Abstract
Disturbance releases space and allows the growth of opportunistic species, excluded by the old stands, with a potential to alter community dynamics. In coral reefs, abundances of fast-growing, and disturbance-tolerant sponges are expected to increase and dominate as space becomes available following acute coral mortality events. Yet, an increase in abundance of these opportunistic species has been reported in only a few studies, suggesting certain mechanisms may be acting to regulate sponge populations. To gain insights into mechanisms of population control, we simulated the dynamics of the common reef-excavating sponge Cliona tenuis in the Caribbean using an individual-based model. An orthogonal hypothesis testing approach was used, where four candidate mechanisms—algal competition, stock-recruitment limitation, whole and partial mortality—were incorporated sequentially into the model and the results were tested against independent field observations taken over a decade in Belize, Central America. We found that releasing space after coral mortality can promote C. tenuis outbreaks, but such outbreaks can be curtailed by macroalgal competition. The asymmetrical competitive superiority of macroalgae, given by their capacity to pre-empt space and outcompete with the sponge in a size-dependant fashion, supports their capacity to steal the opportunity from other opportunists. While multiple system stages can be expected in coral reefs following intense perturbation macroalgae may prevent the growth of other space-occupiers, such as bioeroding sponges, under low grazing pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interspecific competition is a common structuring process in many ecosystems (Connell 1983; Schoener 1983). Competitive attributes such as size and growth can confer a large advantage to one competitor when resources are limited, leading to asymmetric competition (Weiner 1990). For example, large plants can pre-empt resources (e.g. light) in proportion to their size, thus compromising the survivorship and growth of smaller plants (Berger et al. 2008). Such competitive asymmetry can also be observed on the coral reef benthos (Sandin and McNamara 2012), where space is an essential and finite resource (Connell 1983), and therefore ‘pre-emptable’. Organisms capable of disproportionately high growth rates and rapid colonisation, such as macroalgae (Connell et al. 2004), have the capacity to limit the growth of other benthic organisms in a size-dependent fashion (Ferrari et al. 2012a). Also, space pre-emption by macroalgae limits the population replenishment of other benthic groups by occupation of space available for larval recruitment (Arnold et al. 2010; Doropoulos et al. 2015). Such competitive superiority of macroalgae is complemented by allelopathic properties that can compromise the growth, recruitment and mortality of other benthic organisms (Paul et al. 2011; Rasher and Hay 2010).
Although there is no doubt that competition is an important process in structuring benthic communities, uncertainty arises when trying to predict how complex competitive networks determine the trajectory of perturbed ecosystems (Karlson and Jackson 1981). In particular, the consideration of multispecies asymmetrical interactions and the dynamic nature of such hierarchical interactions are especially complex and difficult to predict (Connell 1983). Yet it is important to understand the outcome of multispecies interactions to predict the response of ecosystems to disturbance (Hastings 1980).
Complex community responses to disturbance have previously been explored in terrestrial systems, where the outbreak of disturbance-resistant species is dampened by the competitive strength of other players (Lenssen et al. 2004). In space-limited systems, such as benthic marine environments (Muko et al. 2001; Roughgarden et al. 1985), disturbance often favours the rapid colonization of ‘fugitive’ or opportunistic species (Hutchinson 1951). This can occur through release from competition (Horn and MacArthur 1972) or by altering the competitive hierarchy among species (Suding and Goldberg 2001). Opportunistic species generally colonize vacated space quicker than others, because of their fast growth rates and high recruitment rates (Armstrong 1989). Many ecosystems possess multiple opportunistic species and although many are relatively weak competitors (Paine 1988), asymmetric competition among opportunistic species could have a strong bearing on community dynamics (Platt and Weis 1985). In highly disturbed systems, released space brings the opportunity for a number of species to rapidly grow and dominate (Platt and Weis 1985; Suding and Goldberg 2001), potentially leading to alternative stable states reinforced by feedback mechanisms (Mumby et al. 2007). Given the complexity of competitive interactions in natural systems, predicting the trajectory of these systems following perturbation remains an exciting challenge in ecology (Petraitis and Hoffman 2010).
Coral reefs are disturbance-driven ecosystems (Rogers 1993) and early colonizers include fleshy macrophytes, such as Lobophora variegata and Dictyota spp. (Mumby 2006), and sponges (Rützler 2002). Indeed, phase shifts of community structure following acute disturbance have been described from coral dominance to either macroalgae (Mumby et al. 2007) or other space occupiers, including sponges (González-Rivero et al. 2011; Norström et al. 2009). This raises the question, what is the role of asymmetric competition, primarily between macroalgae and sponges, in determining community dynamics after acute events of coral mortality? Given the rapid colonization potential of macroalgae following perturbation (Mumby et al. 2005), and their capacity to overgrow excavating sponges (González-Rivero et al. 2012; López-Victoria et al. 2006), we hypothesize that macroalgae should largely prevent the outbreak of opportunistic sponge species, in particular the excavating sponge Cliona tenuis (Zea and Weil 2003). Among other benthic components (e.g. soft corals, corallimorpharians, ascidians), bioeroding sponges were chosen as a model group, primarily because of: (1) their predicted increase after coral decline (Schönberg and Ortiz 2009), (2) their opportunistic nature (Rützler 2002) and competitive strength (López-Victoria et al. 2006), (3) their capacity to promote alternative stable states (González-Rivero et al. 2011), and (4) their significant negative impact on reef erosion (Scoffin et al. 1980).
The specific context of our study is that acute coral mortality, driven by disturbances such as hurricanes (Rogers 1993), coral bleaching (Mumby 1999) and sedimentation (Rogers 1990) provides available substrate for excavating sponges, primarily of the family Clionaidae. Few studies have documented an increase in the abundance of clionaids over large temporal scales (Rützler 2002; Schönberg and Ortiz 2009; Ward-Paige et al. 2005), and the biological controls of these populations, including the role of competition, remain enigmatic. A key limitation is that few data (if any) are available on demographic rates of clionaids, so the relative importance of competition, mortality (Hixon et al. 2002), recruitment limitation (Roughgarden et al. 1985) and partial tissue mortality (Bak et al. 2005) are unknown, even though they are often key drivers structuring marine sessile organisms. In contrast, demographic processes of macroalgae have been relatively well studied (Mumby 2006; van Steveninck et al. 1988; Williams et al. 2001).
Here, we combine field observations with an ecological model to explain a decade-long pattern of C. tenuis population dynamics, following a major disturbance to a Caribbean coral reef. In doing so, we take an inferential approach to understanding why the populations of bioeroding sponges, which are expected to outbreak following coral mortality, have remained at steady low levels despite a substantial decline in coral cover at Glover’s Atoll, Belize. The study provides a mechanistic understanding of system behaviour following perturbation, in particular consideration of structuring processes such as asymmetric competition in a complex multi-species and spatially explicit context.
Materials and methods
Study species
Cliona tenuis is a common photosymbiotic bioeroding sponge on Caribbean reefs that belongs to the highly competitive Cliona viridis species complex (Zea and Weil 2003). Photosymbiotic clionaids are fast-growing species whose capacity to outcompete corals may be due to their ability to undermine polyps (López-Victoria et al. 2006). While C. tenuis is one of the fastest growing clionaid in the Caribbean (López-Victoria et al. 2006), competition can reduce its lateral expansion rate in a species-specific fashion, and macroalgal species such as Lobophora variegata often overgrow the sponge (González-Rivero et al. 2012). Reproduction occurs once a year and clionaid larvae show weak swimming abilities, tending to crawl over the substrate, which supports the closed stock-recruitment relationship observed in temperate photosymbiotic species of the same complex (González-Rivero et al. 2013; Mariani et al. 2006).
The depth of tissue penetration into the reef substrate for species of the C. viridis complex is mostly limited to 1–2 cm (Rützler 1974). For modelling purposes, however, we considered individuals as bi-dimensional structures, whose growth is chiefly defined by their lateral expansion. Predation does not appear to impact the somatic growth of adult sponges, though incidental predation may influence the number of juveniles in the population (González-Rivero et al. 2012). This group of clionaid species has a high tolerance against typical disturbances on coral reefs such as thermally induced bleaching (Schönberg et al. 2008; Vicente 1990), sedimentation (Carballo 2006; Schönberg 2015) and hurricanes (López-Victoria and Zea 2004). Their superior competitiveness for space coupled with their strong resistance to environmental stress has been used to explain observed increases in the abundance (cover and density) on reef systems that experience modern regimes of disturbance (López-Victoria and Zea 2004; Rützler 2002; Schönberg and Ortiz 2009; Ward-Paige et al. 2005).
Study area
Field data were collected at Glover’s Reef Atoll, Belize. Surveys were conducted within the boundary of the Glover’s Atoll Marine Reserve, on shallow (8 to 10-m) fore-reefs dominated by Orbicella spp. corals. Orbicella-dominated habitat (formerly Montastraea reef) is one of the most important habitats of Caribbean reefs. Previous studies across the region have found that these habitats consistently exhibit the highest abundance and richness of sessile invertebrates and fish (Mumby et al. 2008). Corals are the foundation species in tropical reefs (Wild et al. 2011) but the gradual loss of corals on many Caribbean reefs (Gardner et al. 2003), and the study site in particular (Mumby et al. 2005), means that macroalgae have become dominant competitors for space. Sponges are another key component of Caribbean reefs; their abundance and biotic interactions with their surrounding environment highlight their relevance among benthic groups (Díaz and Rützler 2001).
In recent decades, coral reefs at Glover’s Atoll have been impacted by a number of hurricanes and bleaching events (Mumby et al. 2005). Consequently, structural changes to the benthic community have been observed on the fore-reefs (Mumby et al. 2005; Roff et al. 2015). Model parameterisation and validation were performed with in situ data collected by independent field surveys both in time and space. Data for model parameterisation were collected from repetitive surveys on tagged individuals from the windward side at Glover’s Atoll during 2009, while model validation was conducted using data from 1998 to 2007 from three locations (Table 1; Online Resource 3).
The ecosystem model was parameterised with four functional groups of corals representative of the dominant composition of Caribbean coral assemblages. These functional groups disaggregate corals based on their reproductive mode, growth rate and susceptibility to disturbance (Darling et al. 2012). The model also includes three groups of algae: (a) cropped turf algae, a functional group of algae that undertake weak competitive interactions and facilitate the growth and recruitment of other benthic species (Box and Mumby 2007; González-Rivero et al. 2012; Mumby 1999); (b) seasonally constrained macroalgae, a group of upright fleshy algae represented by the genus Dictyota that exhibit seasonal mortality in winter (Ferrari et al. 2012b; Renken et al. 2010) and dominate the macroalgal composition at Glover’s Atoll (Online Resource 1); and (c) Lobophora variegata, a common competitor with corals and sponges (Box and Mumby 2007; González-Rivero et al. 2012), and the second most dominant species at Glover’s Atoll (Online Resource 1). Both groups of macroalgae have been reported to be major competitors of corals and sponges (Chadwick and Morrow 2011; González-Rivero et al. 2012). Lastly, the model incorporates the dominant bioeroding sponge C. tenuis. An earlier version of this model (without sponges) has been shown to emulate coral reef dynamics faithfully when compared to long-term and independent field data (Mumby et al. 2007).
Population modelling of C. tenuis
Overview
The spatial simulation model by Mumby et al. (2007) was extended to include bioeroding sponges and to simulate their population dynamics. Parameterization for sponge demographic rates and initial population structure was undertaken using field data gathered on the windward side of Glover’s Atoll in 2009 (Table 1). Model outputs (population size-structure of C. tenuis) were validated against independent field observations covering the simulated period (1998–2007). The relative importance of processes driving sponge population structure was explored using a perturbation analysis (inclusion/exclusion), whereby the simulated sponge size structure was compared to field observations. Below, we: (1) give an overview of the simulation model, (2) explain the candidate drivers of C. tenuis populations included in the model, and (3) describe the procedures used to test the model performance and compare the relative roles of the putative drivers of C. tenuis population dynamics.
Ecosystem model
We modelled the dynamics of the main benthic components occurring over 6-month time steps as a result of recruitment, growth, mortality and competitive rates in each component. The model is structured in a square lattice of 400 cells, each of which represents 0.25 m2 of reef and can be occupied by a combination of living organisms and dead substrata. Individual cells in the model comprise multiple coral colonies, algal patches and C. tenuis individuals, so that ecological interactions occur at individual scales as they do in situ. Grazing affects all algal classes and grazed patches become algal turf. Corals and sponges are subject to size-dependent fecundity and mortality. Competition between corals, sponges and macroalgae assumes physical contact among species within a cell, and results in the loss of colonised area to the benefit of the winner(s). The size-asymmetric nature of competitive interactions among macroalgae, corals, and/or sponges arises as an emergent property of the model, and it essentially depends on the parameterisation of individual species traits and behaviours (Online Resource 5, Table 5). Competition coefficients quantify the area that each inferior competitor loses due to overgrowth from a superior competitor every 6 months. Such coefficients were determined from experimental and field observations on tagged sponge individuals over time (see Online Information 3 for detailed calculations). Acute disturbance occurs from hurricanes, and coral bleaching is considered in the background whole-colony mortality. Percent cover and size structure of corals and sponges, and percent cover of macroalgae gave the initial conditions of each simulation in 1998. Each parameter varies stochastically at each time step, given a probability function. Each model simulation was run for 11 years, and 50 iterations were used to determine the average outputs for every population (Online Resource 5).
Field observations
The population structure of C. tenuis and the abundance of other benthic components were evaluated over 11 years at Glover’s Atoll (during 1998, 2003, 2007 and 2009). Data were gathered using videos analysis of belt-transects (10 m2) and random quadrats (1 m2) at two sites on the windward side of the atoll, and one site on the leeward side of the atoll (Online Resource 3). Footage were analysed using the software VidAna (http://www.marinespatialecologylab.org) to calculate the size and number of C. tenuis individuals within each sampling unit.
Population metrics of C. tenuis, such as size frequency distribution, kurtosis, skewness, geometric mean size, percent cover and population size, were evaluated at the four time periods. We divided the sponge population into seven size classes: I = 0–100 cm2, II = 100–300 cm2, III = 300–500 cm2, IV = 500–700 cm2, V = 700–900 cm2, VI = 900–1100 cm2, VII > 1100 cm2. Changes over time in the mean size of individuals (geometric mean) for each site were evaluated using a mixed effects regression model, including site as a random effect to account for repeated measurements, and temporal autocorrelation was modelled using the corARMA function (Pinheiro and Bates 2000).
Testing the model performance
The overall performance of the model was visually inspected against the in situ patterns of coral, sponge and macroalgal cover from 1998 to 2009 (section above). To test how well the model captured sponge population dynamics, we compared the simulated and observed size-frequency classes for the end year (2009). Here we used two metrics of goodness-of-fit to evaluate the performance of the model in describing the population structure of C. tenuis: residual sum of squares (RSS) and model likelihood (LL) estimated between the modelled and observed size structure. RSS measures the discrepancy between the observed and predicted data, so that the smaller the RSS the better the fit, based on a least squares approach. Equation 1 gives RSS where \(y_{i}\) is the observed abundance for the ith size class out of a total of k (8), and \(\hat{y}_{i}\) is the predicted values for the same category
On the other hand, the LL can be defined as the probability of the observed data given a model where a maximum LL estimate indicates a better fit of the model to the data. For discrete model outcomes (number of individuals) for multiple size categories, the probability of a given category (size class) from the model can be used to denote the LL (Haddon 2001). Therefore, we can define the sum of the LLs by using a multinomial probability function, where p is the probability for each ith possible model outcome, x i is the number of times an event of type i occurred (field data) in n number of observations (size categories).
Evaluating the role of regulatory mechanisms
We hypothesized that four mechanisms could drive the population structure of C. tenuis: (a) stock-recruitment dynamics of a closed population, (b) whole-individual mortality, (c) partial tissue mortality, and (d) interspecific macroalgal competition, as summarized in Table 1. The first two mechanisms can be considered as intrinsic processes related to the life history of the sponge, while competition acts as an extrinsic factor. For partial mortality, extrinsic factors such as disease and predation cause mortality of sections of the tissue while the individual remains alive (González-Rivero et al. 2012, 2013). However, partial tissue mortality also involves intrinsic processes, associated with the life history of the species, such as senescence. Therefore, for comparative analysis, partial tissue mortality can be considered as both an intrinsic and extrinsic parameter. Model implementation of the candidate mechanisms of sponge population regulation is briefly described below (and Table 1), and detailed in Online Resource 4.
Competition
The effect of different competitors (e.g. corals and algae species) on the somatic growth of C. tenuis was modelled by dividing the perimeter of the sponge into sections for each competitor, and calculating the resulting linear growth rate of the sponge for each section (Table 1). The proportion of the perimeter in contact with a competitor is given by the local abundance of each competitor (five-cell mean cover). Assuming a radial expansion of C. tenuis, the final size of the sponge after every time step (6 months) thus results from the sum of the linear somatic growth of the individual sections in contact with each competitor (Table 1).
The linear extension of C. tenuis sections in contact with cropped algae (i.e. turf) varies as a function of the developing state of this algal community, indicating that the competitive strength of turf increases as it gets thicker and taller (González-Rivero et al. 2012). Here we estimate growth of the sponge in competition with the average cropped algal state at Glover’s Atoll (tall turf algae, based on field observations). Competition among sponges is also considered, where the larger individual overtops the smaller one, thereby reducing the underlying sponge size by the overtopping area of the winner (Online Resource 5).
Stock-recruitment dynamics
Modelled populations of C. tenuis are assumed to be sustained by stock-recruitment dynamics determined by the number and fecundity of adult sponges. A positive and linear fecundity-size relationship is assumed, whereby the number of propagules and larvae produced will proportionately increase with the individual size of the sponge.
Whole-individual mortality
The mortality of C. tenuis was modelled as a negative power function of sponge size (Peterson and Wroblewski 1984):
where the parameters were estimated by the non-linear least square regression fit to data from tagged sponges monitored during 2009 (Online Resource 4).
Partial mortality
To calculate the per capita probability of occurrence and intensity of partial mortality we repeatedly surveyed C. tenuis individuals that were not subjected to macroalgal or coral competition throughout the year in 2009. These sponges had 95.6 ± 0.2 % [mean ± confidence interval (CI)0.95, n = 27] of the perimeter in contact with short turf, therefore minimizing any possible confounding effect of competition in the estimates of partial mortality. The probability of partial mortality was then calculated as a proportion of shrinking sponges versus those that did not change in size.
To explore the relative importance of these four mechanisms in regulating the population dynamics of C. tenuis, we compared the simulated and observed population size structure that was observed at the end of the simulation period (2009) when each candidate mechanism was incorporated into the model. A null model was used as a baseline (i.e. null hypothesis) that included only sponge growth rates (1.65 cm year−1; linear extension over short turf) and a constant recruitment rate (2.5 individuals year−1 m−2). This approach resembles a hypothesis testing design, whereby a ‘null model’ is used to simulate the scenario where none of the regulatory mechanisms were incorporated. We then used an orthogonal design that iteratively included each mechanism independently as well as their interactions. For simplicity of representation, we show here only four simulation scenarios: (a) null model, (b) intrinsic mechanisms of regulation, (c) macroalgal competition as an extrinsic mechanism, and (d) the interaction between both mechanisms. Note that intrinsic mechanisms refer here to whole-individual mortality, stock recruitment, and partial mortality, even when, as explained before, partial mortality can also be driven by external factors. The design of simulations is fully detailed in Online Resource 6.
Models for each scenario were compared using two-model selection criteria, based on model LLs: (1) Bayesian information criterion (BIC), and (2) adjusted amount of deviance (D) accounted for by a model (\(\bar{D}^{2}\)). BIC tends to favour more parsimonious models, and is therefore ideal for descriptive models, based on an assessment of relative importance. Given the LL estimate of a model, the number of parameters added to the model (x) and the number of observations (n), BIC can then be calculated as (Wit et al. 2012):
Deviance (D) is a quality-of-fit statistic often used for statistical hypothesis testing, when compared against a saturated model. Here, the model with the lowest RSS is used as the saturated model, which includes all four explanatory variables. The D is then used to compare each model scenario against the fully saturated model. Therefore, given the LL estimates for a model m (LL m ) and for the saturated model (LL s ), D of a model m is defined as (Nelder and Wedderburn 1972):
Like the coefficient of determination, the amount of D accounted by a model can be calculated as a direct comparison among different models, as a metric of variance explained, or D reduction (\(\bar{D}^{2}\)). Given the null D, defined as the D without the proposed regulation mechanisms (D null), and the residual D, as the D that remains unexplained after the variables have been included (D m), \(\bar{D}^{2}\) can be defined as:
Results
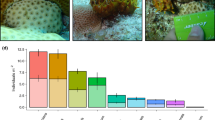
Throughout the observations at Glover’s Atoll over 11 years, live coral cover exhibited a reduction of 52 ± 33 % of the original cover in 1998 (mean ± 95 % CI). Macroalgae steadily dominated the benthic community over time, averaging 36 ± 7 % (mean ± 95 % CI across sites and time) and mainly comprised two species: Dictyota spp and Lobophora variegata (Fig. 1a, c). In contrast, sponges only represented an average of 1.7 ± 0.6 % (mean ± 95 %-CI across sites and time), where C. tenuis dominated the composition of sponges and other clionaids (averaging 1.1 ± 0.6 %; Online Resource 1). Macroalgae comprised the main competitors, averaging a percentage contact with the sponge of 37 ± 8.4 %. Although some variability in algal composition was observed between the two observation periods, Dictyota pulchella and L. variegata represented 78 % of the total macroalgal abundance (Fig. 1c) and interacted, on average, with 27 % of the perimeter of each individual sponge at Glover’s Atoll (Fig. 1d).
Spatial patterns of benthic composition and intensity of competition with Cliona tenuis at Glover’s Atoll, Belize. Relative abundance of a major functional groups and c macroalgal groups, derived from photo-quadrat surveys in 2007, is presented for each surveyed location (n = 93). Proportion of the sponge perimeter in contact with each b functional group, and d macroalgal species, is shown as the relative importance of each competitor. Data derived from field measurements of the sponge perimeter in contact with each competitor, on tagged individuals, over 286 days in 2009. Macroalgae species are categorised into major groups (e.g. Chlorophyta, Phaenophyta and Rodophyta) with the exception of Lobophora variegata, Dictyota spp. and Halimeda spp., being the most dominant species (c). A higher taxonomic resolution of each competing algal group is detailed for two seasons: start of the dry season (late January), and end of the wet season (early December). Dpul Dictyota pulchella, Lvar Lobophora variegata, Amat algal mat, Hsp Hypnea spp., Jadh Jania adherens, Shys Sargassum hystrix, Cyan cyanobacteria, F. rhodo filamentous Rhodophyta. See Online Resources 1–4 for details
The population of C. tenuis maintained its size structure and density over time. Observed size distribution of sponges followed a lognormal distribution, being strongly skewed to the right and typically leptokurtic—described by a low variation around the mean and a high ‘peakedness’ (Online Resource 2). On average, the population was represented by individuals smaller than 100 cm2, with a geometric mean of 14.3 ± 2.6 cm2 (mean ± SE), which did not show statistical differences among observation periods (mixed-effect regression model, t-value = 0.968, df = 8, p = 0.3614).
The model successfully simulated the dynamics of the dominant benthic taxa (corals, algae and sponges) over the 11-year period (Fig. 2). Despite a decrease in coral cover (Fig. 2a) and a consistent dominance of macroalgae (Fig. 2b), the abundance of C. tenuis did not vary either in cover (Fig. 2c) or density (Fig. 2d). The model successfully captured this static behaviour and reproduced the size-frequency distribution observed on C. tenuis populations in 2009 (Fig. 2f).
Model inspection when contrasted against the observed trend of the coral reefs at Glover’s Atoll over the past 11 years. Left panels show temporal changes in the cover of a corals, b macroalgae and c sponges (C. tenuis). The density of sponge individuals is depicted in d, as well as the size distribution of the population in 2009 in e; 95 % confidence intervals, among sites and years (n = 10–40, Table S2, Online Resource 2), are shown in black, while the modelled attributes are shown in light grey. Note that for e grey bars represent observed values, indicating the mean (horizontal bar) and range of values (maximum and minimum) as depicted by the length of the bar
To compare the relative importance of each of the four candidate mechanisms in controlling the observed population structure of C. tenuis, we iteratively included each mechanism in the model and compared the results against those of a null model (Fig. 3; see Online Resource 6 for full model comparisons). When all the hypothesized regulating processes were negligible (null hypothesis model), the population size structure rapidly shifted to a left-skewed distribution, where large individuals represented the bulk of the population and pre-empted space (Fig. 3a). Including mortality, partial mortality and stock-recruitment dynamics in the model increased the model goodness-of-fit, represented by an increase in the BIC and reduction of the RSS, so that 43.9 % of the D was explained (\(\bar{D}^{2} = 0.439\); Fig. 3b). However, if only algal competition was considered, the LL increased (and RSS decreased), while accounting for 98.5 % of the D (Fig. 3c). After including competition, model simulations offered a better fit to the observed size structure, with a strong positive increase in the BIC by about six units. When including all four putative mechanisms of population control in the model, the simulated population structure was constrained to small sizes, providing the best fit (Fig. 3d). However, while the inclusion of all mechanisms into the model largely increased the fit (RSS = 0.013, and LL = −129.45), the BIC only increased by three units, compared to the last model (competition model). Overall, constraints on the somatic growth of the sponge, imposed by macroalgal competition, were the most relevant mechanisms determining the observed size structure and abundance of C. tenuis at Glover’s Atoll, Belize.
The relative role of each candidate driver of the size structure of C. tenuis, showing simulated and observed results. Horizontal grey lines indicate the mean observed number of individuals per size class, while grey bars represent the variability between study sites at Glover’s Atoll in 2009 (n = 20). The simulated number of individuals per size class is depicted by black dots, where the SD among simulations is shown in the error bars. Also included are the likelihood estimate (LL), residual sum of squares (RSS), Bayesian information criterion (BIC) and the adjusted amount of deviance accounted by the model (D 2) for the following modelling scenarios: a null hypothesis, when none of the hypothesised drivers are modelled; b adding only intrinsic mechanisms of population regulation—stock recruitment, mortality and partial mortality; c adding macroalgal competition only; and d including the interaction between intrinsic mechanisms and competition
Discussion
This study captured the benthic community dynamics of a common reef habitat found in the Caribbean region, dominated by corals of the genus Orbicella (Mumby et al. 2008), where macroalgae and bioeroding sponges are also common and functionally relevant species (González-Rivero et al. 2011; Perry et al. 2014). Our results emphasize the complexity of competitive outcomes that emerge when a third competitor, other than corals and algae, is included in the system. This highlights the importance of asymmetrical competition—represented here by disparities in size, competitive strength and colonization rate—as a regulatory process of excavating sponge populations after coral mortality; competition with macroalgae prevented sponges from taking advantage of greater colonisable space.
Between 1998 and 2001, coral populations in Belize were heavily impacted by hurricanes, and then by coral bleaching in 2005 (Mumby et al. 2005), freeing up considerable space for alternative taxa. Despite the increase of substrate suitable for clionaid sponge colonisation in the form of dead coral skeleton, C. tenuis experienced no net increase in abundance and its size structure remained unchanged over an 11-year period. Models that only integrated recruitment, growth and mortality rates of C. tenuis could not explain this observation. The lack of expansion by C. tenuis was accurately reproduced once competition with macroalgae was added to the model, which strongly suggests that asymmetric competition with macroalgae (particularly L. variegata) is responsible for the control of sponge populations after coral mortality events. These results confirm the superior ability of macroalgae to outcompete sessile reef organisms under reduced herbivory, as already shown with corals (Hughes 1994; Knowlton and Jackson 2008; Mumby et al. 2007).
C. tenuis exhibited a lognormal size distribution, which is common in many taxa (Kirchner et al. 1980). For sessile organisms, a lognormal size-frequency distribution can be explained by extrinsic processes (e.g. competition) that constrain the transition of juvenile individuals to larger size classes (Muko et al. 2001). Alternatively, such size distribution can be attributed to partial tissue mortality, increasing with body size, thus limiting the abundance of large individuals (Bak et al. 2005; Hughes and Jackson 1985). In the case of C. tenuis, we have shown that both extrinsic and intrinsic mechanisms limit the growth of the sponge and can explain the stable but skewed size-frequency distribution of sponges over time. Although extrinsic processes such as predation and disease-induced mortality (integrated here within partial mortality) have the potential to influence the population dynamics of sponges (Hughes and Connell 1987; Muko et al. 2001), macroalgal competition largely explained the observed patterns in the sponge populations.
The combination of rapid growth, perhaps high recruitment rates, and tolerance to disturbance is thought to support opportunistic population growth of Cliona spp. (Rützler 2002; Schönberg and Ortiz 2009; Ward-Paige et al. 2005). Our simulations can confirm this; the stabilizing effect of macroalgal competition on sponge populations found in our study is proportional to algal cover. High percentage cover of macroalgae at Glover’s Atoll, perhaps attributable to low herbivore pressure (Mumby et al. 2011), can explain the observed trends and population size structure. Low levels of macroalgal cover, on the other hand, allowed a rapid positive response of sponges to large size classes in the model, which is consistent with several field observations after coral reef disturbance in the Caribbean (Lopez-Victoria and Zea 2005; Rützler 2002; Williams et al. 1999). Similar patterns have been reported in the Mediterranean and the Great Barrier Reef where grazers limited the growth of algae thus facilitating the expansion of other Cliona viridis species (Cebrian 2010; Cebrian and Uriz 2006; Sammarco et al. 1987). Such transitions have also occurred elsewhere in Belize following bleaching-induced coral mortality where herbivory from sea urchins prevented a strong macroalgal response but failed to prevent a shift in dominance from corals to the encrusting sponge Chondrilla nucula (Aronson et al. 2002). This suggests that altering the competitive network of interactions may strongly affect community dynamics.
By hindering the rapid positive response of bioeroding sponges, the stabilizing effect of macroalgae, under current low grazing pressure (Mumby et al. 2011), can influence the contribution of these opportunistic sponges to the overall system dynamics. Although macroalgal dominance has proven to have a profound and deleterious effect on coral reef systems (Mumby et al. 2013; Steneck et al. 2014), macroalgae-sponge interactions may down-regulate the capacity of bioeroding sponges to rapidly displace corals by competition (Lopez-Legentil and Pawlik 2009; López-Victoria et al. 2006) and consequently increase the net erosion of the reef carbonate matrix (Perry et al. 2014). Failing to regulate bioeroding sponge populations, on the other hand, may promote a rapid positive increase in sponge population size (null model), enhancing reef erosion (Kennedy et al. 2013) and contributing to alternative stable states (González-Rivero et al. 2011).
While this study focuses on the dynamics of C. tenuis, other sponges may respond differently to coral reef decline (Pawlik et al. 2013), including sponges dominating cryptic habitats (de Goeij et al. 2013; Pawlik et al. 2013), which are underrepresented in this study. Looking forward, further studies will be needed to evaluate the role of structuring drivers on the overall dynamics of other abundant reef-dwelling groups, within a multi-species context.
The degree to which competitive outcomes between macroalgae and C. tenuis will vary geographically and temporally will depend, at least in part, on regional variations in interaction strength and the abundance of each taxon. While some competitive interactions appear to be consistent across the Caribbean, such as the superior competitive ability of Lobophora over some weedy corals (Mumby and Harborne 2010; Nugues and Bak 2006), our knowledge of algal-sponge interactions remains limited. Moreover, a recent study reveals that the outcome of inter-taxon competition can be strikingly different among major coral reef biogeographic provinces (Mumby et al. 2015). Whether this extends to sponge-algal interactions remains to be seen. It is worth noting that both the habitat type and benthic species included in this study have a pan-regional distribution. Thus, we would expect our results to have a bearing on community dynamics throughout the Caribbean. It would be useful to study how interaction strength varies in a context-dependent fashion, where the sign or magnitude of the effect on fitness changes as a function of the biotic or abiotic context in which the interaction occurs (Chamberlain et al. 2014). This includes biotic and abiotic drivers such as herbivory (Burkepile and Hay 2006), wave energy (Chollett and Mumby 2012), temperature and nutrients (Rützler 2002; Ward-Paige et al. 2005).
Considering a third species can influence the context dependency of competitive outcomes in marine systems (Chadwick and Morrow 2011), where intransitive interactions, for example, alter the competitive hierarchy in a system (Edwards and Schreiber 2010). An intriguing implication of a third competitor might arise if it becomes involved in reinforcing feedback mechanisms. For example, studies of alternative attractors of Caribbean reefs have focused on the reinforcing competitive feedbacks between corals and macroalgae (Mumby et al. 2007, 2013). One reinforcing mechanism that drives increased coral is the intensification of fish herbivory on non-coral substrates as coral cover increases (Williams et al. 2001), thereby accelerating the loss of macroalgae and reinforcing coral recovery. If the ‘third taxon’ were also avoided by herbivorous fish, and therefore served to intensify herbivory, then the coral attractor might strengthen. Alternatively, if the third taxon was consumed it would play a destabilising role in the resilience of coral and algae, and potentially lead to the emergence of unexpected system states (González-Rivero et al. 2011).
References
Armstrong RA (1989) Fugitive coexistence in sessile species: models with continuous recruitment and determinate growth. Ecology 70:674–680
Arnold SN, Steneck R, Mumby PJ (2010) Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser 414:91. doi:10.3354/meps08724
Aronson R, Precht W, Toscano M, Koltes K (2002) The 1998 bleaching event and its aftermath on a coral reef in Belize. Mar Biol 141:435–447
Bak RP, Nieuwland G, Meesters EH (2005) Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curacao and Bonaire. Coral Reefs 24:475–479. doi:10.1007/s00338-005-0009-1
Berger U, Piou C, Schiffers K, Grimm V (2008) Competition among plants: concepts, individual-based modelling approaches, and a proposal for a future research strategy. Perspect Plant Ecol Evolut Syst 9:121–135. doi:10.1016/j.ppees.2007.11.002
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149. doi:10.3354/meps342139
Burkepile DE, Hay ME (2006) Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology 87:3128–3139
Carballo JL (2006) Effect of natural sedimentation on the structure of tropical rocky sponge assemblages. Ecoscience 13:119–130. doi:10.2980/1195-6860(2006)13[119:eonsot]2.0.co;2
Cebrian E (2010) Grazing on coral reefs facilitates growth of the excavating sponge Cliona orientalis (Clionaidae, Hadromerida). Mar Ecol 31:533–538. doi:10.1111/j.1439-0485.2010.00401.x
Cebrian E, Uriz MJ (2006) Grazing on fleshy seaweeds by sea urchins facilitates sponge Cliona viridis growth. Mar Ecol Prog Ser 323:83–89. doi:10.3354/meps323083
Chadwick NE, Morrow KM (2011) Competition among sessile organisms on coral reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Amsterdam, pp 347–371
Chamberlain SA, Bronstein JL, Rudgers JA (2014) How context dependent are species interactions? Ecol Lett 17:881–890
Chollett I, Mumby PJ (2012) Predicting the distribution of Montastraea reefs using wave exposure. Coral Reefs 31:493–503. doi:10.1007/s00338-011-0867-7
Connell JH (1983) On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat 122:661–696. doi:10.1086/284165
Connell JH, Hughes TE, Wallace CC, Tanner JE, Harms KE, Kerr AM (2004) A long-term study of competition and diversity of corals. Ecol Monogr 74:179–210. doi:10.1890/02-4043
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378–1386. doi:10.1111/j.1461-0248.2012.01861.x
de Goeij JM et al (2013) Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–110
Díaz MC, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Doropoulos C, Roff G, Bozec Y-M, Zupan M, Werminghausen J, Mumby PJ (2015) Characterising the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol Monogr “Preprint”. doi: 10.1890/15-0668.1
Edwards KF, Schreiber SJ (2010) Preemption of space can lead to intransitive coexistence of competitors. Oikos 119:1201–1209. doi:10.1111/j.1600-0706.2009.18068.x
Ferrari R, Gonzalez-Rivero M, Mumby PJ (2012a) Size matters in competition between corals and macroalgae. Mar Ecol Prog Ser 467:77. doi:10.3354/meps09953
Ferrari R, Gonzalez-Rivero M, Ortiz JC, Mumby PJ (2012b) Interaction of herbivory and seasonality on the dynamics of Caribbean macroalgae. Coral Reefs 31:683–692. doi:10.1007/s00338-012-0889-9
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960. doi:10.1126/science.1086050
González-Rivero M, Yakob L, Mumby PJ (2011) The role of sponge competition on coral reef alternative steady states. Ecol Model 222:1847–1853. doi:10.1016/j.ecolmodel.2011.03.020
González-Rivero M, Ferrari R, Schönberg CHL, Mumby PJ (2012) Impacts of macroalgal competition and parrotfish predation on the growth of a common bioeroding sponge. Mar Ecol Prog Ser 444:133–142. doi:10.3354/meps09424
González-Rivero M, Ereskovsky AV, Schönberg CHL, Ferrari R, Fromont J, Mumby PJ (2013) Life-history traits of a common Caribbean coral-excavating sponge, Cliona tenuis (Porifera: Hadromerida). J Nat Hist 47:2815–2834. doi:10.1080/00222933.2013.802042
Haddon M (2001) Modelling and quantitative methods in fisheries, 2nd edn. CRC, Florida
Hastings A (1980) Disturbance, coexistence, history, and competition for space. Theor Popul Biol 18:363–373. doi:10.1016/0040-5809(80)90059-3
Hixon MA, Pacala SW, Sandin SA (2002) Population regulation: historical context and contemporary challenges of open vs. closed systems. Ecology 83:1490–1508. doi:10.1890/0012-9658
Horn HS, MacArthur RH (1972) Competition among fugitive species in a harlequin environment. Ecology 53:749–752. doi:10.2307/1934797
Hughes TP (1994) Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551. doi:10.1126/science.265.5178.1547
Hughes TP, Connell JH (1987) Population-dynamics based on size or age—a reef-coral analysis. Am Nat 129:818–829
Hughes TP, Jackson JBC (1985) Population dynamics and life histories of foliaceous corals. Ecol Monogr 55:141–166
Hutchinson GE (1951) Copepodology for the onithologist. Ecology 32:571–577. doi:10.2307/1931745
Karlson RH, Jackson JB (1981) Competitive networks and community structure: a simulation study. Ecology 62:670–678. doi:10.2307/1937735
Kennedy E et al (2013) Avoiding coral reef functional collapse requires local and global action. Curr Biol 23:912–918. doi:10.1016/j.cub.2013.04.020
Kirchner TB, Anderson RV, Ingham RE (1980) Natural selection and the distribution of nematode sizes. Ecology 61:232–237
Knowlton N, Jackson JB (2008) Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol 6:e54. doi:10.1371/journal.pbio.0060054
Lenssen JPM, van de Steeg HM, de Kroon H (2004) Does disturbance favour weak competitors? Mechanisms of changing plant abundance after flooding. J Veg Sci 15:305–314. doi:10.1658/1100-9233(2004)015
Lopez-Legentil S, Pawlik JR (2009) Genetic structure of the Caribbean giant barrel sponge Xestospongia muta using the I3-M11 partition of COI. Coral Reefs 28:157–165. doi:10.1007/s00338-008-0430-3
Lopez-Victoria M, Zea S (2005) Current trends of space occupation by encrusting excavating sponges on Colombian coral reefs. Mar Ecol 26:33–41. doi:10.1111/j.1439-0485.2005.00036.x
López-Victoria M, Zea S (2004) Storm-mediated coral colonization by an excavating Caribbean sponge. Clim Res 26:251–256. doi:10.3354/cr026251
López-Victoria M, Zea S, Weil E (2006) Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Mar Ecol Prog Ser 312:113–121. doi:10.3354/meps312113
Mariani S, Uriz M-J, Turon X, Alcoverro T (2006) Dispersal strategies in sponge larvae: integrating the life history of larvae and the hydrologic component. Oecologia 149:174–184. doi:10.1007/s00442-006-0429-9
Muko S, Sakai K, Iwasa Y (2001) Size distribution dynamics for a marine sessile organism with space-limitation in growth and recruitment: application to a coral population. J Anim Ecol 70:579–589. doi:10.1046/j.1365-2656.2001.00513.x
Mumby PJ (1999) Bleaching and hurricane disturbances to populations of coral recruits in Belize. Mar Ecol Prog Ser 190:27–35. doi:10.3354/meps190027
Mumby PJ (2006) The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl 16:747–769. doi:10.1890/1051-0761(2006)016[0747:tioegs]2.0.co;2
Mumby PJ, Harborne AR (2010) Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One 5:e8657. doi:10.1371/journal.pone.0008657
Mumby PJ, Foster N, Fahy E (2005) Patch dynamics of coral reef macroalgae under chronic and acute disturbance. Coral Reefs 24:681–692. doi:10.1007/s00338-005-0058-5
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101. doi:10.1038/nature06252
Mumby PJ et al (2008) Coral reef habitats as surrogates of species, ecological functions, and ecosystem services. Conserv Biol 22:941–951. doi:10.1111/j.1523-1739.2008.00933.x
Mumby PJ et al (2011) Fishing down a Caribbean food web relaxes trophic cascades. Mar Ecol Prog Ser 445:13–24
Mumby PJ, Steneck RS, Hastings A (2013) Evidence for and against the existence of alternate attractors on coral reefs. Oikos 122:481–491
Mumby PJ, Steneck RS, Adjeroud M, Arnold SN (2015) High resilience masks underlying sensitivity to algal phase shifts of Pacific coral reefs. Oikos. doi:10.1111/oik.02673
Nelder JA, Wedderburn RWM (1972) Generalized linear models. J R Stat Soc Ser A (General) 135:370–384. doi:10.2307/2344614
Norström AV, Nyström M, Lokrantz J, Folke C (2009) Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar Ecol Prog Ser 376:295–306. doi:10.3354/meps07815
Nugues MM, Bak RPM (2006) Differential competitive abilities between Caribbean coral species and a brown alga: a year of experiments and a long-term perspective. Mar Ecol Prog Ser 315:75–86. doi:10.3354/meps315075
Paine RT (1988) Habitat suitability and local population persistence of the sea palm Postelsia palmaeformis. Ecology 69:1787–1794. doi:10.2307/1941157
Paul V, Kuffner I, Walters L, Ritson-Williams R, Beach K, Becerro M (2011) Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar Ecol Prog Ser 426:161–170. doi:10.3354/meps09032
Pawlik JR, Loh T-L, McMurray SE, Finelli CM (2013) Sponge communities on Caribbean coral reefs are structured by factors that are top-down, not bottom-up. PLoS One 8:e62573
Perry CT et al (2014) Changing dynamics of Caribbean reef carbonate budgets: emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc R Soc B Biol Sci 281:20142018
Peterson I, Wroblewski JS (1984) Mortality rate of fishes in the pelagic ecosystem. Can J Fish Aquat Sci 41:1117–1120. doi:10.1139/f84-131
Petraitis PS, Hoffman C (2010) Multiple stable states and relationship between thresholds in processes and states. Mar Ecol Prog Ser 413:189–200. doi:10.3354/meps08691
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Platt WJ, Weis IM (1985) An experimental study of competition among fugitive prairie plants. Ecology 66:708–720. doi:10.2307/1940532
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not controlled by herbivores. PNAS 107:9683–9688. doi:10.1073/pnas.0912095107
Renken H, Mumby PJ, Matsikis I, Edwards HJ (2010) Effects of physical environmental conditions on the patch dynamics of Dictyota pulchella and Lobophora variegata on Caribbean coral reefs. Mar Ecol Prog Ser 403:63–74. doi:10.3354/meps08441
Roff G, Jx Zhao, Mumby PJ (2015) Decadal-scale rates of reef erosion following El Niño-related mass coral mortality. Glob Change Biol 21:4415–4424. doi:10.1111/gcb.13006
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Progr Ser 62:185–202. doi:10.3354/meps062185
Rogers CS (1993) Hurricanes and coral reefs: the intermediate disturbance hypothesis revisited. Coral Reefs 12:127–137. doi:10.1007/bf00334471
Roughgarden J, Iwasa Y, Baxter C (1985) Demographic theory for an open marine population with space-limited recruitment. Ecology 66:54–67. doi:10.2307/1941306
Rützler K (1974) The burrowing sponges of Bermuda. Smithson Contrib Zool 165:1–32. doi:10.5479/si.00810282.165
Rützler K (2002) Impact of crustose clionid sponges on Caribbean reef corals. Acta Geol Hisp 37:61–72
Sammarco PW, Risk MJ, Rose C (1987) Effects of grazing and damselfish territoriality on internal bioerosion of dead corals: indirect effects. J Exp Mar Biol Ecol 112:185–199
Sandin SA, McNamara DE (2012) Spatial dynamics of benthic competition on coral reefs. Oecologia 168:1079–1090. doi:10.1007/s00442-011-2156-0
Schoener TW (1983) Field experiments on interspecific competition. Am Nat 122:240–285. doi:10.1086/284133
Schönberg CHL (2015) Happy relationships between marine sponges and sediments—a review and some observations from Australia. J Mar Biol Assoc UK 1–22. doi: 10.1017/S0025315415001411
Schönberg CHL, Ortiz JC (2009) Is sponge bioerosion increasing? In: Proceedings of the 11th International Coral Reef Symposium, vol 1, Ft. Lauderdale, Florida, pp 527–530
Schönberg CHL, Suwa R, Hidaka M, Loh WKW (2008) Sponge and coral zooxanthellae in heat and light: preliminary results of photochemical efficiency monitored with pulse amplitude modulated fluorometry. Mar Ecol 29:1–12. doi:10.1111/j.1439-0485.2007.00216.x
Scoffin TP et al (1980) Calcium-carbonate budget of a fringing-reef on the west-coast of Barbados. 2. Erosion, sediments and internal structure. Bull Mar Sci 30:475–508
Steneck RS, Arnold SN, Mumby PJ (2014) Experiment mimics fishing on parrotfish: insights on coral reef recovery and alternative attractors. Mar Ecol Prog Ser 506:115–127
Suding KN, Goldberg D (2001) Do disturbances alter competitive hierarchies? Mechanisms of change following gap creation. Ecology 82:2133–2149. doi:10.1890/0012-9658(2001)082[2133:ddachm]2.0.co;2
van Steveninck EDD, Vanmulekom LL, Breeman AM (1988) Growth-inhibition of Lobophora variegata (Lamouroux) Womersley by scleractinian corals. J Exp Mar Biol Ecol 115:169–178. doi:10.1016/0022-0981(88)90101-3
Vicente VP (1990) Response of sponges with autotrophic endosymbionts during the coral-bleaching episode in Puerto Rico. Coral Reefs 8:199–202. doi:10.1007/bf00265011
Ward-Paige CA, Risk MJ, Sherwood OA, Jaap WC (2005) Clionid sponge surveys on the Florida Reef Tract suggest land-based nutrient inputs. Mar Pollut Bull 51:570–579. doi:10.1016/j.marpolbul.2005.04.006
Weiner J (1990) Asymmetric competition in plant populations. Trends Ecol Evol 5:360–364. doi:10.1016/0169-5347(90)90095-U
Wild C et al (2011) Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar Freshwater Res 62:205–215. doi:10.1071/mf10254
Williams EH, Bartels PJ, Bunkley-Williams L (1999) Predicted disappearance of coral-reef ramparts: a direct result of major ecological disturbances. Glob Change Biol 5:839–845
Williams ID, Polunin NVC, Hendrick VJ (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–196
Wit E, Evd Heuvel, Romeijn J-W (2012) ‘All models are wrong…’: an introduction to model uncertainty. Stat Neerl 66:217–236. doi:10.1111/j.1467-9574.2012.00530.x
Zea S, Weil E (2003) Taxonomy of the Caribbean excavating sponge species complex Cliona caribbaea—C. aprica—C. langae (Porifera, Hadromerida, Clionaidae). Caribb J Sci 39:348–370
Acknowledgments
The authors would like to thank the contribution of five anonymous reviewers and the handling editor, S. Sandin, to the final version of the manuscript. This study was funded by: (1) the Fondo Nacional de Ciencia, Tecnología e Innovación to MGR, (2) the Wildlife Conservation Society and Khaled bin Sultan Living Oceans Foundation to R. F., and (3) the Natural Environment Research Council and ARC Laureate Fellowship to P. J. M. We are grateful to the WCS Belize staff and volunteers for their generous field assistance, and to S. O’Farrell, A. Harborne, E. Johnston and C. Doropoulos for editorial comments.
Author contribution statement
M. G. R. and P. J. M. conceived and designed the field surveys and the ecosystem model. M. G. R., R. F. and P. J. M. performed the field surveys. M. G. R., Y. M. B., I. C. and P. J. M. designed and performed the data analysis. M. G. R., P. J. M., I. C. and Y. M. B. wrote the manuscript; other authors provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stuart Sandin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González-Rivero, M., Bozec, YM., Chollett, I. et al. Asymmetric competition prevents the outbreak of an opportunistic species after coral reef degradation. Oecologia 181, 161–173 (2016). https://doi.org/10.1007/s00442-015-3541-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3541-x