Abstract
Purpose
Circulating TGF-β1 levels were found to be a predictor of delayed bone healing and non-union. We therefore aimed to investigate some factors that can influence the expression of TGF-β1. The correlation between the expression of TGF-β1 and the different socio-demographic parameters was analysed.
Methods
Fifty-one patients with long bone fractures were included in the study and divided into different groups according to their age, gender, cigarette smoking status, diabetes mellitus and regular alcohol intake. TGF-β1 levels were analysed in patient’s serum and different groups were retrospectively compared.
Results
Significantly lower TFG-β1 serum concentrations were observed in non-smokers compared to smokers at week 8 after surgery. Significantly higher concentrations were found in male patients compared to females at week 24. Younger patients had significantly higher concentrations at week 24 after surgery compared to older patients. Concentrations were significantly higher in patients without diabetes compared to those with diabetes at six weeks after surgery. Patients with chronic alcohol abuse had significantly higher concentrations compared to those patients without chronic alcohol abuse.
Conclusion
TGF-β1 serum concentrations vary depending upon smoking status, age, gender, diabetes mellitus and chronic alcohol abuse at different times and therefore do not seem to be a reliable predictive marker as a single-point-in-time measurement for fracture healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite new concepts in fracture treatment, long bone fractures are at risk of poor fracture healing with a rate of non-union ranging from 10 to 30 % [1–7]. Detection of delayed- or non-union at the earliest time point is of crucial importance for the implementation of early therapeutic interventions. Currently, early diagnosis of bone healing disturbance is only based on the patient’s symptoms, such as exercise pain. However, clinical criteria alone are imprecise for the early detection of delayed union. Examination of a tissue sample by callus biopsy can diagnose delayed union, but this procedure is invasive and unethical. An ideal marker of fracture healing should have the properties of being quick, easy and non-invasively obtainable, able to be repeatedly measured, and both sensitive and specific. Serological markers would best fit these criteria and could complement clinical features for more accurate and rapid recognition of delayed or non-union.
Evidence exists that the local and systemic concentrations of different osteogenic growth factors are increased during fracture healing [8–17]. Among these factors TGF-β1 is known to be pivotal for the bone healing. In a recent study our group demonstrated a significant increase in the TGF-β1 concentration in fracture haematoma and in serum of patients with long bone fracture. These results indicated the importance of this cytokine for fracture healing and confirmed other clinical and experimental studies [8–17]. Circulating TGF-β1 levels were found to be a predictor of delayed bone healing and non-union [11]. However, the reliability of TGF-β1 as a marker of the fracture healing is unexplored. An ideal marker of bone healing must reflect the status of bone healing and is not influenced by any other factor. Influencing the expression of systemic growth factors that are not directly related to the fracture healing would weaken the validity of the marker. The aim of this study was to find out if the expression of TGF-β1 after fracture of long bones is solely influenced by the healing process. We therefore analysed the correlation between the expression of TGF-β1 and the socio-demographic differences such as age and gender. We further analysed the correlation between the expression of TGF-β1 differences in patient’s habits such as cigarette smoking, chronic alcohol consumption and the existence of diabetes mellitus.
Patients and methods
This study was approved by the Ethics Committee of the Medical University of Vienna and conducted in accordance with the declaration of Helsinki. Patients gave informed written consent to be enrolled in the study, and were 18–90 years old. The recruitment parameters, sample collection schedule, matching process, patient demographics and exclusion criteria of this study have been previously published in detail [8–10]. In brief, between 2006 and 2008 a consecutive series of 113 patients with meta-/ diaphyseal fractures of long bone (humerus, femur, lower leg and forearm) and surgical treatment were included. In order to have a homogenous study group and due to the strict selection criteria 67 patients with incomplete data were excluded from further investigation. Finally the data of 51 patients were analysed. Patient’s serum was collected following a standardised time schedule. TGF-β1 levels were then measured in patient’s serum. Patient’s history with special focus on cigarette smoking, diabetes mellitus and regular alcohol intake were recorded. All patients were followed-up for at least six months after the operation. Follow-up examination was based on clinical and radiological examination at one, two, four, six, eight, 12, and 24 weeks after trauma. A total of 22 male and 29 female patients formed the study population. Mean age of the patients was 59.3 years (range 17–90). Mean serum TGF-β1 level was respectively evaluated dependant on whether patients were smokers or non-smokers, male or female, young or old. Moreover, TGF-β1 levels were respectively evaluated in patients with and without diabetes mellitus and in patients with or without chronic alcohol abuse. Smoking status was defined based on self-reported daily cigarette consumption (more than five cigarettes/day). Smokers consuming fewer than five cigarettes per day were not enlisted. Patients below 50 were considered as “young” and those over 50 years as “old”. Chronic use of alcohol was defined based on the self-reported daily alcohol intake in combination with pathological liver parameters. Diabetics were those patients who required oral anti-diabetics or insulin therapy.
Blood samples
Peripheral venous blood was obtained from each patient at one, two, four, six, eight, 12 and 24 weeks after surgery and stored at –80 °C until analysis. Haematoma was removed manually before any manipulation or irrigation, avoiding contamination by blood in the operating field, and placed in sterile containers. These specimens were centrifuged immediately and the resulting supernatant was stored at −80 °C until assayed.
Measurement of TGF-β1
TGF-β1 concentrations were measured by a commercially available antibody (Quantikine, RD Systems, Minneapolis, MN, USA) in enzyme-linked immunosorbent assay (ELISA). All analytical steps were performed according to the manufacturer’s recommended protocol. The TGF-β1 assay detects specifically the biologically active form of the protein. Concentrations are presented as mean of duplicate measurements. To avoid inter-assay variability, samples of the corresponding matching partner were analysed with the same assay. The comparison of the measurements using different kits for the same time points of the study measurements indicates the low range of variability of the assays.
Statistical analysis
Comparisons between groups of continuous variables were performed by nonparametric Mann–Whitney U-test. Spearman's correlation coefficient (young, old, male, female, smoker, non-smoker, DM, no-DM, alcohol, no-alcohol) and test were used to examine the relationship between the variables: young, old, male, female, smoker, non-smoker, DM, no-DM, alcohol, no-alcohol. Statistical analyses were performed using SPSS for Windows 17.0. Data are presented as means ± SEM (standard error of the mean). The statistical significance level was set at p < 0.05.
Results
Cigarette smoking and growth factor expression
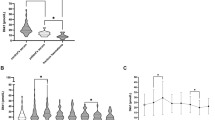
Significantly lower TFG-β1 serum concentration (28,765 ± 22,73.1 pg/ml) was observed in non-smokers compared to smokers (36,650.8 ± 1,352.7 pg/ml) at week eight after surgery (p = 0.02). Non-smokers also had a lower overall serum concentration (30,204 ± 928.8 pg/ml) than patients who smoked (31291.5 ± 1498.9 pg/ml). However, there was no statistically significant difference of the overall serum TFG-β1 concentrations between both groups (p = 0.37) (Fig. 1).
Gender and growth factor expression
Gender specific differences of the TGF-β1 expression were observed at week 24 after surgery. At this time point mean TGF-β1 serum concentrations were significantly higher in males (34,765.4 ± 2,988.5 pg/ml) than in females (24,127 ± 2,029.2 pg/ml) (p = 0.008). However, the mean overall TGF-β1 serum concentrations did not significantly differ between both groups (Fig. 2).
Age and growth factor expression
Mean TGF-β1 concentrations differed significantly between younger and older patients. TGF-β1 serum concentrations were found to be higher in younger patients (40,658.1 ± 3,529.9 pg/ml) compared to the older ones (28,297 ± 1,588.2 pg/ml) at 24 weeks after surgery (p = 0.008). No significant differences in the overall concentrations between young and old patients were observed (Fig. 3).
Diabetes mellitus and growth factor expression
TGF-β1 serum concentrations were higher in patients without diabetes (31,216.8 ± 2,046.8 pg/ml) compared to those with diabetes (18,429 ± 4,709.5 pg/ml) at six weeks after surgery (p = 0 0.014). However, there were no significant differences in the overall concentrations between patients with and without diabetes mellitus (Fig. 4).
Chronic alcohol abuse and growth factor expression
Patients with chronic alcohol abuse had significantly higher TGF-β1 serum levels (38,495 ± 4,607.4 pg/ml) compared to those patients without chronic alcohol abuse (26,752.2 ± 2,042.8 pg/ml) at 24 weeks after surgery (p = 0.04). Mean overall TGF- β1 serum concentration was also higher in patients with chronic alcohol abuse (33,855.7 ± 2,051.4 pg/ml) than in those without chronic alcohol abuse (29,979 ± 851.4 pg/ml). However, this difference was not significant (p = 0.06) (Fig. 5).
Discussion
This study demonstrates alterations of the post traumatic TGF-β1 serum level dependent on age, gender, cigarette smoking, diabetes mellitus and chronic alcohol abuse in patients with long bone fractures. Our data show that smoking significantly increases the expression of TGF-β1 in the eighth post traumatic week. These results are in conflict with another study reporting an increase of TGF-β1 serum concentrations in non-smokers [18]. However, that study has a major limitation since only 28 patients were reported. In our study lower overall TGF-β1 concentration was observed in non-smokers. In a recently published study the expression of TGF-β1, BMP-2, PDGFAA and VEGF have been shown to be inhibited by nicotine at high concentrations, but with no significant difference at low concentration in rabbits. Moreover, nicotine suppressed osteoblast proliferation and inhibited the expression of some key osteogenic and angiogenic mediators [19]. In another experimental study the expression of TGF-β1, PDGF-A and FGF in rabbits was inhibited by nicotine resulting in compromised bone regeneration [20]. Giorgetti et al [13] analysed the expression of alkaline phosphatase, bone morphogenetic proteins (BMP)-2 and -7, receptor activator of nuclear factor-B ligand, and osteoprotegerin in rats that were exposed to cigarette smoke and those that were not. They found a trend toward the down regulation of BMP-2 and ALP and the up-regulation of the RANKL/OPG ratio for exposed animals. Cigarette smoking resulted in a signif-icant increase of BMP-7 in rats that were exposed to smoke. Since it is known that TGF-β1 intensifies the effect of the BMPs [14, 15], it is conceivable that the high expression of BMP-7 in the smoke-exposed animals is caused by a high expression of TGF-β1 as shown in our study. Nicotine in high doses is directly toxic to proliferating osteoblasts al-though low-dose nicotine may be stimulatory [21]. In an in vivo experiment, tobacco extract which did not contain nicotine significantly reduced the mechanical strength of healing femoral fractures in rats, while nicotine alone did not affect the mechanical properties [22]. Other studies have reported that nicotine stimulates osteoblastic proliferation, ALP activity, collagen and protein synthesis, and minerali-zation of the osteoblastic cells [16, 23]. Higher levels of TGF-β1 in smoker’s serum as found in our patients suggest the stimulating effect of nicotine on the osteoblasts. However, the mechanism, by which TGF-β1 is elevated in smokers, cannot be explained from our data. Another issue addressed in our study was the possible gender specific alteration of the TGF-β1 expression during fracture healing. In a study with 1,133 patients, female sex was identified as a major risk factor for compromised fracture healing [24]. This was confirmed by other studies [25–27]. However, the cellular or molecular reasons for the sex-specific differences in fracture healing remain elusive. In our study TGF-β1 serum concentrations differed significantly at the 24th post traumatic week between both sexes. In accordance with other reported results male patients had higher levels of TGF-β1 compared to female patients [28–31]. In a previous study, a diminished number of nucleated cells from human iliac crest aspirates were observed for both sexes with increasing age. However, the number of osteoblasts per aspirate decreased with increasing age in the female group but was constant in males [28]. In addition, testosterone has been reported to increase TGF-β1 production in human osteoblastic cells [29]. Higher testos-terone in male patients could explain the increased TGF-β1 expression in these patients, and higher TGF-β1 levels explain the higher number of osteoblasts. We further investigated whether age can influence the expression of TGF-β1 in patients after fracture of long bones. Evidence exists that increasing age leads to the diminution of fracture healing in animals and humans. Studies of fracture healing in rats have shown that the formation of cartilage and bone, and cartilage resorption were delayed in elderly animals [32]. There was evidence that accretion of mineral into the callus was reduced in elderly animals [33, 34]. Lu et al. reported age-related changes in fracture healing in rats [35]. Street et al. found that angiogenesis at the fracture site and the response of growth factor to fracture in the elderly human was preserved [36]. Moreover, age was considered as factor for non-union after internal fixation of femoral neck fractures and clavicular non-union [37, 38]. In addition, the amount of TGF-β in cortical bone was found to decrease with age [31]. In our study TGF-β1 concentrations differed signifi-cantly between younger and older patients. As expected the concentrations were found to be higher in younger patients compared to the older ones at 24 weeks after surgery. These data confirm the results of the above-mentioned studies. It seems that the decrease of TGF-β1 expression in older patients is one of the reasons for the slowing in the process of repair.
Another aim of our study was to examine if diabetes mellitus has an influence on the expression of TGF-β1 in patients with long bone fracture. The effect of diabetes mellitus on fracture healing has been evaluated in various experimental models. Diabetic rats with bone fracture showed a 29 % decrease in tensile strength and a 50 % decrease in stiffness of the callus after two weeks of healing, compared with the controls [39]. In the same study the tensile strength and stiffness of the callus recovered in those diabetic animals that were treated with insulin. Other animal studies showed reduced cellular proliferation, reduced osteoblast activity and reduced collagen synthesis and content in the diabetic compared with control animals [40–42]. Clinical studies have demonstrated a significantly higher incidence of delayed union, non-union, and a doubling of the consolidation time of the fracture in diabetic compared with non-diabetic patients [43–45]. Evidence exists that some of the influences of diabetes on fracture repair are related to the inhibition of growth factors, although the underlying mechanism is largely unknown. It has been postulated that in diabetes there is reduced cell proliferation in the early phase of fracture healing as a result of decreased expression of platelet-derived growth factor [46]. The levels of TGF-β1 and other growth factors (IGF-1, VEGF) have also been shown to be significantly reduced in diabetic animals [47]. We observed significantly lower TGF-β1 serum concentrations in patients with diabetes mellitus in the sixth post-traumatic week. These clinical results correlate with the results of the experimental data of the previous studies for the first time.
The last aspect that was evaluated in the present study was the possible effect of chronic alcohol abuse on the systemic levels of TGF-β1 during fracture healing. Alcohol abuse can diminish levels of vitamin D metabolites and decrease levels of serum osteocalcin causing a suppression of osteoblasts, and can even cause hypocalcemia and hypocalciuria. These alterations affect bone healing [48]. Insufficient nutrition is an important physiological aspect of patients reporting alcohol abuse [49, 50], altering hemostasis and slowing bone repair [51], which ultimately exerts varying effects on the results of fracture treatment. Alcoholics experience not only an increased incidence of fractures from falls, but also delays in healing compared with non-alcoholics. In fracture healing, the effect of alcohol is to suppress synthesis of an ossifiable matrix, possibly because of inhibition of cell proliferation and poor differentiation of mesenchymal cells in the repair tissue. Alcohol has been shown to have an anti proliferative and inhibitive effect on osteoblastic cells in vitro and in vivo [52–55]. In our study significantly higher TGF-β1 serum concentrations were observed in alcoholics at the 24th week after trauma. In addition, overall TGF-β1 serum concentration was higher in patients with chronic alcohol consumption. Elevated TGF-β1 serum concentrations in alcoholic patients could be a response to the low number of the osteoblasts and body's response to stimulate osteoblastic activity that has been suppressed by alcoholic consumption.
In summary, the overall trends showed no statistically significant difference between the groups. However, at various time points, there were statistically significant differences in all of the control groups. The major limitation of this study is the low number of patients. Nevertheless, to our knowledge, this is the first study to investigate the effect of age, gender, diabetes mellitus and chronic alcohol abuse on the TGF-β1 expression after long bone fracture. Although the overall TGF-ß1 concentration is not significantly influenced by exogenous, endogenous, socio-demographical and patient specific factors, these factors still can significantly influence the TGF-ß1 expression at various time points. TGF-β1 serum concentrations vary dependent upon smoking status, age, gender, diabetes mellitus and chronic alcohol abuse at different time points. One cannot exclude the possibility that the expression of TGF-β1 in serum is influenced by various other factors than the factors investigated in this study.
References
Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, Schipper IB (2011) Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop 35:1271–1280
Pecina M, Haspl M, Jelic M, Vukicevic S (2003) Repair of a resistant tibial non-union with a recombinant bone morphogenetic protein-7 (rh-BMP-7). Int Orthop 27:320–321
Pećina M, Giultaij LR, Vukičević S (2001) Orthopaedic applications of osteogenic protein-1 (BMP-7). Int Orthop 25:203–208
Ong CT, Choon DS, Cabrera NP et al (2002) The treatment of open tibial fractures and of tibial non-union with a novel external fixator. Injury 33:829–834
Gustilo RB, Mendoza RM, Williams DN (1984) Problems in the management of type III (severe) open fracture: a new classification of type III open fractures. J Trauma 24:742–746
Warren SB, Brooker AF (1992) Intramedullary nailing of tibial nonunion. Clin Orthop 285:236–243
Wu CC, Shih CH (1992) Treatment of 84 cases of femoral nonunion. Acta Orthop Scand 63:57–60
Sarahrudi K, Thomas A, Mousavi M et al (2011) Elevated transforming growth factor-beta 1(TGF-B1) levels in human fracture healing. Injury 42:833–837
Sarahrudi K, Mousavi M, Thomas A et al (2010) Elevated levels of macrophage colony stimulating factor in human fracture healing. J Orthop Res 28(5):671–676
Sarahrudi K, Thomas A, Braunsteiner T et al (2009) VEGF serum concentrations in patients with long bone fractures: a comparison between impaired and normal fracture healing. J Orthop Res 27(10):1293–1297
Zimmermann G, Henle P, Kusswetter M et al (2005) TGF-beta 1 as a marker of delayed fracture healing. Bone 36:779–785
Giannoudis P, Psarakis S, Kontakis G (2007) Can we accelerate fracture healing? A critical analysis of the literature. Injury 38(1):81–89
Giorgetti AP, César Neto JB, Ruiz KG et al (2010) Cigarette smoke inhalation modulates gene expression in sites of bone healing: a study in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110(4):447–452
Bostrom MP (1998) Expression of bone morphogenetic proteins in fracture healing. Clin Orthop 355:116–123
Gazit D, Zilberman Y, Turgeman G et al (1999) Recombinant TGF-beta 1 stimulates bone marrow osteoprogenitor cell activity and bone matrix synthesis in osteopenic, old male mice. J Cell Biochem 73:379–389
Yuhara S, Kasagi S, Inoue A et al (1999) Effects of nicotine on cultured cells suggest that it can influence the formation and resorption of bone. Eur J Pharmacol 383:387–393
Harley BJ, Beaupre LA, Jones CA et al (2002) The effect of time to definitive treatment on the rate of non-union and infection in open fractures. J Orthop Trauma 16:484–490
Moghaddam A, Weiss S, Wölfl CG et al (2010) Cigarette smoking decreases TGF-ß1 serum concentrations after long bone fracture. Injury 41(19):1020–1025
Ma L, Zheng LW, Sham MH et al (2011) Effect of platelet-rich plasma on a rabbit model of nicotine-compromised bone healing. J Oral Maxillofac Surg 69(1):28–35
Ma L, Zwahlen RA, Zheng LW et al (2011) Influence of nicotine on the biological activity of rabbit osteoblasts. Clin Oral Impl Res 22(3):338–342
Gullihorn L, Karpman R, Lippiello L (2005) Differential effects of nicotine and smoke condensate on bone cell metabolic activity. J Orthop Trauma 19:17–22
Skott M, Andreassen TT, Ulrich-Vinther M et al (2006) Tobacco extract but not nicotine impairs the mechanical strength of fracture healing in rats. J Orthop Res 24:1472–1479
Preira ML, Carvalho JC, Peres A et al (2010) Simultaneous effects of nicotine, acrolein, and acetaldehyde on osteogenic-induced bone marrow cells cultured on plasma-sprayed titanium implants. Int J Oral Maxillofac Implants 25:112–122
Parker MJ, Raghavan R, Gurusamy K (2007) Incidence of fracture-healing complications after femoral neck fractures. Clin Orthop Relat Res 458:175–179
Chang MA, Bishop AT, Moran SL et al (2006) The outcomes and complications of 1,2-intercompartmental supraretinacular artery pedicled vascularized bone grafting A of scaphoid nonunions. J Hand Surg Am 31:387–396
Li Z, Zhang W, Li ZB et al (2006) Abnormal union of mandibular fractures: a review of 84 cases. J Oral Maxillofac Surg 64:1225–1231
David V, Lafage-Proust MH, Laroche N et al (2006) Two week longitudinal survey of bone architecture alteration in the hindlimb unloaded A rat model of bone loss: sex differences. Am J Physiol Endocrinol Metab 290:440–447
Muschler GF, Nitto H, Boehm CA et al (2001) Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 19:117–125
Benz DJ, Haussler MR, Thomas MA et al (1991) High-affinity androgen binding and androgenic regulation of alpha 1(I)-procollagen and transforming growth factor-beta steady state messenger ribonucleic acid levels in human osteoblast-like osteosarcoma cells. Endocrinology 128(6):2723–2730
Strube P, Mehta M, Baerenwaldt A et al (2009) Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone 45:1065–1072
Nicolas V, Prewett A, Bettica P et al (1994) Age related decrease in insulin-like growth factor and transforming growth factor-β in femoral cortical bone from both men and women: implication for bone loss with aging. J Clin Endocrinol Metab 78:1011–1016
Aho AJ (1966) Electron microscopic and histologic studies on fracture repair in old and young rats. Acta Chir Scand Suppl 357:162–165
Meyer RA Jr, Tsahakis PJ, Martin DF et al (2001) Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res 19:428–435
Meyer RA Jr, Meyer MH, Tenholder M et al (2003) Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am 85:1243–1254
Lu C, Miclau T, Hu D et al (2005) Cellular basis for age-related changes in fracture repair. J Orthop Res 23:1300–1307
Street JT, Wang JH, Wu QD et al (2001) The angiogenic response to skeletal injury is preserved in the elderly. J Orthop Res 19:1057–1066
Parker MJ (1994) Prediction of fracture union after internal fixation of intracapsular femoral neck fractures. Injury 25(2):3–6
Robinson CM, Court-Brown CM, McQueen MM et al (2004) Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am 86:1359–1365
Macey LR, Kana SM, Jingushi S et al (1989) Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg Am 71:722–733
Beam HA, Parsons JR, Lin SS (2002) The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res 20:1210–1216
Funk JR, Hale JE, Carmines D et al (2000) Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res 18:126–132
Herbsman H, Powers JC, Hirschman A et al (1968) Retardation of fracture healing in experimental diabetes. J Surg Res 8:424–431
Cozen L (1972) Does diabetes delay fracture healing? Clin Orthop 82:134–140
Levin ME, Boisseau VC, Avioli LV (1976) Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med 294:241–245
Loder RT (1988) The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop 232:210–216
Tyndall WA, Beam HA, Zarro C et al (2003) Decreased platelet derived growth factor expression during fracture healing in diabetic animals. Clin Orthop 408:319–330
Gandhi A, Doumas C, O’Connor JP et al (2006) The effects of local platelet rich plasma delivery on diabetic fracture healing. Bone 38:540–546
Manus RC Jr, Dodson TB, Miller EJ Jr et al (2000) Nutritional status on substance abusers with mandible fractures. J Oral Maxillofac Surg 58:153
Serena-Gómez E, Passeri LA (2008) Complications of mandible fractures related to substance abuse. J Oral Maxillofac Surg 66(10):2028–2034
Santolaria F, Pérez-Manzano JL, Milena A et al (2000) Nutritional assessment in alcoholic patients. Its relationship with alcoholic intake, feeding habits, organic complications and social problems. Drug Alcohol Depend 59:295
Sandler NA (2001) Patients who abuse drugs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:12
Klein RF (1997) Alcohol-induced bone disease: impact of ethanol on osteoblast proliferation. Alcohol Clin Exp Res 21:392–399
Nyquist F, Karlsson MK, Obrant KJ et al (1997) Osteopenia in alcoholics after tibia shaft fractures. Alcohol 32:599–604
Chavassieux PS, Serre CM, Vernaud P et al (1993) In vitro evaluation of dose-dependant effect of ethanol on human osteoblastic cells. Bone Miner 22:95–103
Dyer SA, Buckendahl P, Sampson HW (1998) Alcohol consumption inhibits osteoblastic cell proliferation and activity in vivo. Alcohol 16:337–341
Acknowledgments
This study was supported by grants from the Lorenz Böhler Foundation and the Austrian Science Fund (FWF grant #: P19188-B09) to K. Sarahrudi.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaiser, G., Thomas, A., Köttstorfer, J. et al. Is the expression of Transforming Growth Factor-Beta1 after fracture of long bones solely influenced by the healing process?. International Orthopaedics (SICOT) 36, 2173–2179 (2012). https://doi.org/10.1007/s00264-012-1575-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-012-1575-9