAPHO is the identification of its various clinical components, which require a careful differential diagnosis. The association of non-infectious, inflammatory osteitis with PPP skin lesions is a finding of cardinal importance for diagnosis. ACW syndrome is the typical manifestation of the disease, but it is unspecific [39]. The criteria described by Benhamou et al. [11] provide a helpful guideline to differential diagnosis. Infectious causes of ACW and spondylodiscitis and/or skin disorders should be ruled out, with emphasis on Propionibacterium acnes and Staphylococcus aureus. In some cases, bone biopsy may be necessary to discern SAPHO syndrome from neoplastic, granulomatous, or other bone disorders, especially when there is involvement of the soft tissues [10]. Imaging techniques are extremely helpful in the differential diagnosis, in particular magnetic resonance imaging, which can help differentiate active bone lesions, and computed tomography, which may document osteitis/hyperostosis or other ACW complications [40]. Whole body bone scintigraphy allows identification of the classic scintigraphic “bull’s head sign” in about a third of patients, helping to confirm the diagnosis in those suspected to have the condition [41].
Conventional Treatments
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used as first-line treatment for pain relief or during the diagnostic phase, but in most cases they are not sufficient. Intra-articular or systemic corticosteroids are transiently effective in the majority of patients [42], but their chronic long-term use is associated with well-known complications. Moreover, relapses with skin and/or bone involvement are frequent at withdrawal. Considering the possible role of P. acne as one of the possible triggers, antibiotic treatment (in particular doxycycline) is another alternative treatment. However, only a small proportion of patients respond [8•, 9, 10], and the effect is often partial and lost after treatment withdrawal [43]. Recently, the efficacy of the antibiotic cefcapene pivoxil hydrochloride has been described in pustulotic arthro-osteitis [44], a clinical entity close to SAPHO [45, 46].
In contrast to antibiotics, bisphosphonates, especially pamidronate, have consistent and rapid efficacy but transient activity on bouts of bone inflammation [8•]. In a portion of patients, a partial or complete sustained remission over time has been described [8•, 47, 48]. Increased serum cross laps might be a prognostic marker for a positive clinical response in SAPHO patients treated with pamidronate [48].No efficacy on cutaneous complaints or the induction of exacerbations has been seldom reported [49, 50]. Immunosuppressive drugs such as methotrexate, sulfasalazine, cyclosporine and leflunomide have been widely used, obtaining mixed responses [9, 27, 51, 52]. Regardless of the effectiveness of these treatments, the majority of patients may require additional treatments often in long-term or in multiple courses.
No predictors of efficacy of treatment with antibiotics, bisphosphonates, or immunosuppressive drugs are available.
To date, we have identified one ongoing trial investigating various treatment options on SAPHO syndrome. Researchers from the Peking Union Medical College Hospital are carrying out a non-randomized, open label single centric intervention in adults aged 18–70 years old aimed at assessing the long-term efficacy of intravenous bisphosphonates for bone marrow oedema in patients with SAPHO (Clinical Trials Identifier NCT02544659). The results of this study are expected to be available in 2017. These findings will add to the recent discussion on the effectiveness of bisphosphonates for SAPHO syndrome [53].
Treatment with Biologicals
Anti-TNF-α Agents
Since 2002, various case series and case reports described the use of anti-TNF-α agents as a therapeutic option for SAPHO cases unresponsive or refractory to conventional drugs [54, 55]. These treatments have shown efficacy for bone, skin, and joint manifestations at standard doses and achieving complete remission and promising results in most of cases [56, 57]. TNF-α is a pivotal pro-inflammatory cytokine and potent regulator of cytokines including IL-1 β, IL-6, and IL-8, which are crucial in inflammation, acute-phase response induction, and chemotaxis [58]. The rationale for the off-label use of anti-TNF drugs relies on the increased expression of TNF-α in bone specimens [55], on the altered levels of cytokines and of neutrophil responses. The upregulation of TNF-alpha release to functional stimuli has been shown to be modulated by etanercept, as assessed in ex-vivo studies [24]. There is also strong evidence suggesting that TNF-α is implicated in the pathogenesis of neutrophilic dermatoses [31] and that anti-TNF treatment has improved pustular psoriasis/PPP and hidradenitis suppurativa [59–62].
Infliximab (INFX), an anti-TNF-α monoclonal antibody, has been increasingly used in SAPHO patients, especially after the failure of conventional therapeutic approaches. Its mechanism of action involves blocking TNF-α action and inducing apoptosis of T-cells that express TNF-α. It has also been reported to be helpful in cases of associated neutrophilic dermatoses [63]. Case reports and case series published demonstrate in a large proportion of the infliximab-treated patients a marked amelioration of bone, joints, and skin inflammatory manifestations [56, 64–68].
However, investigators reported the recurrence or worsening of skin lesions in 3/6 reported cases [65, 69]. In other chronic inflammatory diseases, infliximab is preferred over other TNF-α blockers because of its rapid onset of action. Nevertheless, we and other authors did not notice a clearly different time span of improvement of SAPHO syndrome comparing the available anti-TNF drugs [65, 70].
Etanercept (ETN) is a recombinant soluble TNF receptor and one of the first biological response modifier drugs that has proven effective in SAPHO syndrome [24, 55, 63, 71].
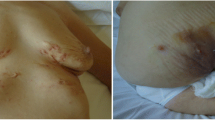
Adalimumab (ADA) is a fully human monoclonal antibody directed to TNF-α, which is usually given as fortnightly subcutaneous injection. Its efficacy in the treatment of SAPHO patients has been demonstrated in several studies [69, 72–74] and in the authors’ personal experience (Fig. 1).
Ben Abdelghani and colleagues showed in their case series the efficacy of TNF-α inhibition, achieving with the above mentioned drugs a clinical response in two thirds of their patients [69]. In their interesting preliminary results on a large cohort of patients, Hayem et al. reported the partial or complete remission in most of patients treated with TNF-α-blockers [57].
Paradoxical flares of PPP or hidradenitis suppurativa may occur in patients treated with these drugs [57, 69, 72]. It is known that these phenomena occur irrespectively of SAPHO as underlying conditions [75].
Subcutaneous injection of certolizumab pegol, a PEGylated Fc-free anti-TNF, has been recently employed in monotherapy in a patient affected by SAPHO syndrome, with rapid amelioration of articular and skin symptoms [76].
Subcutaneous golimumab, another monoclonal antibody targeting TNF-α [22, 77, 78], was safely administered and well-tolerated, but halted after 6 months for complete lack of efficacy in a patient with previous failure of other anti-TNF biologicals.
Patients with disease worsening or unresponsive to anti-TNF-α drugs have been described [74].
Anti-IL-1 Agents
The seminal demonstration of the P2X7–IL1β axis dysregulation in a SAPHO patient supported the rationale to consider the use of drugs targeting this cytokine [79]. Anakinra, a recombinant IL-1 receptor antagonist that provides inhibition of IL-1 signaling, has been proven effective in a small number of published patients by two groups of researchers [79, 80•]. In fact, the two groups reported the efficacy of this drug both as first-line biological and in patients who failed to respond to TNF-inhibitors. Follow-up data of these patients at medium-term are still not available, but awaited with great interest. Also, the extent to which the efficacy of anakinra is due to the blocking of IL-1α or IL-1β is largely unknown.
Use of Other Biological Drugs and Recent Developments
To date, there are scant published reports on the use of biological drugs other than TNF-α antagonists and anakinra.
Recently, the use of tocilizumab at 8 mg/kg has been reported as first biological treatment in a patient affected by SAPHO and AA amyloidosis, showing an initial response on bone pain, but also the onset of chest neutrophilic sterile abscess with neutrophilic infiltration at skin biopsy [81]. No other reports have been published and the efficacy and safety profiles of this drug might be further analyzed when treating SAPHO syndrome.
We previously reported a patient switched from anakinra (skin drug reaction) to golimumab, a subcutaneous monoclonal antibody targeting TNF-α [22, 77, 78]. This drug was safely administered and well-tolerated, but halted after 6 months for complete lack of efficacy. Then same patient underwent a treatment with ustekinumab, a novel monoclonal antibody inhibiting the p40 subunit common for IL-12 and IL-23 cytokines, approved for treatment of psoriasis and PsA. Its efficacy has been described also for PPP [82]. Subcutaneous monotherapy with ustekinumab 90 mg allowed achieving a significant improvement of skin and osteoarticular symptoms after 2 years of treatment, without adverse effects.
Open Issues Related to the Use of Biological Drugs in SAPHO Syndrome
One of the main triggers of SAPHO has been hypothesized to be a primitive “reactive” osteitis in genetically predisposed subjects, elicited by P. acne and other germs [83, 84]. Data available may indicate both a condition of defective immune response or pathogen clearance and an immune response with the features of autoinflammation [85].
Data on long-term evolution of bone processes of treated patients are scant or mainly derive from limited follow-up, but up to about 50 % of patients may develop new foci or show persistence of bone lesions [65, 68, 73, 74].
The demonstration that the efficacy of antibiotic treatment for SAPHO syndrome is lost after its discontinuation indicates that other undisclosed factors perpetuate the bouts of bone inflammation [43]. Biologicals have been employed usually after failure of NSAIDs, multiple antibiotic courses, and bisphosphonates have failed to halt this chronic condition. The association of long-term antibiotic treatment plus biologicals may be proposed on the basis of this rationale [85].
In the absence of solid data and shared guidelines for treatment with biologicals, maintaining or adding a synthetic disease-modifying antirheumatic drug (DMARD), in particular MTX, might help to improve the efficacy and reduce anti-drug antibodies formation and secondary failure of biological treatment [86–88]. To date, the retention rate of biological drugs in SAPHO or CRMO patients has not been reported in detail. Primary failures of biological drugs have been reported, in particular with anti-TNFs [70, 74].
The existence of patients responsive to anakinra, as first shown by Colina et coll., has given evidence of a subset of SAPHO patients in whom there is a pathological role of either IL-1α or IL-1β that can be blocked antagonizing IL-1 [89].
Inflammation markers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are usually normal or only slightly heightened during disease flares [10], often in contrast with imaging data. Potential biomarkers are currently under investigation in order to identify reliable markers of activity and characterize the disease subsets [90].
Conclusions
Although the pathogenesis of SAPHO is still unclear, there is increasing agreement that it could be classified within the spectrum of AIDs, as a consequence of the complex interactions between mono- or poly-genic factors (e.g., elements of innate immunity, inflammasomes) and various exogenous factors, mostly still undisclosed, that could trigger the disease.
NSAIDs, glucocorticosteroids, antibiotics, bisphosphonates, and DMARDs have variable degrees of efficacy and of reported range of beneficial effect over time.
In the last decade, however, the successful use of biological treatments for SAPHO syndrome has given novel and effective weapons to the therapeutic armamentarium for this rare disease.
The inhibition of TNF-α and of IL-1, as previously shown, underlines the central role and the potential of targeting the immune dysregulation underlying SAPHO syndrome [79]. At present, most of the diagnostic and therapeutic approach to treating SAPHO is based in the analogy to SpA and published data provide a low level of evidence. Biological drugs have been primarily employed in patients with a chronic course of the disease, mainly when multiple lines of conventional treatments have been partially efficacious or failed. To date, there are no clear data regarding early treatment with biologics and their association with DMARDs. Anti-TNF-α agents have proved to be a safe and effective therapeutic option, especially for patients with the prolonged course of the disease with multiple, prolonged disease flares or with evidence of chronic inflammation [91••]. Occurrences of osteoarticular or skin disease worsening or unresponsive to anti-TNF-α drugs have been reported, and among responders many achieve only a partial control of the disease. These empirical observations are mainly derived from small cohorts and could be linked to the differences in the mechanism of action of the different biologicals drugs inhibiting TNF-α, as observed in other diseases [92]. Also, the presence of subtypes of the disease could be linked to variations in the response to biologicals.
The efficacy of the IL-1 antagonist anakinra gives novel insights on the mechanisms of the disease. Apart from SAPHO, anakinra has been successfully used in CRMO and DIRA [21, 93]. Nevertheless, the pathogenesis of SAPHO is still elusive, but there is increasing understanding that it could be classified within the spectrum of polygenic AIDs [18]. An important role of IL-1β in the differentiation of TH17 lineage has been demonstrated, and recent data from humans and mice highlight the role of IL-1β hypersecretion in AIDs [25, 94••]. Alternative and different effects of IL-1 or TNF-α blockade are known [95] and may also involve the P2X7 receptor [96]. The interaction between dysregulated innate and adaptive immune systems and bone homeostasis in the course of SAPHO plays a central role [23, 97, 98].
In addition, the efficacy of ustekinumab in a single SAPHO case with failure or intolerance to multiple treatments has been described, showing that the anti-IL12/IL23 agents can be a promising therapeutic option for SAPHO, similarly to PPP and hidradenitis [99, 100]. Targeting the dysregulated IL23/TH17 pathway should be further analyzed in larger studies [22]. Furthermore, a rationale emerges for the use of the new anti-IL-1 antagonists or the IL-17 blockade, which now may be considered as possible therapeutic options in the most difficult-to-treat SAPHO cases.
To date, no predictable differences in efficacy, long-term outcome, adverse events, or loss of efficacy over time emerged from reports of patients with SAPHO syndrome treated with etanercept, adalimumab, infliximab, or anakinra. On the basis of the data available, we can only speculate on the mechanisms underlying primary or secondary failures of immunosuppressant or biological drugs.
Double-blind randomized controlled studies regarding the use of biologic drugs or small molecules for this rare disorder are still awaited.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME. The SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):254–65. doi:10.1016/j.semarthrit.2012.05.006.
McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3(8), e297. doi:10.1371/journal.pmed.0030297.
Braun-Falco M, Ruzicka T. Skin manifestations in autoinflammatory syndromes. J Dtsch Dermatol Ges. 2011;9(3):232–46. doi:10.1111/j.1610-0387.2010.07580.x.
Stern SM, Ferguson PJ. Autoinflammatory bone diseases. Rheum Dis Clin North Am. 2013;39(4):735–49. doi:10.1016/j.rdc.2013.05.002.
Wipff J, Adamsbaum C, Kahan A, Job-Deslandre C. Chronic recurrent multifocal osteomyelitis. Joint Bone Spine. 2011;78(6):555–60. doi:10.1016/j.jbspin.2011.02.010.
Beretta-Piccoli BC, Sauvain MJ, Gal I, Schibler A, Saurenmann T, Kressebuch H, et al. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood: a report of ten cases and review of the literature. Eur J Pediatr. 2000;159(8):594–601.
Rohekar G, Inman RD. Conundrums in nosology: synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome and spondylarthritis. Arthritis Rheum. 2006;55(4):665–9. doi:10.1002/art.22087.
Aljuhani F, Tournadre A, Tatar Z, Couderc M, Mathieu S, Malochet-Guinamand S, et al. The SAPHO syndrome: a single-center study of 41 adult patients. J Rheumatol. 2015;42(2):329–34. doi:10.3899/jrheum.140342. A recent case-series of patients with SAPHO syndrome providing clinical and therapeutic insights.
Hayem G, Bouchaud-Chabot A, Benali K, Roux S, Palazzo E, Silbermann-Hoffman O, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum. 1999;29(3):159–71.
Colina M, Govoni M, Orzincolo C, Trotta F. Clinical and radiologic evolution of synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: a single center study of a cohort of 71 subjects. Arthritis Rheum. 2009;61(6):813–21. doi:10.1002/art.24540.
Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol. 1988;6(2):109–12.
Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases. Rev Rhum Mal Osteoartic. 1987;54(3):187–96.
Ombrello MJ. Advances in the genetically complex autoinflammatory diseases. Semin Immunopathol. 2015;37(4):403–6. doi:10.1007/s00281-015-0498-0. Updated view on the current classification of autoinflammatory diseases, their genetic basis and relevant undisclosed issues.
Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147(3):155–74. doi:10.1016/j.clim.2013.03.016.
Ferguson PJ, Bing X, Vasef MA, Ochoa LA, Mahgoub A, Waldschmidt TJ, et al. A missense mutation in pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone. 2006;38(1):41–7. doi:10.1016/j.bone.2005.07.009.
Cassel SL, Janczy JR, Bing X, Wilson SP, Olivier AK, Otero JE, et al. Inflammasome-independent IL-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci U S A. 2014;111(3):1072–7. doi:10.1073/pnas.1318685111. This paper shows the role of cytokine IL-1β in the Pstpip2-deficient mice affected by chronic multifocal osteomyelitis.
Hurtado-Nedelec M, Chollet-Martin S, Chapeton D, Hugot JP, Hayem G, Gerard B. Genetic susceptibility factors in a cohort of 38 patients with SAPHO syndrome: a study of PSTPIP2, NOD2, and LPIN2 genes. J Rheumatol. 2010;37(2):401–9. doi:10.3899/jrheum.090456.
Colina M, Pippucci T, Moro MA, Marconi C, Magini P, Ciancio G, et al. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome: is PTPN22 involved? Clin Exp Rheumatol. 2012;30(3):451.
Koenders MI, Devesa I, Marijnissen RJ, Abdollahi-Roodsaz S, Boots AM, Walgreen B, et al. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2008;58(11):3461–70. doi:10.1002/art.23957.
Shepherd J, Little MC, Nicklin MJ. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J Invest Dermatol. 2004;122(3):665–9. doi:10.1111/j.0022-202X.2004.22305.x.
Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360(23):2426–37. doi:10.1056/NEJMoa0807865.
Firinu D, Barca MP, Lorrai MM, Perra S, Cabras S, Muggianu E, et al. TH17 cells are increased in the peripheral blood of patients with SAPHO syndrome. Autoimmunity. 2014;47(6):389–94. doi:10.3109/08916934.2014.906582.
Hofmann SR, Morbach H, Schwarz T, Rosen-Wolff A, Girschick HJ, Hedrich CM. Attenuated TLR4/MAPK signaling in monocytes from patients with CRMO results in impaired IL-10 expression. Clin Immunol. 2012;145(1):69–76. doi:10.1016/j.clim.2012.07.012.
Hurtado-Nedelec M, Chollet-Martin S, Nicaise-Roland P, Grootenboer-Mignot S, Ruimy R, Meyer O, et al. Characterization of the immune response in the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Rheumatology (Oxford). 2008;47(8):1160–7. doi:10.1093/rheumatology/ken185.
Lasiglie D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6(5), e20014. doi:10.1371/journal.pone.0020014.
Ovadia A, Livneh A, Feld O, Ben-Zvi I, Kukuy E, Kivity S, et al. T helper 17 polarization in familial Mediterranean fever. Genes Immun. 2013;14(4):212–6. doi:10.1038/gene.2013.6.
Zuo RC, Schwartz DM, Lee CC, Anadkat MJ, Cowen EW, Naik HB. Palmoplantar pustules and osteoarticular pain in a 42-year-old woman. J Am Acad Dermatol. 2015;72(3):550–3. doi:10.1016/j.jaad.2014.07.014.
Wallach D, Vignon-Pennamen MD. From acute febrile neutrophilic dermatosis to neutrophilic disease: forty years of clinical research. J Am Acad Dermatol. 2006;55(6):1066–71. doi:10.1016/j.jaad.2006.07.016.
Marzano AV, Ishak RS, Saibeni S, Crosti C, Meroni PL, Cugno M. Autoinflammatory skin disorders in inflammatory bowel diseases, pyoderma gangrenosum and Sweet’s syndrome: a comprehensive review and disease classification criteria. Clin Rev Allergy Immunol. 2013;45(2):202–10. doi:10.1007/s12016-012-8351-x.
Borella E, Palma L, Zen M, Bettio S, Nalotto L, Gatto M, et al. The body against self: autoinflammation and autoimmunity. Isr Med Assoc J. 2014;16(10):608–10.
Marzano AV, Cugno M, Trevisan V, Lazzari R, Fanoni D, Berti E, et al. Inflammatory cells, cytokines and matrix metalloproteinases in amicrobial pustulosis of the folds and other neutrophilic dermatoses. Int J Immunopathol Pharmacol. 2011;24(2):451–60.
Scarpa R, Lubrano E, Cozzi R, Ames PR, Oriente CB, Oriente P. Subcorneal pustular dermatosis (Sneddon-Wilkinson syndrome): another cutaneous manifestation of SAPHO syndrome? Br J Rheumatol. 1997;36(5):602–3.
Marzano AV, Cugno M, Trevisan V, Fanoni D, Venegoni L, Berti E, et al. Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin Exp Immunol. 2010;162(1):100–7. doi:10.1111/j.1365-2249.2010.04201.x.
Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180(11):7423–30.
Marzano AV, Tavecchio S, Berti E, Gelmetti C, Cugno M. Paradoxical autoinflammatory skin reaction to tumor necrosis factor alpha blockers manifesting as amicrobial pustulosis of the folds in patients with inflammatory bowel diseases. Medicine (Baltimore). 2015;94(45), e1818. doi:10.1097/MD.0000000000001818.
Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279(4):2559–67. doi:10.1074/jbc.M308809200.
Skov L, Beurskens FJ, Zachariae CO, Reitamo S, Teeling J, Satijn D, et al. IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplantar pustulosis. J Immunol. 2008;181(1):669–79.
Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115(2):335–43. doi:10.1182/blood-2009-04-216085.
Kalke S, Perera SD, Patel ND, Gordon TE, Dasgupta B. The sternoclavicular syndrome: experience from a district general hospital and results of a national postal survey. Rheumatology (Oxford). 2001;40(2):170–7.
Depasquale R, Kumar N, Lalam RK, Tins BJ, Tyrrell PN, Singh J, et al. SAPHO: what radiologists should know. Clin Radiol. 2012;67(3):195–206. doi:10.1016/j.crad.2011.08.014.
Fu Z, Liu M, Li Z, Fan Y, Zhang J, Zhang X, et al. Is the bullhead sign on bone scintigraphy really common in the patient with SAPHO syndrome? A single-center study of a 16-year experience. Nucl Med Commun. 2015. doi:10.1097/MNM.0000000000000451.
Jung J, Molinger M, Kohn D, Schreiber M, Pfreundschuh M, Assmann G. Intra-articular glucocorticosteroid injection into sternocostoclavicular joints in patients with SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):266–70. doi:10.1016/j.semarthrit.2012.03.012.
Assmann G, Kueck O, Kirchhoff T, Rosenthal H, Voswinkel J, Pfreundschuh M, et al. Efficacy of antibiotic therapy for SAPHO syndrome is lost after its discontinuation: an interventional study. Arthritis Res Ther. 2009;11(5):R140. doi:10.1186/ar2812.
Murakami M, Masuda K, Utsunomiya R, Oda F, Namba C, Sayama K. Cefcapene pivoxil hydrochloride is a potentially new treatment for palmoplantar pustulosis with pustulotic arthro-osteitis. Dermatology. 2015;231(4):304–11. doi:10.1159/000439401.
Yabe H, Ohshima H, Takano Y, Koyanagi T, Usui H, Nojiri K, et al. Mucosal lesions may be a minor complication of SAPHO syndrome: a study of 11 Japanese patients with SAPHO syndrome. Rheumatol Int. 2010;30(10):1277–83. doi:10.1007/s00296-009-1138-6.
Yamamoto T. Pustulotic arthro-osteitis associated with palmoplantar pustulosis. J Dermatol. 2013;40(11):857–63. doi:10.1111/1346-8138.12272.
Amital H, Applbaum YH, Aamar S, Daniel N, Rubinow A. SAPHO syndrome treated with pamidronate: an open-label study of 10 patients. Rheumatology (Oxford). 2004;43(5):658–61. doi:10.1093/rheumatology/keh149.
Solau-Gervais E, Soubrier M, Gerot I, Grange L, Puechal X, Sordet C, et al. The usefulness of bone remodelling markers in predicting the efficacy of pamidronate treatment in SAPHO syndrome. Rheumatology (Oxford). 2006;45(3):339–42. doi:10.1093/rheumatology/kei160.
Colina M, La Corte R, Trotta F. Sustained remission of SAPHO syndrome with pamidronate: a follow-up of fourteen cases and a review of the literature. Clin Exp Rheumatol. 2009;27(1):112–5.
Delattre E, Guillot X, Godfrin-Valnet M, Prati C, Wendling D. SAPHO syndrome treatment with intravenous pamidronate. Retrospective study of 22 patients. Joint Bone Spine. 2014. doi:10.1016/j.jbspin.2014.01.017.
Hayama K, Inadomi T, Fujisawa D, Terui T. A pilot study of medium-dose cyclosporine for the treatment of palmoplantar pustulosis complicated with pustulotic arthro-osteitis. Eur J Dermatol. 2010;20(6):758–62. doi:10.1684/ejd.2010.1109.
Kundu BK, Naik AK, Bhargava S, Srivastava D. Diagnosing the SAPHO syndrome: a report of three cases and review of literature. Clin Rheumatol. 2013;32(8):1237–43. doi:10.1007/s10067-013-2251-1.
Gorecki P, Stockmann P, Distler JHW, Wuest W, Schmidt D, Neukam FW, et al. Implication of bisphosphonate use in the treatment of SAPHO syndrome: case report and discussion of current literature. J Med Hypotheses Ideas. 2015;9(2):72–8. doi:10.1016/j.jmhi.2015.04.002.
Olivieri I, Padula A, Ciancio G, Salvarani C, Niccoli L, Cantini F. Successful treatment of SAPHO syndrome with infliximab: report of two cases. Ann Rheum Dis. 2002;61(4):375–6.
Wagner AD, Andresen J, Jendro MC, Hulsemann JL, Zeidler H. Sustained response to tumor necrosis factor alpha-blocking agents in two patients with SAPHO syndrome. Arthritis Rheum. 2002;46(7):1965–8. doi:10.1002/art.10539.
Burgemeister LT, Baeten DL, Tas SW. Biologics for rare inflammatory diseases: TNF blockade in the SAPHO syndrome. Neth J Med. 2012;70(10):444–9.
Hayem G, M’Barek RB, Toussirot E, Compaore C, Pham T, Houvenagel E et al. Abstracts of the American College of Rheumatology & Association of Rheumatology Health Professionals, Annual Scientific Meeting. November 6–11, 2010. Atlanta, Georgia, USA. Arthritis Rheum. 2010;62 Suppl 10:1. doi:10.1002/art.30032.
Laveti D, Kumar M, Hemalatha R, Sistla R, Naidu VG, Talla V, et al. Anti-inflammatory treatments for chronic diseases: a review. Inflamm Allergy Drug Targets. 2013;12(5):349–61.
Robinson A, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG, Bebo Jr BF, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67(2):279–88. doi:10.1016/j.jaad.2011.01.032.
Haslund P, Lee RA, Jemec GB. Treatment of hidradenitis suppurativa with tumour necrosis factor-alpha inhibitors. Acta Derm Venereol. 2009;89(6):595–600. doi:10.2340/00015555-0747.
Adisen E, Gurer MA. Therapeutic options for palmoplantar pustulosis. Clin Exp Dermatol. 2010;35(3):219–22. doi:10.1111/j.1365-2230.2009.03520.x.
Delage M, Samimi M, Atlan M, Machet L, Lorette G, Maruani A. Efficacy of infliximab for hidradenitis suppurativa: assessment of clinical and biological inflammatory markers. Acta Derm Venereol. 2011;91(2):169–71. doi:10.2340/00015555-1025.
Vilar-Alejo J, Dehesa L, de la Rosa-del Rey P, Novoa-Medina J, Valeron Almazan P, Santana Medina N, et al. SAPHO syndrome with unusual cutaneous manifestations treated successfully with etanercept. Acta Derm Venereol. 2010;90(5):531–2. doi:10.2340/00015555-0895.
Moll C, Hernandez MV, Canete JD, Gomez-Puerta JA, Soriano A, Collado A, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum. 2008;37(5):299–306. doi:10.1016/j.semarthrit.2007.08.004.
Massara A, Cavazzini PL, Trotta F. In SAPHO syndrome anti-TNF-alpha therapy may induce persistent amelioration of osteoarticular complaints, but may exacerbate cutaneous manifestations. Rheumatology (Oxford). 2006;45(6):730–3. doi:10.1093/rheumatology/kei221.
De Souza A, Solomon GE, Strober BE. SAPHO syndrome associated with hidradenitis suppurativa successfully treated with infliximab and methotrexate. Bull NYU Hosp Jt Dis. 2011;69(2):185–7.
Salles M, Olive A, Perez-Andres R, Holgado S, Mateo L, Riera E, et al. The SAPHO syndrome: a clinical and imaging study. Clin Rheumatol. 2011;30(2):245–9. doi:10.1007/s10067-010-1560-x.
Fruehauf J, Cierny-Modre B, Caelen Lel S, Schwarz T, Weinke R, Aberer E. Response to infliximab in SAPHO syndrome. BMJ Case Rep. 2009;2009. doi: 10.1136/bcr.10.2008.1145.
Ben Abdelghani K, Dran DG, Gottenberg JE, Morel J, Sibilia J, Combe B. Tumor necrosis factor-alpha blockers in SAPHO syndrome. J Rheumatol. 2010;37(8):1699–704. doi:10.3899/jrheum.091086.
Firinu D, Murgia G, Lorrai MM, Barca MP, Peralta MM, Manconi PE, et al. Biological treatments for SAPHO syndrome: an update. Inflamm Allergy Drug Targets. 2014;13(3):199–205.
Zhang LL, Zhao JX, Liu XY. Successful treatment of SAPHO syndrome with severe spinal disorder using entercept: a case study. Rheumatol Int. 2012;32(7):1963–5. doi:10.1007/s00296-011-1916-9.
Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernandez-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol. 2010;90(3):301–2. doi:10.2340/00015555-0822.
Garcovich S, Amelia R, Magarelli N, Valenza V, Amerio P. Long-term treatment of severe SAPHO syndrome with adalimumab: case report and a review of the literature. Am J Clin Dermatol. 2012;13(1):55–9. doi:10.2165/11593250-000000000-00000.
Henriques CC, Sousa M, Panarra A, Riso N. The dark side of SAPHO syndrome. BMJ Case Rep. 2011. doi:10.1136/bcr.11.2011.5197.
Brunasso AM, Laimer M, Massone C. Paradoxical reactions to targeted biological treatments: a way to treat and trigger? Acta Derm Venereol. 2010;90(2):183–5. doi:10.2340/00015555-0777.
Kamata Y, Minota S. Successful treatment of a patient with SAPHO syndrome with certolizumab pegol. Rheumatol Int. 2015;35(9):1607–8. doi:10.1007/s00296-015-3263-8.
Chimenti MS, Teoli M, Saraceno R, Dattola A, Ventura A, Chiricozzi A, et al. Golimumab in patients affected by moderate to severe psoriatic arthritis: an open-label study in thirty-two patients previously treated with other biologics. Dermatology. 2013;227(4):305–10. doi:10.1159/000354263.
Firinu D, Lorrai MM, Barca MP, Peralta MM, Mura MN, Perra S, et al. Increased peripheral T(H)17 Cells in SAPHO syndrome: a novel target for treatment? Allergy. 2013;68:198–9.
Colina M, Pizzirani C, Khodeir M, Falzoni S, Bruschi M, Trotta F, et al. Dysregulation of P2X7 receptor-inflammasome axis in SAPHO syndrome: successful treatment with anakinra. Rheumatology (Oxford). 2010;49(7):1416–8. doi:10.1093/rheumatology/keq074.
Wendling D, Prati C, Aubin F. Anakinra treatment of SAPHO syndrome: short-term results of an open study. Ann Rheum Dis. 2012;71(6):1098–100. doi:10.1136/annrheumdis-2011-200743. The largest published series of SAPHO patients treated with Anakinra.
Fujita S, Kosaka N, Mito T, Hayashi H, Morita Y. Development of aseptic subcutaneous abscess after tocilizumab therapy in a patient with SAPHO syndrome complicated by amyloid A amyloidosis. Int J Rheum Dis. 2015;18(4):476–9. doi:10.1111/1756-185X.12525.
Gerdes S, Franke J, Domm S, Mrowietz U. Ustekinumab in the treatment of palmoplantar pustulosis. Br J Dermatol. 2010;163(5):1116–8. doi:10.1111/j.1365-2133.2010.09897.x.
Edlund E, Johnsson U, Lidgren L, Pettersson H, Sturfelt G, Svensson B, et al. Palmoplantar pustulosis and sternocostoclavicular arthro-osteitis. Ann Rheum Dis. 1988;47(10):809–15.
Jappe U, Boit R, Farrar MD, Ingham E, Sandoe J, Holland KT. Evidence for diversity within Propionibacterium acnes: a comparison of the T-cell stimulatory activity of isolates from inflammatory acne, endocarditis and the laboratory. J Eur Acad Dermatol Venereol. 2004;18(4):450–4. doi:10.1111/j.1468-3083.2004.00950.x.
Hayem G, Hurtado-Nedelec M, Chollet-Martin S. The immune response in SAPHO syndrome: deficiency, hyper- responsiveness, or both? Curr Rheumatol Rev. 2013;9(1):11–4.
Benucci M, Saviola G, Baiardi P, Manfredi M, Sarzi-Puttini P, Atzeni F. Efficacy and safety of leflunomide or methotrexate plus subcutaneous tumour necrosis factor-alpha blocking agents in rheumatoid arthritis. Int J Immunopathol Pharmacol. 2011;24(1):269–74.
Garces S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. 2013;72(12):1947–55. doi:10.1136/annrheumdis-2012-202220.
Meyer MW, Zachariae C, Bendtzen K, Skov L. Lack of anti-drug antibodies in patients with psoriasis well-controlled on long-term treatment with tumour necrosis factor inhibitors. Acta Derm Venereol. 2012;92(4):362–4. doi:10.2340/00015555-1376.
Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–52. doi:10.1038/nrd3800.
Sanna M, Firinu D, Manconi PE, Pisanu M, Murgia G, Piras V, et al. The salivary proteome profile in patients affected by SAPHO syndrome characterized by a top-down RP-HPLC-ESI-MS platform. Mol Biosyst. 2015. doi:10.1039/c4mb00719k.
Colina M, Trotta F. Clinical and radiological characteristics of SAPHO syndrome. Curr Rheumatol Rev. 2013;9(1):22–7. A paper describing the key aspects of the disease.
Marotte H, Cimaz R. Etanercept—TNF receptor and IgG1 Fc fusion protein: is it different from other TNF blockers? Expert Opin Biol Ther. 2014. doi:10.1517/14712598.2014.896334.
Eleftheriou D, Gerschman T, Sebire N, Woo P, Pilkington CA, Brogan PA. Biologic therapy in refractory chronic non-bacterial osteomyelitis of childhood. Rheumatology (Oxford). 2010;49(8):1505–12. doi:10.1093/rheumatology/keq122.
Lukens JR, Gross JM, Calabrese C, Iwakura Y, Lamkanfi M, Vogel P, et al. Critical role for inflammasome-independent IL-1beta production in osteomyelitis. Proc Natl Acad Sci U S A. 2014;111(3):1066–71. doi:10.1073/pnas.1318688111. This paper shows the role of IL-1β dysregulation and identifies PSTPIP2 as a negative regulator of caspase-1–autonomous IL-1β production in chronic multifocal osteomyelitis of mice.
Pham TN, Rahman P, Richardson VJ. Divergent effects of infliximab and anakinra therapies on macrophage phenotype from patients with refractory rheumatoid arthritis. Int J Immunopathol Pharmacol. 2010;23(2):491–501.
Castrichini M, Lazzerini PE, Gamberucci A, Capecchi PL, Franceschini R, Natale M, et al. The purinergic P2x7 receptor is expressed on monocytes in Behcet’s disease and is modulated by TNF-alpha. Eur J Immunol. 2013. doi:10.1002/eji.201343353.
Scholtysek C, Kronke G, Schett G. Inflammation-associated changes in bone homeostasis. Inflamm Allergy Drug Targets. 2012;11(3):188–95.
Abu-Amer Y, Darwech I, Otero J. Role of the NF-kappaB axis in immune modulation of osteoclasts and bone loss. Autoimmunity. 2008;41(3):204–11. doi:10.1080/08916930701694543.
Sharon VR, Garcia MS, Bagheri S, Goodarzi H, Yang C, Ono Y, et al. Management of recalcitrant hidradenitis suppurativa with ustekinumab. Acta Derm Venereol. 2012;92(3):320–1. doi:10.2340/00015555-1229.
Hermanns-Le T, Berardesca E, Pierard GE, Lesuisse M, Pierard-Franchimont C. Challenging regional psoriasis and ustekinumab biotherapy: impact of the patterns of disease. J Biomed Biotechnol. 2012;2012:413767. doi:10.1155/2012/413767.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with the Helsinki declaration and its amendments and institutional research committee standards.
Additional information
Topical Collection on Orphan Diseases
Rights and permissions
About this article
Cite this article
Firinu, D., Garcia-Larsen, V., Manconi, P.E. et al. SAPHO Syndrome: Current Developments and Approaches to Clinical Treatment. Curr Rheumatol Rep 18, 35 (2016). https://doi.org/10.1007/s11926-016-0583-y
Published:
DOI: https://doi.org/10.1007/s11926-016-0583-y