Abstract
In African savannahs, mound-building termites induce higher diversity in plant communities. Biotic and abiotic filters, such as nutrients and disturbances (for example, herbivory or fire), may influence the distinct vegetation on termite mounds; however, seed dispersal has not yet been evaluated as a filter in this ecosystem. This study examined the effects of seed dispersal, particularly animal seed dispersal, on the distinct woody plant community on termite mounds in a mopane woodland in north-western Namibia. We compared the functional traits of woody plants related to dispersal, as well as responses to resource availability and disturbance, between plant communities on and those off termite mounds. We conducted vegetation surveys of woody plants in 13 paired mound–savannah plots and measured their functional traits. Soil samples were also collected from 10 of the 13 plots for soil chemical analysis to compare the differences between mound and savannah plots. Drupe-type fruits and dispersal by animals, including mammals and birds, were more dominant in plant communities on termite mounds, whereas pod and winged fruits and wind-dispersed species were typical in matrix communities. Termite mounds were rich in soil nutrients compared with the surrounding matrix, and soil phosphorus was associated with mound soil. We conclude that dispersal mechanisms contribute to the distinct and diverse woody plant community on termite mounds. Seed dispersal by animals is likely to be more common in the distinct woody plant community of the mounds, whereas the community in the surrounding matrix was characterised by wind dispersal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In savannah ecosystems, spatial heterogeneity plays an important role (Scholes 1990) in maintaining a high level of biodiversity (Mittermeier and others 1998; Scholes and Biggs 2005). This heterogeneity is found at different spatial scales and is driven by different abiotic and biotic factors (Pickett and others 2003). At the continental to regional scales, spatial and temporal variations in precipitation drive vegetation dynamics (Wiegand and others 2005), whereas fire, geology and soil factors (Scholes 1990; Higgins and others 2000; Bond and others 2005) and herbivory (Cromsigt and Olff 2008) become more important at the regional to landscape scales. The landscape-scale heterogeneity positively affects species richness, especially in severe environments (Yang and others 2015), by providing niche space for species that have different ecological strategies (Bergholz and others 2017).
In African savannahs, mound-building termites are important agents producing fine-scale heterogeneity through the reallocation of nutrients and subsoil particles (Sileshi and others 2010; Gosling and others 2012). Termite-induced heterogeneity often creates a distinct woody plant assembly, with high species richness and woody biomass (Loveridge and Moe 2004; Traore and others 2008) compared with the surrounding savannah. However, why plant communities on termite mounds are so different from the surrounding savannah is poorly understood.
Plant species differ in their environmental requirements for successful establishment and survival; thus, the environment acts as a filter, removing species that lack traits allowing their persistence under a particular set of conditions (Keddy 1992). The process of seed dispersal determines which species reach a site (that is, dispersal limitation, Nathan and Muller-Landau 2000; Kraft and others 2015). Then, biotic and abiotic filters determine which species establish under conditions formed by the environment and other organisms (Weiher and Keddy 1995; Belyea and Lancaster 1999). The observed community composition is an outcome of the sum of these filters, which sort the species by functional traits.
Numerous abiotic and biotic filters contribute to the unique vegetation on termite mounds. Enriched soil (Sileshi and others 2010; Erpenbach and others 2013), higher soil moisture (Dangerfield and others 1998; Konate and others 1999; Sileshi and others 2010), and elevated topography, which acts as a refugium from fire (Moe and others 2009; Joseph and others 2011, 2013), and savannah flooding (Dangerfield and others 1998; McCarthy and others 1998) have been suggested as causes for the distinct plant communities found on termite mounds. Herbivory acts as a biotic filter to the plant community on mounds. Herbivores prefer to browse plants on mounds, which results in higher deposition of urine and dung on mounds as a result of longer or more frequent visits, creating a positive feedback loop (Holdo and McDowell 2004; Loveridge and Moe 2004). Furthermore, the higher browsing activity alters herbaceous and woody plant communities (Okullo and Moe 2012b; Stoen and others 2013). In addition to these herbivores, small vertebrate (Fleming and Loveridge 2003) and avian species (Joseph and others 2011) also frequently visit termite mounds for browsing and nesting spots, which leads to seed deposition on mounds. However, the effects of dispersal on the distinct plant assembly of termite mounds have not been sufficiently evaluated.
In north-eastern Namibia, large Macrotermes (Isoptera) termite mounds are sparsely distributed (Coaton and Sheasby 1972). The vegetation in this region is classified as mopane, Colophospermum mopane ((J. Kirk ex Benth.) J. Kirk ex J. Léonard) woodland (White 1984). Although C. mopane is a dominant species both on and off termite mounds, there is higher density and species richness of woody plants on these mounds than in the surrounding savannah matrix (Yamashina 2013). Avian seed dispersal may contribute to this unique woody plant community (Yamashina 2014); however, clear evidence is lacking.
We examined the effects of seed dispersal, particularly by animals, on the community composition of termite mound vegetation by exploring woody plant functional traits related to dispersal. The plant community on termite mounds will also reflect the effects of filters other than dispersal; therefore, we assessed the effects of traits related to soil resource use and disturbance. The soil nutrient environment, a well-studied factor affecting termite mound vegetation, was also analysed as an underlying factor. We used community-weighted mean (CWM) trait values (that is, the mean of the trait values weighted by the relative abundances of species), which are adequate to summarise shifts in mean trait values within a given community (Ricotta and Moretti 2011).
We examined three questions by measuring plant traits, including the traits related to seed dispersal and other processes, and analysing the relationship between soil components and woody species composition on and off mounds: (1) Do the functional traits of woody plant communities differ between termite mounds and the surrounding savannah matrix? (2) If so, which traits differ in woody plant communities between the mound and savannah matrices? (3) Does seed dispersal by animals contribute to the distinct woody plant community on termite mounds?
Methods
Study Site
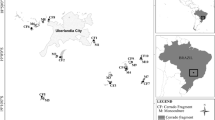
Fieldwork was conducted in the Muyako Community Forest (17.88°S, 24.40°E) in the Zambezi region of Namibia in November 2012, November 2014, January 2015 and November 2015. The altitude is approximately 1000 m above sea level, and the topography is nearly flat. The annual precipitation is greater than 650 mm (Mendelsohn and others 2002), with most rain falling between October and March. The average temperature ranges between 20 and 22 °C. September is the warmest month (range, 14.1–33.8 °C) and June is the coolest (range, 6.1–24.5 °C) (Namibia Meteorological Service 2013). Large areas of the landscape are covered by eutric Fluvisols and fertile soils with high base saturation are found along large river margins and valleys (Mendelsohn and others 2002). The research site lies between the Zambezi and Chobe Rivers, downstream from the Kwando River. The site is located on the boundary between the Kwando and Zambezi drainage basins; some areas were inundated during the rainy season. Lyambezi Lake, which contained water all year round during the study period, lies to the west of the study site. The local vegetation contains mopane woodland, acacia woodland, riparian forest and floodplains, which cover small areas of the landscape (Mendelsohn and Roberts 1997). The understory vegetation of mopane veldt, including grasses and herbs, is poorly developed (Werger and Coetzee 1978); therefore, we only surveyed woody plants.
Field Sampling
This study included 13 paired mound–savannah plots (20 × 20 m). Within our study site, there were several types of mound shapes: (1) mostly active cone-shaped mounds; (2) sometimes active cone-shaped areas with surrounding erosion skirts; and (3) mostly inactive dome-shaped mounds without a prominent cone-shaped area. Our plots focused on the latter two types, and we selected mounds at least 20 m in diameter, based on accessibility in the forest and low human impact due to far distances from residential areas. Termite samples, including soldiers and workers, were collected from two mounds and were identified as Macrotermes michaelseni (Sjöstedt) by a termite expert in the National Museum of Namibia. Although we could not identify the builders of all of the mounds in our samples, a previous study conducted in a floodplain in Botswana suggested that dome-shaped mounds with a large diameter originated from the cone-shaped mounds built by M. michaelseni (McCarthy and others 1998). Savannah plots were set in the surrounding savannah matrix at least 50 m from each mound.
In each plot, all woody plants taller than 1 m were counted and identified by their leaves, flowers and fruit, using Wyk and Wyk (1997) as a reference. Leaf, fruit/pod and twig samples were collected for trait measurements. We collected 10 paired mound–savannah soil cores (> 200 g per core) between 0 and 10 cm in depth for soil chemical analysis.
Trait Selection and Measurement
To examine if functional traits of woody plants differ between termite mounds and the surrounding matrix, we selected 16 traits related to seed dispersal, and responses to soil nutrient availability or disturbance (Table 1) including growth form, plant height, phenology, specific leaf area (SLA), leaf area (LA), leaf thickness, leaf dry matter content (LDMC), twig dry matter content (TDMC), leaf palatability (for mammal herbivores), spinescence, seed mass, fruit/pod palatability, fruit type (drupe, pod, winged), dispersal mode (mammal, bird, wind), nitrogen (N)-fixing ability, leaf N content and leaf carbon (C) content. We followed standardised protocols to measure functional traits (Cornelissen and others 2003; Perez-Harguindeguy and others 2013). To determine LA and leaf thickness, 10 leaves from 3–6 individuals (n = 157) were collected from each species (n = 31). Each leaf was weighed fresh and photographed using a reference scale in the field. LA was measured using the Photoshop CS6 software. Leaf thickness was measured using a digital micrometre (Mitsutoyo, Tokyo, Japan). Leaves were oven-dried at 75 °C for at least 48 h, and dry weight was measured to 0.001 g precision. SLA was expressed as the ratio of fresh LA to dry mass of the leaf sample (mm2 mg−1). LDMC was expressed as the ratio of dry mass to fresh mass. Leaf samples were collected at both mound and savannah plots where possible. Other traits including N-fixing ability, phenology, growth form and fruit type were obtained from the literature (Defaria and others 1989; Campbell 1996; Jacobs and others 2007; Roux and Muller 2009; Cramer and others 2010).
We measured three traits related to seed dispersal: seed mass, fruit type and dispersal mode. Seed mass is related to dispersal and establishment, with smaller-seeded species producing more seeds per reproductive bout to increase the chance of dispersal, whereas species with lager seeds are more likely to establish in competitive environments (Westoby 1998). Dispersal agents can be inferred from seed morphology (Levin and others 2003). In this study, we classified the plant seeds as either drupes, pods, or winged. Seeds with wings are dispersed by wind. Drupes are fleshy fruits dispersed by birds and mammals (Perez-Harguindeguy and others 2013). Pods occur mostly in the Fabaceae family and are eaten and dispersed by livestock, ungulates, elephants and other wildlife (Miller 1996; Dudley 1999). Species with a higher LDMC tend to be resistant to physical damage, such as herbivory and fire, and are associated with low-nutrient environments (Perez-Harguindeguy and others 2013; Wigley and others 2016). The leaf C/N ratio and SLA also reflect the soil resource, that is, a higher leaf C/N ratio tends to be associated with a low-nutrient environments, whereas lower C/N ratio tends to be associated with productive environments (Wigley and others 2016). N fixation is a costly process that consumes carbon (Vitousek and Howarth 1991), and N-fixing ability reduces the need for this in rich environments (Van der Plas and others 2013).
Soil and Leaf Analysis
Soil samples were analysed for pH (H2O), conductivity (EC), calcium carbonate equivalent (% CaCO3), organic C, organic matter, phosphorus (P), sodium (Na), potassium (K), magnesium (Mg), calcium (Ca) and total N at the Analytical Laboratory Services in Namibia. Soil pH was measured in a supernatant suspension with a 2:5 soil/water ratio using a hydrogen-selective electrode and pH meter (WTW MultiLab 540, Weilheim, Germany). Conductivity (with a soil/water ratio) was measured using a specific conductivity meter (WTW MultiLab 540). The calcium carbonate equivalent was measured by neutralising the 2:5 soil/HCl (1 M) suspension with standardised 1 M sodium hydroxide and titrating the supernatant. The determination of soil organic C was based on the Walkley–Black chromic acid wet oxidation method. P was measured by the Olsen method using a spectrophotometer (UVmini-1240, Shimadzu, Kyoto, Japan). Soil organic C was converted to soil organic matter using a van Bemmelen factor of 1.724. Soil exchangeable bases (Na, K, Mg and Ca) were analysed using 1 M ammonium acetate (pH 7.0) by inductively coupled argon plasma optical emission spectroscopy (Optima 7000 DV, Perkin Elmer, Waltham, MA, USA). The Kjeldahl method was used to determine the total N content in soil samples. Soil particle analyses were also performed using a pipette method. Leaf C and N were measured using an NC Analyser at a laboratory in Kyoto University (Sumigraph NC-22F Sumika Chemical Analysis Service, Tokyo, Japan) with ground-dried leaves processed after dry weight measurements had been taken.
Statistical Analysis
All statistical analyses were performed using R software (R Development Core Team 2012). To compare the plant communities on termite mounds with those in the surrounding matrix, we calculated the density, species richness and species diversity index (alpha diversity, evenness and beta diversity) of the woody plant community on each plot. Species richness was expressed as the total number of species in each plot. For alpha diversity, we used the Shannon–Wiener index, calculated as H′ = − \( \sum P_{i} \left( {\ln P_{i} } \right), \) where Pi is the proportion of each species ‘i’ in the sample. Evenness was calculated as H′/lnS, where ‘S’ is the total number of species in each plot. Beta diversity was assessed as among-plot dissimilarity in community composition. We used the Bray–Curtis index, computed using the ‘vegdist’ function in the ‘vegan’ R-package (Oksanen and others 2016). To test for differences in these indices, the CWMs of each functional trait and the soil components between mound and matrix plots, we used paired t-tests or the Wilcoxon signed-rank test following Shapiro–Wilk tests. Functional traits and soil components were standardised, and multicollinearity of functional traits and soil components were assessed using a correlation matrix of all the functional traits and soil parameters (Pearson’s correlation coefficient) to examine possible linkages between variables before subsequent analyses.
To assess differences in dispersal-related traits between mound and matrix plots, we defined an ‘indicator species’ for each site using typical species and their dispersal mode. To identify indicator species, an indicator value (Dufrene and Legendre 1997) was calculated using the ‘labdsv’ package (Roberts 2016) in R with 100,000 iterations.
We conducted a detrended correspondence analysis (DCA), which indicated that the gradient length of the first axis was long (5.2 standard deviation), suggesting a unimodal distribution response of species assemblages to environmental variables (ter Braak and Schaffers 2004). To explore whether differences in woody plant communities can be explained by soil nutrients and functional traits, we used a canonical correspondence analysis (CCA). CCA is a constrained ordination method that detects key variables accounting for the variation (ter Braak 1986). Variables that significantly explained the variance in species assemblages (p < 0.05) were selected using the ‘ordistep’ function in the ‘vegan’ package (Oksanen and others 2016) of R. A separate CCA was used to evaluate the influence of soil components and functional traits on species composition. Explanatory variables with p < 0.05, P and Na for soil components and dispersal mode (endozoochory, bird and wind) for functional traits, were selected for a separate CCA analysis. A permutation of 1000 iterations was used to evaluate significance in CCA. The CCA and DCA were also conducted using the ‘vegan’ package.
Results
Termite mounds exhibited higher density of woody plants (t = 3.6, p < 0.01), species richness (t = 7.2, p < 0.0001), alpha diversity (t = 5.4, p < 0.0001) and evenness (t = 3.6, p < 0.001) of woody plants than the matrix; however, beta diversity did not differ between the mound and matrix plots (Table 2). We extracted 10 indicator species (p < 0.05) to represent the mound plots. Species with significant indicator values (> 60%) were considered characteristic mound species (McGeoch and others 2002). Thus, the top six indicator species were characteristic of mounds; Salvadora persica had the highest indicator value of 92% (Table 3). Four indicator species were extracted to represent matrix plots; however, the indicator values were not significant (Table 3). All of the indicator species in mound plots were bird- and/or mammal-dispersed, whereas matrix plots were characterised mostly by wind-dispersed species and one mammal-dispersed species (Table 3).
CWMs of woody species exhibiting traits related to mammal (W = 128, p < 0.05) and bird dispersal (W = 159, p < 0.001) were higher on mounds, whereas wind-dispersed species (W = 38, p < 0.05) were more common in the matrix (Table 4). CWMs of woody species bearing drupe-type fruits were higher on mounds (W = 160, p < 0.0001), whereas those of pod and winged seeds were higher in the matrix (W = 14, p < 0.001; W = 41, p < 0.05, respectively). Woody species in the matrix plots had heavier seeds than the species in mound plots (t = − 2.6, p < 0.05). Leaf traits with high CWMs in the matrix included leaf palatability (W = 14, p < 0.0001), LA (t = –2.1, p < 0.05), LDMC (W = 37, p < 0.05), leaf C content (W = 0, p < 0.0001) and leaf C/N (W = 23, p < 0.01), whereas SLA (W = 124, p < 0.05) and leaf thickness (W = 150, p < 0.001) had high CWMs on mounds. There were no differences in CWM of leaf N content between mound and matrix plots. The CWM of evergreens was higher on mounds than in the matrix (W = 165, p < 0.0001), and the CWM of deciduous species was conversely higher in the matrix than on mounds (W = 4, p < 0.0001); CWMs of plant height (W = 40, p < 0.05) and N-fixing ability (W = 13, p < 0.001) were higher in the matrix. The tree growth form was common in the matrix (W = 15, p < 0.001), whereas shrubs and climbers were typical on mounds (W = 155, p < 0.0001; W = 118, p < 0.05, respectively). The CWMs of TDMC and spinescence showed no difference between the mound and matrix plots.
Values of soil pH, EC, total N, P, K, Ca, CaCO3, organic C and organic matter were higher on mounds than in the matrix (all p < 0.05). The soil on mounds contained more silt than the matrix (Appendix 1 of electronic supplementary material). In the evaluation of the effect of soil components on species composition, the first two axes explained 58% of the species composition (Figure 1A; ANOVA: F = 3.8, p < 0.01; eigenvalues for axes 1 and 2, 0.361 and 0.219, respectively). The first axis separated mound plots from matrix plots. P values tended to be associated with mound plots, whereas Na was associated with Terminalia spp. and Acacia spp., which produce winged or pod fruits. In the evaluation of the relationship between functional traits and species composition, the first two axes explained 64% of the species composition (Figure 1B; ANOVA: F = 5.1, p < 0.05; eigenvalues for axes 1 and 2, 0.475 and 0.161, respectively). The first axis was separated by traits related to endozoochory or wind. Mound plots were strongly associated with traits related to endozoochory and bird dispersal, whereas matrix plots tended to be associated with traits related to wind dispersal. Indicator species on termite mounds were also strongly associated with traits related to bird dispersal.
CCA of woody plant species composition with statistically significant explanatory variables for A soil components and B functional traits. Abbreviations indicate the indicator species names of woody plants: A.n., Acacia nigrescens; B.d., Berchemia discolor; C.g., Commiphora glandulosa; C.h., Combretum hereroense; C.i., Combretum imberbe; C.m., Colophospermum mopane; C.t., Capparis tomentosa; Cordia, Cordia sp.; D.c., Dichrostachys cinereal; Ehretia, Ehretia sp.; Grewia: Grewia sp.; L.d., Lannea discolor; S.p., Salvadora persica; X.a., Ximenia americana. Italics indicate the indicator species of the mounds, and underline indicates the indicator species of the matrix.
Discussion
We used woody plant functional traits related to seed dispersal to understand the contribution of seed dispersal by animals in determining the woody plant community species composition on termite mounds in an African savannah. We found that termite mounds in the savannah have a diverse woody plant community, which is consistent with the findings of previous studies (for example, Joseph and others 2014). Many functional traits of woody plants and soil components differed between the mound and matrix.
Seed dispersal by animals, including mammals and birds, and drupe-type fruits were defined as the traits of the community composition on the mounds, whereas woody species with winged seeds dispersed by wind were common in the matrix. These results would indicate the contribution of animal seed dispersal in forming the distinct woody plant community found on termite mounds. In the indicator species on these mounds, S. persica and Capparis tomentosa bear drupes at the beginning of the rainy season, and many avian species visit fruiting plants and consume these fruits (Yamashina 2014). In addition, many mammal species were observed on termite mounds; African elephants (Loxodonta africana) fed on the leaves of Commiphora sp., vervet monkeys (Chlorocebus pygerythrus) ate the fruits of Ximenia americana, and aardvarks (Orycteropus afer) hunted termites (personal observation using automatic sensor cameras in October for 1 month). Genets (Genetta genetta or Genetta maculata), common warthogs (Phacochoerus africanus), cape porcupines (Hystrix africaeaustralis) and mongoose (species unknown) were also observed on termite mounds (same method as above), and their nests were also found on mounds, except for genets (personal direct observation on mounds). Studies have suggested that large herbivores and small mammals utilise termite mounds, more than the surrounding matrix, as browsing and nesting sites (Loveridge and Moe 2004; Okullo and others 2013). Avian species nesting in woody species on termite mounds may disperse seed among the mounds (Joseph and others 2011). Of the animal species observed at our site, the genet, vervet monkey, elephant and mongoose have been suggested to act as seed dispersers (Jackson and Gartlan 1965; Debussche and Isenmann 1989; Clevenger 1996; Barnes 2001; Tews and others 2004). Additional observational studies on matrix need to compare the relative abundance of potential seed dispersers between mounds and surrounding matrix. However, these mammals, in addition to avian species, visited S. persica and C. tomentosa, possibly dispersing seeds that shape the unique vegetation on termite mounds, because some of them feed on the indicator species on mounds and utilise the mounds as nesting sites.
Soil components would contribute to the differences in the species compositions of distinct vegetation on mounds. As many studies have shown (for example, Sileshi and others 2010), Macrotermes mounds are relatively resource-rich islands with higher total N, P, K, Ca and organic matter contents compared with the surrounding matrix. Of these components, P was associated with mound plots and indicated the difference in species composition between mound and matrix plots. We also found that N-fixing ability, LDMC, LA and leaf C/N were higher in the resource-poor matrix, whereas SLA and leaf thickness were higher on resource-rich mounds. These results are consistent with the findings of previous studies, which showed that species growing in resource-rich environments generally have higher SLA and lower LDMC values (Cornelissen and others 2003), whereas species growing in resource-poor environments have higher leaf C/N and LDMC values and larger leaves (Perez-Harguindeguy and others 2013; Wigley and others 2016). Van der Plas and others (2013) suggested that the presence of fewer N fixers in mounds indicates the role of the mounds as refugia for woody plant species that are less adapted to environments with relatively poor nutrient availability, which is consistent with our results.
We found more evergreen species on mounds and more deciduous species in the matrix. In recent studies, termite mounds have been shown to act as buffers against drought by enhancing revegetation and plant growth (Bonachela and othersand others 2015) and woody species associated with the mounds have been shown to exert cooling effects, which modulate temperature and humidity in African savannahs (Joseph and others 2016). Deciduous species have traits associated with drought avoidance, whereas evergreen species are less well adapted to drought (Lebrija-Trejos and others 2010). Therefore, these mound-induced environments would be advantageous for the establishment of drought-sensitive evergreen species and act as revegetation foci during drought periods, resulting in non-uniform distributions of evergreen and deciduous species and further distinction of the vegetation patterns on mounds and the surrounding matrix. Termite mounds might also act as refugia for fire-prone evergreen species (Van der Plas and others 2013). The vegetation on termite mounds experiences less fire damage due to low fuel load (Joseph and others 2013), high water availability (Konate and others 1999) and elevated topography (Moe and others 2009; Sileshi and others 2010). Although grass cover did not differ significantly between termite mounds and the surrounding matrix at this site (Yamashina 2013), water availability, elevated topography and fire frequency should be examined to assess their filtering effects.
Large herbivores have been suggested to influence the vegetation community of termite mounds, as a disturbance factor (Okullo and Moe 2012a), and have been shown to negatively affect mound woody plant diversity at high densities, with browsers having a greater impact than grazers (Joseph and others 2015). In miombo woodland, termite mounds provide preferred forage for large herbivores (Loveridge and Moe 2004; Mobaek and others 2005) because of their nutrient-rich foliage (Holdo and McDowell 2004), although the reverse pattern, in which the plants on mounds are less preferred by mammal herbivores, was found in mosaic vegetation in South Africa (Van der Plas and others 2013). Davies and others (2016) found that the importance of termite mounds for herbivores as foraging sites varied with nutrient availability and season, and is likely to be more important in nutrient-poor environments in the wet season, and more important in nutrient-rich environments in the dry season. In this study, we found no differences in leaf N content, fruit/pod palatability, or spinescence between mound and matrix, and leaf palatability was higher in the matrix plots. Thus, we found no evidence from functional traits that termite mounds should act as browsing hotspots, which may be explained by the fact that the leaves and pods of C. mopane, the sole dominant species at the site, are the most important resource for herbivores in mopane vegetation ecosystems. The lack of difference in spinescence, which acts as physical protection against herbivores, also indicates no difference in browsing pressure on the woody species on the mound and in the matrix. Therefore, we found no filtering effects due to herbivory in this study.
This study suggested that dispersal processes, especially animal seed dispersal, contribute more to the distinct and diverse woody plant community found on termite mounds than that in the surrounding matrix. Certainly, this non-uniform distribution of woody species with animal dispersal traits could be an incidental result of other filters rather than dispersal mechanisms. Actually, soil can act as a filter of vegetation from the soil P in mound plots. This study, however, found a clear association only between soil P and woody species composition on mounds, despite the significant differences in many soil components between mounds and matrix. Then, animal seed dispersal was suggested as a filter. The seed dispersal process, including direct measurement of seed dispersal, during the creation of diverse plant assemblies on the termite mounds (Pringle and others 2010; Sileshi and others 2010; Erpenbach and others 2013) should be evaluated in future studies, in addition to biotic and abiotic filters, such as the soil environment (for example, Konate and others 1999; Sileshi and others 2010), topography (Dangerfield and others 1998; McCarthy and others 1998) and disturbance (Holdo and McDowell 2004; Loveridge and Moe 2004; Moe and others 2009; Joseph and others 2013). Animals contribute to the development of this hotspot via nutrient input (Holdo and McDowell 2004; Loveridge and Moe 2004) and seed deposition, whereas termite mounds act as browsing and nesting hotspots for mammals and birds (Holdo and McDowell 2004; Loveridge and Moe 2004; Joseph and others 2011). Therefore, more studies are needed to explore the interaction between animals and the plant community on termite mounds to understand the heterogeneity induced by mound-building termites (Sileshi and others 2010; Erpenbach and others 2013).
References
Barnes ME. 2001. Seed predation, germination and seedling establishment of Acacia erioloba in northern Botswana. J Arid Environ 49:541–54.
Belyea LR, Lancaster J. 1999. Assembly rules within a contingent ecology. Oikos 86:402–16.
Bonachela JA, Pringle RM, Sheffer E, Coverdale TC, Guyton JA, Caylor KK, Levin SA, Tarnita CE. 2015. Termite mounds can increase the robustness of dryland ecosystems to climatic change. Science 347:651–5.
Bond WJ, Woodward FI, Midgley GF. 2005. The global distribution of ecosystems in a world without fire. New Phytol 165:525–37.
Bergholz K, May F, Giladi I, Ristow M, Ziv Y, Jeltsch F. 2017. Environmental heterogeneity drives fine-scale species assembly and functional diversity of annual plants in a semi-arid environment. Perspect Plant Ecol Evolut Syst 24:138–46.
Campbell BM. 1996. The miombo in transition: woodlands and welfare in Africa. Bogor, Indonesia: CIFOR.
Clevenger AP. 1996. Frugivory of Martes martes and Genetta genetta in an insular Mediterranean habitat. Rev Ecol-Terre Vie 51:19–28.
Coaton WGH, Sheasby JL. 1972. Preliminary report on a survey of the termites (ISOPTERA) of Southwest Africa. Cimbebasia Memoir No. 2.
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–80.
Cramer MD, Cauter AV, Bond WJ. 2010. Growth of N2-fixing African savanna acacia species is constrained by below-ground competition with grass. J Ecol 98:156–67.
Cromsigt JPGM, Olff H. 2008. Dynamics of grazing lawn formation: an experimental test of the role of scale-dependent processes. Oikos 117:1444–52.
Dangerfield JM, McCarthy TS, Ellery WN. 1998. The mound-building termite Macrotermes michaelseni as an ecosystem engineer. J Trop Ecol 14:507–20.
Davies AB, Baldeck CA, Asner GP. 2016. Termite mounds alter the spatial distribution of African savanna tree species. J Biogeogr 43:301–13.
Debussche M, Isenmann P. 1989. Fleshy fruit characters and the choices of bird and mammal seed dispersers in a Mediterranean region. Oikos 56:327–38.
Defaria SM, Lewis GP, Sprent JI, Sutherland JM. 1989. Occurrence of nodulation in the leguminosae. New Phytol 111:607–19.
Dudley JP. 1999. Seed dispersal of acacia erioloba by african bush elephants in hwange national park, Zimbabwe. Afr J Ecol 37:375–85.
Dufrene M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–66.
Erpenbach A, Bernhardt-Römermann M, Wittig R, Thiombiano A, Hahn K. 2013. The influence of termite induced heterogeneity on savanna vegetation along a climatic gradient in West Africa. J Trop Ecol 29:11–23.
Fleming PA, Loveridge JP. 2003. Miombo woodland termite mounds: resource islands for small vertebrates? J Zool 259:161–8.
Gosling CM, Cromsigt JPGM, Mpanza N, Olff H. 2012. Effects of erosion from mounds of different termite genera on distinct functional grassland types in an African savannah. Ecosystems 15:128–39.
Higgins SI, Bond WJ, Trollope WSW. 2000. Fire, resprouting and variability: a recipe for grass-tree coexistence in savanna. J Ecol 88:213–29.
Holdo RM, McDowell LR. 2004. Termite mounds as nutrient-rich food patches for elephants. Biotropica 36:231–9.
Jackson G, Gartlan JS. 1965. The flora and fauna of Lolui Island, Lake Victoria—a study of vegetation, men and monkeys. J Ecol 53:573–97.
Jacobs SM, Pettit NE, Naiman RJ. 2007. Nitrogen fixation by the savanna tree Philenoptera violacea (Klotzsch) Schrire (Apple leaf) of different ages in a semi-arid riparian landscape. S Afr J Bot 73:163–7.
Joseph GS, Cumming GS, Cumming DHM, Mahlangu Z, Altwegg R, Seymour CL. 2011. Large termitaria act as refugia for tall trees, deadwood and cavity-using birds in a miombo woodland. Landsc Ecol 26:439–48.
Joseph GS, Makumbe M, Seymour CL, Cumming GS, Mahlangu Z, Cumming DHM. 2015. Termite mounds mitigate against 50 years of herbivore-induced reduction of functional diversity of savanna woody plants. Landsc Ecol 30:2161–74.
Joseph GS, Seymour CL, Coetzee BWT, Ndlovu M, De la Torre A, Suttle R, Hicks N, Oxley S, Foord SH. 2016. Microclimates mitigate against hot temperatures in dryland ecosystems: Termite mounds as an example. Ecosphere 7:10.
Joseph GS, Seymour CL, Cumming GS, Cumming DHM, Mahlangu Z. 2014. Termite mounds increase functional diversity of woody plants in African savannas. Ecosystems 17:808–19.
Joseph GS, Seymour CL, Cumming GS, Mahlangu Z, Cumming DHM. 2013. Escaping the flames: large termitaria as refugia from fire in miombo woodland. Landsc Ecol 28:1505–16.
Keddy PA. 1992. Assembly and response rules—two goals for predictive community ecology. J Veg Sci 3:157–64.
Konate S, Le Roux X, Tessier D, Lepage M. 1999. Influence of large termitaria on soil characteristics, soil water regime, and tree leaf shedding pattern in a West African savanna. Plant Soil 206:47–60.
Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM. 2015. Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol 29:592–9.
Lebrija-Trejos E, Perez-Garcia EA, Meave JA, Bongers F, Poorter L. 2010. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 91:386–98.
Levin SA, Muller-Landau HC, Nathan R, Chave J. 2003. The ecology and evolution of seed dispersal: A theoretical perspective. Annu Rev Ecol Evolut Syst 34:575–604.
Loveridge JP, Moe SR. 2004. Termitaria as browsing hotspots for African megaherbivores in miombo woodland. J Trop Ecol 20:337–43.
McCarthy TS, Ellery WN, Dangerfield JM. 1998. The role of biota in the initiation and growth of islands on the floodplain of the Okavango alluvial fan, Botswana. Earth Surf Proc Land 23:291–316.
McGeoch MA, Van Rensburg BJ, Botes A. 2002. The verification and application of bioindicators: a case study of dung beetles in a savanna ecosystem. J Appl Ecol 39:661–72.
Mendelsohn J, Jarvis A, Roberts C, Roberts T. 2002. Atlas of Namibia. Cape Town: David Philip Publishers.
Mendelsohn J, Roberts C. 1997. An environmental profile and atlas of Caprivi. Windhoek: Gamsberg Macmillan Publishers.
Miller MF. 1996. Dispersal of acacia seeds by ungulates and ostriches in an african savanna. J Trop Ecol 12:345–56.
Mittermeier RA, Myers N, Thomsen JB, da Fonseca GAB, Olivieri S. 1998. Biodiversity hotspots and major tropical wilderness areas: Approaches to setting conservation priorities. Conserv Biol 12:516–20.
Mobaek R, Narmo AK, Moe SR. 2005. Termitaria are focal feeding sites for large ungulates in Lake Mburo National Park, Uganda. J Zool 267:97–102.
Moe SR, Mobaek R, Narmo AK. 2009. Mound building termites contribute to savanna vegetation heterogeneity. Plant Ecol 202:31–40.
Namibia Meteorogical Service (PDF). 2013. http://www.meteona.com/attachments/035_Namibia_long-term_climate_statistics_for_specified_places.pdf. Accessed 1 Sept 2013.
Nathan R, Muller-Landau HC. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol 15:278–85.
Oksanen JFGB, Roeland Kindt, Pierre Legendre,, Peter R. Minchin RBOH, Gavin L. Simpson, Peter Solymos,, M. Henry H. Stevens HW. 2016. Package ‘vegan’.
Okullo P, Greve PMK, Moe SR. 2013. Termites, large herbivores, and herbaceous plant dominance structure small mammal communities in savannahs. Ecosystems 16:1002–12.
Okullo P, Moe SR. 2012a. Large herbivores maintain termite-caused differences in herbaceous species diversity patterns. Ecology 93:2095–103.
Okullo P, Moe SR. 2012b. Termite activity, not grazing, is the main determinant of spatial variation in savanna herbaceous vegetation. J Ecol 100:232–41.
Perez-Harguindeguy N, Diaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quetier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC. 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234.
Pickett STA, Cadenasso ML, Benning TL. 2003. Biotic and abiotic variability as key determinants of savanna heterogeneity at multiple spatiotemporal scales. In: Du Toit JT, Rogers KH, Biggs HC, Eds. The Kruger experience: ecology and management of savanna heterogeneity. Washington, DC: Island Press. p 22–40.
Pringle RM, Doak DF, Brody AK, Jocque R, Palmer TM. 2010. Spatial pattern enhances ecosystem functioning in an African savanna. Plos Biol 8:12.
R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ricotta C, Moretti M. 2011. CWM and RAO’s quadratic diversity: a unified framework for functional ecology. Oecologia 167:181–8.
Roberts DW. 2016. Ordination and multivariate analysis for ecology package ‘labdsv’.
Roux PL, Muller M. 2009. Le roux and muller’s field giode to the trees and shrubs of Namibia. Windhoek, Namibia: Macmillan Education Namibia.
Scholes RJ. 1990. The influence of soil fertility on the ecology of southern African dry savannas. J Biogeogr 17:415–19.
Scholes RJ, Biggs R. 2005. A biodiversity intactness index. Nature 434:45–9.
Sileshi GW, Arshad MA, Konate S, Nkunika POY. 2010. Termite-induced heterogeneity in African savanna vegetation: mechanisms and patterns. J Veg Sci 21:923–37.
Stoen OG, Okullo P, Eid T, Moe SR. 2013. Termites facilitate and ungulates limit savanna tree regeneration. Oecologia 172:1085–93.
ter Braak CJF. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167-79.
ter Braak CJF, Schaffers AP. 2004. Co-correspondence analysis: a new ordination method to relate two community compositions. Ecology 85:834-46.
Tews J, Moloney K, Jeltsch F. 2004. Modeling seed dispersal in a variable environment: a case study of the fleshy-fruited savanna shrub Grewia flava. Ecol Model 175:65–76.
Traore S, Nygard R, Guinko S, Lepage M. 2008. Impact of macrotermes termitaria as a source of heterogeneity on tree diversity and structure in a Sudanian savannah under controlled grazing and annual prescribed fire (Burkina Faso). Forest Ecol Manag 255:2337–46.
Van der Plas F, Howison R, Reinders J, Fokkema W, Olff H. 2013. Functional traits of trees on and off termite mounds: understanding the origin of biotically-driven heterogeneity in savannas. J Veg Sci 24:227–38.
Vitousek PM, Howarth RW. 1991. Nitrogen limitation on land and in the sea—how can it occur. Biogeochemistry 13:87–115.
Weiher E, Keddy PA. 1995. The assembly of experimental wetland plant-communities. Oikos 73:323–35.
Werger MJA, Coetzee BJ. 1978. The Sudano-Zembezian Region. Werger MJA editor. Biogeography and ecology of southern Africa, monographiae biologicae: The Hague, p 303–454.
Westoby M. 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–27.
White F. 1984. The vegetation of Africa. Paris: UNESCO.
Wiegand K, Ward D, Saltz D. 2005. Multi-scale patterns and bush encroachment in an arid savanna with a shallow soil layer. J Veg Sci 16:311–20.
Wigley BJ, Slingsby JA, Diaz S, Bond WJ, Fritz H, Coetsee C. 2016. Leaf traits of African woody savanna species across climate and soil fertility gradients: evidence for conservative vs. acquisitive resource use strategies. J Ecol 104:1357–69.
Wyk BV, Wyk PV. 1997. Field guide to trees of Southern Africa. Cape Town: Struik.
Yamashina C. 2013. Variation in savanna vegetation on termite mounds in north-eastern Namibia. J Trop Ecol 29:559–62.
Yamashina C. 2014. Importance of bird seed dispersal in the development of characteristic vegetation on termite mounds in north-eastern Namibia. Tropics 23:33–44.
Yang ZY, Liu XQ, Zhou MH, Ai D, Wang G, Wang YS, Chu CJ, Lundholm JT. 2015. The effect of environmental heterogeneity on species richness depends on community position along the environmental gradient. Sci Rep 5:7.
Acknowledgements
We thank Prof. G. Yamakoshi and S. Ichino of Kyoto University, K. Zamma of Nagano College of Nursing and T. Fujita of the National Institute for Environmental studies, who provided valuable advice during this study. We specially thank Mr. J. Mulofa and all of the villagers in the study area for their hospitality and cooperation and to the Desert Research Foundation of Namibia for their support in obtaining our research permit. This work was financially supported by JSPS Kakenhi (Grants-in-Aid for Scientific Research) Grant Numbers 09J04226 (headed by Chisato Yamashina).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
CY conceived this study, collected and analysed the data and wrote the article. MH participated in the data analysis, particularly the analysis of leaf carbon and nitrogen, and wrote the article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamashina, C., Hara, M. Seed Dispersal by Animals Influences the Diverse Woody Plant Community on Mopane Woodland Termite Mounds. Ecosystems 22, 496–507 (2019). https://doi.org/10.1007/s10021-018-0283-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-018-0283-8