Abstract
Fine-scale spatial heterogeneity influences biodiversity and ecosystem productivity at many scales. In savanna systems, Macrotermes termites, through forming spatially explicit mounds with unique woody plant assemblages, emerge as important sources of such heterogeneity. Despite a growing consensus regarding the importance of functional diversity (FD) to ecosystem processes, no study has quantified how termite mounds affect woody plant FD. We address whether termite mounds alter the distribution of functional traits, and increase FD of woody plant communities within Africa’s largest savanna woodland, the 2.7 million km2 miombo system. Using plant traits that change according to soil resources (for example, water and nutrients), and disturbance (for example, fire and elephant herbivory), we identified response functional groups and compared relative representation of these groups between mound and matrix habitats. We also asked whether mound and matrix habitats differed in their contribution to FD within the system. Although species representing most functional groups were found in both mound and matrix habitats, relative abundance of functional groups differed between mound and matrix. Mound plant assemblages had greater response diversity to soil resources than matrix plots, but there was no difference in response diversity to disturbance. High trait values on mounds included tree height, leaf nitrogen, phosphorus, and palatability. Species with root ectomycorrhizae dominated the matrix. In conclusion, these small patches of nutrient-enriched substrate emerge as drivers of FD in above-ground woody plant communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrient-enriched patches that are small relative to the surrounding landscape can have disproportionate effects on biodiversity. For example, clear effects have been detected from soil licks (Ayotte and others 2006), caves (Arita 1993), savanna trees (Dean and others 1999), old cattle enclosures (Young and others 1995), and roosting sites (Ellsworth and McComb 2003). Similarly, soil organisms can generate ecologically important heterogeneity (Ettema and Wardle 2002). Interactions between small resource patches and their surrounding landscape can act at a variety of scales to influence plant and animal population structure, community composition, and related ecosystem processes (Pickett and Cadenasso 1995). The termitaria constructed by termites of the family Macrotermitinae provide a good example of small, high-nutrient patches that influence the diversity and productivity of entire ecosystems (Pringle and others 2010).
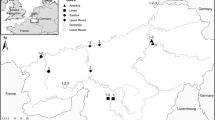
In the dystrophic miombo woodlands of central and southern Africa (Figure 1), Macrotermes (Isoptera) termites concentrate macro- (Sileshi and others 2010) and micro-nutrients (Seymour and others 2014) in their mounds through their foraging activities. These mounds can be up to 9 m high with basal diameters of 30 m (Malaisse 1978). The woody plant communities found on mounds are distinct from those in the surrounding matrix (Figure 2; Joseph and others 2013a), with a greater representation of fruiting and evergreen species, which are often found in eutrophic habitats beyond the miombo system (for example, riparian, rocky, or eutrophic low-veld habitats; Coates Palgrave and Coates Palgrave 2002). Although mounds represent only about 5% of landscape area (Levick and others 2010; Joseph and others 2011), they are browsing hotspots for large ungulates (Holdo and McDowell 2004; Levick and others 2010). Mounds have also been found to harbor a greater diversity of small mammals (Fleming and Loveridge 2003) and cavity-using birds (Joseph and others 2011) than the surrounding habitat. Termites and their mounds influence the fire regime, because termites consume plant material that fuels fires (Sileshi and others 2010). In addition, because fire tends to be excluded from mounds, fire-sensitive species can persist within the miombo woodland, on mounds (Joseph and others 2013b).
The extent of miombo woodlands in southern and central Africa, based on White’s vegetation map (1983). The various lines within the miombo distinguish between wet and dry miombo. The miombo is climatically comparable to Brazilia and Darwin.
There is a growing awareness of the far-reaching influence of Macrotermes foraging on overall biodiversity and productivity. Given that functional diversity (FD) reflects ecosystem pattern and processes better than taxonomic diversity (Díaz and Cabido 2001), and that functional traits of plants differ between mounds and the matrix (Van der Plas and others 2013), the questions of whether mounds are more functionally diverse than the surrounding matrix, or whether they add FD to the system, are both important and, as yet, unanswered.
Here, we define FD as “the value and range of those species and organismal traits that influence ecosystem functioning” (Tilman 2001, p. 109). Functional traits influence the biotic and abiotic environment, and through co-evolution and adaptation, the biotic and abiotic environments in turn influence functional traits (Díaz and Cabido 2001). Given that large termite mounds can harbor plants with different functional traits relative to the surrounding matrix, mound and matrix habitats may differ in their contribution to the overall FD of the system.
Understanding how fine-scale habitat heterogeneity creates fine-scale heterogeneity in FD helps identify the mechanisms underlying community responses to management (Baraloto and others 2012) and environmental change (Podgaiski and others 2013). Species can be classified functionally using traits that are important in their responses to environmental factors (that is, “functional response types”), or their effects on ecosystems (that is, “functional effect types”). Plants that group together because of functional response are those that respond similarly to the abiotic and biotic environment (for example, resource availability and disturbance), whereas functional effect types group together because they have similar effects on ecosystem processes (for example, trophic transfer and primary productivity; Díaz and Cabido 2001).
A greater diversity of responses within a system can protect against loss of ecosystem function when ecosystems undergo disturbance (Elmqvist and others 2003). For example, if species that perform similar ecological functions (for example, nitrogen fixing or seed dispersal) respond differently to a disturbance like fire, then although the relative proportions of those species might change, the functions that they perform will persist (Walker and others 1999).
Here, we asked whether relatively small, nutrient-rich patches, in the form of large termite mounds, might contribute significantly to plant functional response diversity. We also examined the dominant traits of mound and matrix woody plant species to gain insight into their roles within the landscape. Woody cover in savannas is influenced by rainfall, fire, herbivory, and soil nutrients (Sankaran and others 2005). Given the nutrient-concentrating effects of termitaria on miombo soils and frequent seasonal fires in the presence of elephant browsing, we focused on functional groupings based on woody plant responses to (1) soil resources and (2) disturbance (fire and herbivory), and asked:
-
1.
Do woody plants found on mounds belong to different response functional groups from those found in the matrix?
-
2.
Because termitaria are small (~5% of the area) relative to the surrounding matrix, is the contribution to functional response diversity by mound species lower than that of matrix species?
-
3.
Do mounds and the woody plant species that grow upon them add to the functional response diversity of the system, overall? In other words, how does the FD of the combination of mound plus matrix compare to the FD of matrix alone?
-
4.
Which traits are associated with mound species and which are associated with matrix species?
Materials and Methods
Study Site and Field Data
We conducted this study in the miombo woodlands of Chizarira National Park in north-western Zimbabwe, with field visits in November–December 2007, April–May 2009, and November–December 2009. Large termitaria (>2 m tall or >10 m wide) occur in the park at densities of 2 ha−1 (Joseph and others 2011). Woody plant species composition and tree-grass ratios in miombo reserves are influenced by two primary sources of disturbance: herbivory (notably elephant browsing) and fire (Frost 1996). At the time of our study, Chizarira National Park had recently undergone a period of intense elephant herbivory, which had opened up the woodland canopy, allowing an increase in grass cover that supports intense fires (Mapaure and Campbell 2002).
The soils in Chizarira have been identified as Haplic Lixisols and Eutric Leptosols (SADC Food Security Programme 1991). Lixisols, considered “marginal”, contain only low levels of plant nutrients and are highly erodible, whereas Leptosols have very shallow profiles, are also highly erodible and are considered “poor” soils (FAO/IIASA/ISRIC/ISS-CAS/JRC 2012). The wet season in Chizarira is from November to April, followed by a cool dry season from May to July, and a hot dry season from August to November. Mean annual temperature across the park is 20–22.5°C (Torrance 1965). Mean annual rainfall is 600–800 mm (Lineham 1965).
We collected vegetation data from 36 paired large mound-matrix plots, matched for temperature, rainfall, catena, and proximity to drainage channels to minimize environmental variability. We estimated mound surface area by modeling mounds as cones, sampling 100 m2 of mound (connecting the center of the mound to its perimeter, and using this radius as the southernmost aspect of a pie-shape). Each 100 m2 of mound was compared to an adjacent matrix plot measuring 100 m2. Levick and others (2010) found that the effects of large herbivores can be evident at distances of 20 m into the matrix from the mound. Therefore, to capture the variation in response to herbivory within the matrix, each matrix plot was 50 m x 2 m, with the 50 m side radiating out on a random bearing from the mound. Although this design creates higher edge to area ratios for matrix plots, given the large sample size (36 pairs of mound and matrix plots), any important plant–plant interactions (for example, facilitation or competition) have similar probabilities of being incorporated in both mound and matrix plots. For each mound-matrix plot, we recorded woody plant species, their heights, and the number of stems.
Trait Selection and Measurement
Given that within a single trophic level, most theoretical models predict saturation of ecological function at relatively low-species richness (Schwartz and others 2000), we limited our study to woody species that occurred on at least 80% of mound and 80% of matrix plots. These species together represented 90% of aboveground biomass, and were identified using plant height in the formula employed by Higgins and others (2007) to estimate biomass. This yielded 36 species (Figure 3), 15 of which were found predominantly on mounds versus 18 that were found predominantly on matrix plots, with three species common to both. We collected and measured functional trait data following standardized protocols developed by Cornelissen and others (2003), to ensure consistent and replicable evaluation of functional traits.
Functional relationships between the dominant woody plant species in CNP, based on A response to soil resources and B response to disturbance. Letters next to species denote whether they are found primarily on mounds (T), in the matrix (W), or both (B). The three major families are marked using dashed lines: Combretaceae (green dash dotted lines), Fabaceae (red dashed lines) and Ebenaceae (blue dashed lines).
Plant functional and morphological traits reflect edaphic conditions and disturbance regimes (Chapin 2003; Mason and others 2010). Plant species that respond to, or affect, the environment in similar ways share certain sets of traits (Lavorel and Garnier 2002). We selected traits relating to soil resources and disturbance (Table 1) based on guidelines from various sources (Díaz and others 1999; Lavorel and Garnier 2002; Cornelissen and others 2003; Richardson-Kageler 2004; see Supplementary Table 1 for the raw data). For nutrient uptake strategy, we represented the trait by using two categories: those that do or do not fix nitrogen (by any mechanism), and those with and without ectomycorrhizae.
Statistical Analysis
To ensure that the biological variation within each trait was treated equally, we standardized the trait data so that each trait had a mean of zero and a standard deviation of one (Petchey and Gaston 2006). Non-overlapping categorical traits (nitrogen-fixer vs non-nitrogen fixer; ectomycorrhiza vs. no ectomycorrhiza) were quantified as binary data. To reduce bias, we then applied weightings for categorical traits and for single traits for which we had used more than one measurement. For example, seed morphology is a single trait, but we used sphericity, length, and breadth to represent this trait (that is, 3 variables), and thus gave each variable a 1/3 weighting to prevent bias towards seed morphology (Laliberté and Legendre 2010).
To calculate FD, we converted the species by trait matrix into a distance matrix using Gower distance. Gower distance allows individual traits to be weighted differently (Podani 1999), and the use of quantitative and qualitative traits together (Podani and Schmera 2006). We then clustered the matrix using the “average” method in the FD package in R (Laliberté and Shipley 2011), which was chosen because it produced the highest cophenetic correlation (0.75 for response to soil resources and 0.69 for response to disturbance). In this way, each species was given a measure of similarity with every other species, based on the functional traits used.
Comparing Whether Woody Plant Functional Groups Differed Between Mound and Matrix
We placed species into functional groups using the FD (Laliberté and Shipley 2011) and vegan (Oksanen and others 2013) packages in R. To explore the functional relationships of woody plant species, we used the Calinski-Harabasz criterion to first assess the optimal number of functional groups to which species should be allocated. This criterion specified five functional groups for response to soil resources and six functional groups for response to disturbance. Although using functional groups ignore functional differences occurring between species in the same group and the level of difference between groups can be arbitrary (Petchey and Gaston 2006), the groups it identifies can be used to give an overall picture of whether, and how, functional groups might differ between large mounds and woodland habitat. To do this, we used an ANOSIM to ascertain if there was any difference in relative representation of functional groups between mound and matrix plots, based on biomass.
Comparing FD Between Mound and Matrix Plots
The FD of the plant assemblages found in each mound or matrix plot was measured as the total length of branches of the dendrogram needed to connect all of the species in that particular assemblage (Petchey and Gaston 2006). To ascertain if mounds add FD to woody plant communities, we were not only interested in how FD compared between mound and matrix plots, but also in how the FD of matrix plots alone compared with that of mound and matrix together. To compare the FD of matrix, mound, and matrix-plus-mound plots, species richness must first be accounted for. Each time a species is added to an assemblage, the FD can increase. It would be invalid to compare, for example, the FD of a matrix plot with n species to the FD of that matrix plot plus its mound plot (with k species), which would then have n+k species. A null model should ideally produce random values, against which observed values can be compared. Specifically, we compared the FD value for the observed assemblages of n species against 999 random assemblages of n species from the full set of species used in the analysis, using a null model where we shifted taxa labels across the dendrogram. We then calculated the standardized effect size (SES), which is calculated by subtracting the mean of the null distribution from the observed FD value, and dividing by the standard deviation of the null distribution. The SES thus reflects the number of standard deviations that the observed index is above or below the mean (0) of the simulated communities obtained from the null model (Gotelli and Rohde 2002), with higher SES values indicating higher FD. We then used ANOVA to compare SES in functional response to soil resources and disturbance between mound, matrix, and mound-plus-matrix plots. Where these differences were significant, we used pairwise t-tests with Benjamini and Yekutieli (2001) corrections for false discovery rates. All analyses were conducted in R (R Development Core Team 2011).
Species Traits of Mound versus Matrix Species
To identify how the representation of traits differs between mound and matrix species, we carried out a SIMPER analysis in R (package: Vegan, Oksanen and others 2013). SIMPER (SIMilarity PERcentage) analyses use Bray-Curtis distance to compare each sample with every other sample to identify the species that are primarily responsible for the differences between sample groups (in this case, mound vs. matrix). SIMPER identifies the contribution of each species (or other variable) to the observed similarities (or dissimilarities) between sample groups. We used SIMPER to identify the traits that were responsible for differences between woody plant species found on mounds and those found in the matrix.
Results
Of the woody plants surveyed, 36 species, represented by 5,332 individual plants, met the requirements of being present on at least 80% of plots (Cornelissen and others 2003). These are listed in Supplementary Table 1.
Differences in Woody Plant Functional Groups, Mound Versus Matrix
The dendrograms in Figure 3 show the functional relationships between the 36 species in plots in Chizarira National Park, reflecting their responses to soil resources (Figure 3A) and to disturbance (Figure 3B).
Response to Soil Resources
Species with shorter connections between them are more functionally similar. For example, Brachystegia spiciformis and B. boehmii respond in a similar way to soil conditions (Figure 3A), but B. boehmii and Berchemia discolor respond quite differently. Species and the functional groups to which they were allocated are listed in Table 2. Many of the functional groups contained species representative of both mound and matrix. In other words, some matrix species were more similar to some mound species in their response to soil resources than they were to other matrix species. The relative representation of mound and matrix species within functional groups was significantly different, however (ANOSIM: Global R = 0.49, p < 0.0001). Functional groups were also influenced by phylogenetic relatedness. For example, the species in the group from Terminalia brachystemma to Combretum molle are all members of the Combretaceae, and the species from Pterocarpus rotundifolius to B. boehmii are all within the Fabaceae (Figure 3A).
Response to Disturbance
The dendrogram for functional groups based on response to disturbance did not show clear separation between termitaria and matrix woody plant species at low degrees of functional similarity between species, although within these groups, there was some separation between mound and matrix species (Figure 3B). There was also a trend of species grouping according to family. For example, the group that includes P. rotundifolius to Dalbergia melanoxylon are all members of the Fabaceae, and the group from C. zeyheri to C. hereroense are all Combretaceae (Figure 3B). Again, although representatives of mound and matrix were found within most functional groups, when relative abundances are considered, the differences in representation between the functional groups are significantly different (ANOSIM: Global R: 0.589, p < 0.001).
Inspection of Table 2 reveals that 25 of the 36 species (that is, 69.4%) considered here remained in the same functional groups in response to disturbance as to soil resources. Of the remainder, a further seven (19.4%) moved to a new functional group accompanied by one or more species from its original group. Only 11.1% of species moved alone to a new functional group.
Comparison of Response FD Between Mounds Versus Matrix, and Whether Additional FD Contributed by Mounds Increases Overall FD
Response to Soil Resources
There was a significant difference in FD between mound, matrix, and mound and matrix combined (ANOVA: F value = 8.26, Df = 2, p < 0.001). Matrix plots had significantly lower functional response diversity (mean SES ± SD = −0.86 ± 0.82), than mound plots (mean SES = −0.24 ± 0.90; p = 0.006, pairwise t tests with corrections for false discovery rates) and mound and matrix plots together (mean SES = −0.11 ± 0.99, p = 0.001). Mean SES for mound plots was not significantly different to mound and matrix plots together (p = 0.93).
Response to Disturbance
There were no significant differences in FD in terms of response to disturbance between mound, matrix, and mound and matrix plots combined (ANOVA: F value = 0.36, p = 0.70). Mean SES ± SD values were −0.16 ± 0.89 for matrix plots, −0.19 ± 1.04 for mound plots, and −0.02 ± 0.94 for matrix and mound combined.
Differences in Trait Representation Between Mound and Matrix Habitats
For response to soils, SIMPER analysis identified six traits responsible for 75% of differences in traits that respond to soil nutrients between plants found on the matrix and those found on mounds (Table 3), although none of the traits could be considered good discriminators across all sites (that is, the square distance/standard deviation ratio was low), possibly because functional groups were represented on both mound and matrix. Nutrient uptake strategy accounted for 30.24% of the difference explained. Ability to resprout (10.55%) and months/year without leaves (9.4%) were both lower for plants on mounds. Leaf N (9.03%) and leaf P (8.62%) were higher on mounds, while leaf size (8.59%) was higher for the matrix.
Eight different traits explained 75% of the observed differences in response of woody species to disturbance (Table 3). These were resprouting response after fire (lower on termitaria; 11.9%), number of trees larger than 6 m, leaf nitrogen, and leaf phosphorus (all higher on termitaria and each explaining approximately 10% of difference observed), growth form (more trees in the matrix; 9.4%), nutrient uptake strategy (ectomycorrhizal tree species in matrix; 8.49%), and palatability (higher on termitaria, 8.5%).
Discussion
Ecological research is underpinned by a desire to understand how organisms interact with and influence both the biotic and abiotic factors that surround them. Small patches of resources within larger landscapes are known to influence biodiversity pattern, processes, and overall productivity (Dean and others 1999; Ellsworth and McComb 2003; Ayotte and others 2006; Pringle and others 2010). We have found clear support for the hypothesis that Macrotermes termites, in creating fine-scale environmental heterogeneity by building mounds, increase woody plant FD in Africa’s largest savanna woodland.
Although representatives from most functional groups were found both on mounds and in the surrounding woodland matrix, their relative biomass differed between the two habitats, with mounds being dominated by certain functional groups while others dominated the matrix. Functional traits determine not only where species can exist (Keddy 1992; Walker and others 1999; Cornwell and Ackerly 2009), but also their relative abundances across habitats (Cornwell and Ackerly 2010). Our findings support this idea. Recent studies have used the representation of traits within a system to scale up to ecosystem processes (for example, Cortez and others 2007; Suding and others 2008), illustrating the usefulness of understanding the relative representation of traits and functional groups.
Species assemblages on mounds were significantly more diverse than the matrix in terms of response to soil resources, but there were no significant differences between mound and matrix assemblages in terms of their response to disturbance. Contrary to our initial predictions, mounds harbored greater response diversity to soil nutrients, despite occupying a much smaller area than the matrix habitat. Furthermore, the FD in response to soil nutrients of mounds plus matrix was significantly greater than that of the matrix alone, demonstrating that mounds increase FD in the system overall. The relatively low-response diversity to soil resources among matrix species has probably arisen because the nutrient-poor soils of the miombo matrix have acted as a habitat filter, selecting for a few species that can survive under these conditions, whereas mounds, by virtue of being richer and more variable in many macro- and micro-nutrients (Seymour and others 2014), may allow a higher diversity of plant response groups to soil resources. Such considerations remain to be tested. Termite mounds thus represent an example of how small patches may contribute to overall response diversity within a system, but not necessarily to all types of response diversity.
That there were no differences in functional response diversity to disturbance might be because both mound and matrix experience some form of disturbance. The matrix experiences herbivory (by all herbivores) and, when elephant numbers are high, intense and/or frequent fires (Mapaure and Campbell 2002). Mounds tend to experience greater degrees of elephant herbivory (Loveridge and Moe 2004), although they are relatively spared from fires (Joseph and others 2013b). We do not know how these disturbance regimes compare in intensity or frequency between mound and matrix, but perhaps the key point is not so much the degree of disturbance but that disturbances occur at intervals shorter than the lifetimes of most woody plants and at potentially fatal intensities. In addition, many mound plants in our study site are found off-mound in other areas; at a species level, they may be subject to more regular herbivory and/or fire in other parts of their ranges.
Within functional groups, species tended to cluster according to family. This indicates that environmental filtering has acted on shared physiological traits (Webb and others 2002). Here, functional groups tended to be composed of related taxa at the family level, for example, members of the Fabaceae (P. rotundifolius, Philenoptera violacea, Julbernardia globiflora, B. spiciformis, B. boehmii, Colophospermum mopane, Xeroderris stuhlmannii, Cassia abbreviata, Acacia nilotica, and D. melanoxylon); Combretaceae (Combretum and Terminalia species); and, to a lesser extent, Ebenaceae (Diospyros kirkii, D. mespiliformis, and D. quiloensis). That closely related species often grouped together functionally suggests conserved evolution of traits, but until a phylogeny is completed for at least some components of the miombo woodlands, this cannot be accurately assessed.
It is probably not surprising that nutrient uptake strategy accounted for much of the difference in species traits between mound and matrix habitats, given the vast differences between mound and matrix substrate. Ability to resprout was lower on mounds, consistent with earlier findings by Joseph and others (2013b) for woody species’ responses to fire. These results suggest that being able to resprout after disturbance is more important in the matrix. The diversity of responses to disturbance did not differ between mound and matrix despite differences in the types of response between the two habitats. Deciduousness was greater in the matrix than on mounds, perhaps because the species in the two environments have evolved different strategies for dealing with soil nutrient status. Trees in the matrix have evolved to resorb nutrients before leaf fall (Ernst 1975) so that these nutrients are retained; the evergreen species found on mounds do not need this strategy. The substrate of the matrix is low in most nutrients, but both the mound and matrix contain only low concentrations of available P (Seymour and others 2014).
In conclusion, this study emphasizes the disproportionate impact that small patches of unique resources can have on FD. Termite mounds comprise only about 5% of the area in the landscapes in which they are found, yet they increase FD. Given that FD, rather than species richness, can better reflect ecosystem pattern and process (Diaz and Cabido 2001; Suding and others 2008), the increase in woody plant FD because of these small patches could have implications for the entire system.
References
Arita HA. 1993. Conservation biology of the cave bats of Mexico. J Mammal 74:693–702.
Ayotte JB, Parker KL, Arocena JM, Gillingham MP. 2006. Chemical composition of lick soils: functions of soil ingestion by four ungulate species. J Mammal 87:878–88.
Baraloto C, Hérault B, Paine CET, Massot H, Blanc L, Bonal D, Molino J-F, Nicolini EA, Sabatier D. 2012. Contrasting taxonomic and functional responses of a tropical tree community to selective logging. J Appl Ecol 49:861–70.
Benjamini Y, Yekutieli D. 2001. The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–88.
Chapin FS. 2003. Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann Bot 91:455–63.
Clarke KR, Warwick RM. 1994. Change in marine communities: an approach to statistical analysis and interpretation. Natural Environment Research Council. Plymouth: Hutchings & Mason Ltd. 144 pp.
Coates Palgrave K, Coates Palgrave M. 2002. Palgrave’s trees of southern Africa. Cape Town: Struik Publishers.
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, Van Der Heijden MGA, Pausas JG, Poorter H. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–80.
Cornwell WK, Ackerly DD. 2009. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol Monogr 79:109–26.
Cornwell WK, Ackerly DD. 2010. A link between plant traits and abundance: evidence from coastal California woody plants. J Ecol 98:814–21.
Cortez J, Garnier E, Pérez-Harguindeguy N, Debussche M, Gillon D. 2007. Plant traits, litter quality and decomposition in a Mediterranean old-field succession. Plant Soil 296:19–34.
Dean WRJ, Milton SJ, Jeltsch F. 1999. Large trees, fertile islands, and birds in arid savanna. J Arid Environ 41:61–78.
Díaz S, Cabido M, Zak M, Martínez Carretero E, Aranibar J. 1999. Plant functional traits, ecosystem structure and land-use history along a climatic gradient in central-western Argentina. J Veg Sci 10:651–60.
Díaz S, Cabido M. 2001. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–55.
Ellsworth JW, McComb BC. 2003. Potential effects of passenger pigeon flocks on the structure and composition of presettlement forests of eastern North America. Conserv Biol 17:1548–58.
Elmqvist T, Folke C, Nyström M, Peterson G, Bengtsson J, Walker B, Norberg J. 2003. Response diversity, ecosystem change, and resilience. Front Ecol Environ 1:488–94.
Ernst W. 1975. Variation in the mineral contents of leaves of trees in miombo woodland in south central Africa. J Ecol 63:801–7.
Ettema CH, Wardle DA. 2002. Spatial soil ecology. Trends Ecol Evol 17:177–83.
FAO/IIASA/ISRIC/ISS-CAS/JRC. 2012. Harmonized World Soil Database (version 1.2). FAO Rome, Italy and IIASA, Laxenburg, Austria. http://webarchive.iiasa.ac.at/Research/LUC/External-World-soil-database/HTML/. Accessed 3 Nov 2012.
Fleming PA, Loveridge JP. 2003. Miombo woodland termite mounds: resource islands for small vertebrates? J Zool 259:161–8.
Frost P. 1996. The ecology of miombo woodlands. In: Campbell B, Ed. The miombo in transition: woodlands and welfare in Africa. Bogor: Center for International Forestry Research (CIFOR). p 11–58.
Gotelli NJ, Rohde K. 2002. Co-occurrence of ectoparasites of marine fishes: a null model analysis. Ecol Lett 5:86–94.
Higgins SI, Bond WJ, February EC, Bronn A, Euston-Brown DIW, Enslin B, Govender N, Rademan L, O’ Regan S, Potgieter ALF, Scheiter S, Sowry R, Trollope L, Trollope WSW. 2007. Effects of four decades of fire manipulation on woody vegetation structure in savanna. Ecology 88:1119–25.
Holdo RM, McDowell LR. 2004. Termite mounds as nutrient-rich food patches for elephants. Biotropica 36:231–9.
Joseph GS, Cumming GS, Cumming DHM, Mahlangu Z, Altwegg R, Seymour CL. 2011. Large termitaria act as refugia for tall trees, deadwood and cavity-using birds in a miombo woodland. Landsc Ecol 26:439–48.
Joseph GS, Seymour CL, Cumming GS, Cumming DHM, Mahlangu Z. 2013a. Termite mounds as islands: woody plant assemblages relative to termitarium size and soil properties. J Veg Sci 24:702–11. doi:10.1111/j.1654-1103.2012.01489.x.
Joseph GS, Seymour CL, Cumming GS, Mahlangu Z, Cumming DHM. 2013b. Escaping the flames: large termitaria as refugia from fire in miombo woodland. Landsc Ecol 28:1505–16. doi:10.1007/s10980-013-9897-6.
Keddy PA. 1992. Assembly and response rules: two goals for predictive community ecology. J Veg Sci 3:157–64.
Laliberté E, Legendre P. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305.
Laliberté E, Shipley B. 2011. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package 1.0–11.
Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits. Funct Ecol 16:545–56.
Levick SR, Asner GP, Kennedy-Bowdoin T, Knapp DE. 2010. The spatial extent of termite influences on herbivore browsing in an African savanna. Biol Conserv 143:2462–7.
Lineham S. 1965. Rainfall in Rhodesia. In: Collins MO, Ed. Rhodesia: its natural resources and economic development. p. 26–7.
Loveridge JP, Moe SR. 2004. Termitaria as browsing hotspots for African megaherbivores in miombo woodland. J Trop Ecol 20:337–43.
Malaisse F. 1978. High termitaria. In: Werger MJA, Ed. Biogeography and ecology of southern Africa. The Hague: Junk. p 1279–300.
Mapaure IN, Campbell BM. 2002. Changes in miombo woodland cover in and around Sengwa Wildlife Research Area, Zimbabwe, in relation to elephants and fire. Afr J Ecol 40:212–19.
Mason NWH, Peltzer DA, Richardson SJ, Bellingham PJ, Allen RB. 2010. Stand development moderates effects of ungulate exclusion on foliar traits in the forests of New Zealand. J Ecol 98:1422–33.
Oksanen J, Blanchet FG, Roeland Kindt, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2013. Vegan: Community Ecology Package. R package version 2.0-7. http://cran.r-project.org/package=vegan. Accessed 5 May 2013.
Petchey OL, Gaston KJ. 2006. Functional diversity: back to basics and looking forward. Ecol Lett 9:741–58.
Pickett STA, Cadenasso ML. 1995. Landscape ecology: spatial heterogeneity in ecological systems. Science 269:331–4.
Podani J, Schmera D. 2006. On dendrogram-based measures of functional diversity. Oikos 115:179–85.
Podani J. 1999. Extending gower’s general coefficient of similarity to ordinal characters. Taxon 48:331–40.
Podgaiski LR, Joner F, Lavorel S, Moretti M, Ibanez S, Mendonça MDS, Pillar VD. 2013. Spider trait assembly patterns and resilience under fire-induced vegetation change in South Brazilian grasslands. PloS one 8:e60207. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3610671&tool=pmcentrez&rendertype=abstract. Accessed 20 May 2013.
Pringle RM, Doak DF, Brody AK, Jocqué R, Palmer TM. 2010. Spatial pattern enhances ecosystem functioning in an African savanna. PLoS Biol 8:e1000377.
R Development Core Team. 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/. Accessed 4 March 2011.
Richardson-Kageler SJ. 2004. Effects of large herbivore browsing on the functional groups of woody plants in a southern African savanna. Biodivers Conserv 13:2145–63.
SADC Food Security Programme. 1991. Draft soil map of Zimbabwe http://eusoils.jrc.ec.europa.eu/esdb_archive/eudasm/africa/maps/afr_zw2015_so.html. Accessed 20 June 2012.
Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS, Gignoux J, Higgins SI, Le Roux X, Ludwig F, Ardo J, Banyikwa F, Bronn A, Bucini G, Caylor KK, Coughenour MB, Diouf A, Ekaya W, Feral CJ, February EC, Frost PGH, Hiernaux P, Worden J, Zambatis N. 2005. Determinants of woody cover in African savannas. Nature 438:8–11.
Schenk HJ, Jackson RB. 2002. The global biogeography of roots. Ecol Monogr 72:311–28.
Schwartz MW, Brigham CA, Hoeksema JD, Lyons KG, Mills MH, van Mantgem PJ. 2000. Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122:297–305.
Seymour CL, Milewski AV, Mills AJ, Joseph GS, Cumming GS, Cumming DHM, Mahlangu Z. 2014. Do the large termite mounds of Macrotermes concentrate micronutrients in addition to macronutrients in nutrient-poor African savannas? Soil Biol Biochem 68:95–105.
Sileshi GW, Arshad MA, Konate S, Nkunika POY. 2010. Termite-induced heterogeneity in African savanna vegetation: mechanisms and patterns. J Veg Sci 21:923–37.
Suding KN, Lavorel S, Chapin FS, Cornelissen JHC, Díaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navas M-L. 2008. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Change Biol 14:1125–40.
Tilman D. 2001. Functional Diversity. In: Levin SA, Ed. Encyclopedia of Biodiversity, Vol. 3. San Diego, CA: Academic Press. p 109–21.
Torrance JD. 1965. The temperature of Rhodesia. In: Collins MO, editor. Rhodesia: its natural resources and economic development. pp 28–9.
Van der Plas F, Howison R, Reinders J, Fokkema W, Olff H. 2013. Functional traits of trees on and off termite mounds: understanding the origin of biotically-driven heterogeneity in savannas. Bruun HH, editor. J Veg Sci 24:227–38.
Walker BH. 1980. A review of browse and its role in livestock production in southern African savannas. Proc Grassland Soc of southern Africa 11:125–130.
Walker B, Kinzig A, Langridge J. 1999. Plant attribute diversity, resilience, and ecosystem function: The nature and significance of dominant and minor species. Ecosystems 2:95–113.
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Ann Rev Ecol Syst 33:475–505.
White F. 1983. The vegetation of Africa: a descriptive memoir to accompany the UNESCO/AETFAT/UNSO vegetation map of Africa, p. 356. UNESCO, Paris.
Young TP, Patridge N, Macrae A. 1995. Long-term glades in acacia bushland and their edge effects in Laikipia, Kenya. Ecol Appl 5:97–108.
Acknowledgments
This research was funded by an NRF-SADC collaborative grant and the DST/NRF Centre of Excellence at the University of Cape Town. The Director General of the Zimbabwe National Parks and Wildlife Management Authority (ZNPWMA) granted permission to carry out this research under the auspices of a memorandum of understanding between the ZNPWMA and the Tropical Resource Ecology Programme (TREP) at the University of Zimbabwe. Chris Chapano and Anthony Mapaura, and Christien Bredenkamp of the National Herbaria of Harare and Pretoria, respectively, offered assistance and guidance in building a database of functional traits.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author's Contribution
GSJ, GSC conceived of the study; GSJ, CLS, ZM designed the study and gathered data; GSJ, CLS carried out analyses; GSJ, CLS, GSC, DHMC wrote the paper
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joseph, G.S., Seymour, C.L., Cumming, G.S. et al. Termite Mounds Increase Functional Diversity of Woody Plants in African Savannas. Ecosystems 17, 808–819 (2014). https://doi.org/10.1007/s10021-014-9761-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-014-9761-9