Abstract
While positive interactions have been observed to influence patterns of recruitment and succession in marine and terrestrial plant communities, the role of facilitation in macroalgal phase shifts is relatively unknown. In December 2012, typhoon Bopha caused catastrophic losses of corals on the eastern reefs of Palau. Within weeks of the typhoon, an ephemeral bloom of monospecific macroalgae (Liagora sp.) was observed, reaching a peak of 38.6 % cover in February 2013. At this peak, we observed a proliferation of a second macroalgal species, Lobophora variegata. Lobophora was distributed non-randomly, with higher abundances occurring within the shelter of Liagora canopies than on exposed substrates. Bite rates of two common herbivorous fish (Chlorurus sordidus and Ctenochaetus striatus) were significantly higher outside canopies (2.5- and sixfold, respectively), and cage exclusion resulted in a significant increase in Lobophora cover. Experimental removal of Liagora canopies resulted in a 53.1 % decline in the surface area of Lobophora after 12 days, compared to a 51.7 % increase within canopies. Collectively, these results indicate that Liagora canopies act as ecological facilitators, providing a ‘nursery’ exclusion zone from the impact of herbivorous fish, allowing for the establishment of understory Lobophora. While the ephemeral Liagora bloom had disappeared entirely 9 months post-typhoon, the facilitated shift to Lobophora has persisted for over 18 months, dominating ~40 % of the reef substrate. While acute disturbance events such as typhoons have been suggested as a mechanism to reverse algal phase shifts, our results suggest that typhoons may also trigger, rather than just reverse, phase shifts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Positive interactions play an important role in controlling large-scale landscape processes, shaping assemblages and regulating population dynamics in physically stressful habitats (Bertness and Callaway 1994; Bertness and Leonard 1997; Bruno et al. 2003). Positive interactions (or ‘facilitations’) are defined as ‘encounters between organisms that benefit at least one of the participants and cause harm to neither’ (Bruno et al. 2003; Stachowicz 2001), and occur when one organism renders the local environment more favourable for another either directly [e.g. reducing thermal stress via shading (Baumeister and Callaway 2006)] or indirectly [e.g. predator removal (Duffy 2003)]. Facilitation can play an important role across a range of ecological processes, influencing recruitment patterns, regulating species distributions, and facilitating succession in a broad range of communities (Atsatt and O’Dowd 1976; Bertness and Callaway 1994).
Plant communities have traditionally played an important role as a model system for exploring the role of positive interactions (e.g. Brooker et al. 2008; Callaway 1995). Positive interactions have been observed to drive patterns of recruitment in terrestrial plants, resulting in patterns of density-dependent recruitment survivorship (Callaway 1995). Such interactions may occur through habitat amelioration, such as overstory shading by conspecific tree canopies (e.g. Weltzin and McPherson 1999), and enhanced primary productivity (Callaway et al. 1991), or through provision of a nursery environment to protect against herbivory (Fuentes et al. 1986). Facilitation can also influence species distributions in marine ecosystems. For example, the abundance of a palatable species may be positively correlated with the abundance of another less palatable species in both macroalgae (Hay 1986; Pfister and Hay 1988) and sponges (Wulff 1997). Positive interactions are a driving factor in both primary and secondary succession in marine plant assemblages. For example, primary succession of the kelp Macrocystis pyrifera is facilitated by the removal of herbivorous urchins (Harris et al. 1984), and secondary succession of the seagrass Syringodium is facilitated by a rhizophytic algae that stabilises sandy substrates (Williams 1990).
Following major disturbance events on coral reefs (e.g. cyclones, coral bleaching), widespread coral mortality reduces coral cover, in turn opening up new substrate for recruitment (Connell et al. 1997). If levels of herbivory are sufficient, then increased colonisation of coralline algae can, in turn, promote coral recruitment (Edmunds and Carpenter 2001). If substrates are insufficiently grazed by herbivores, then macroalgae can establish and become dominant (e.g. Hughes et al. 2007), inhibiting coral recruitment (e.g. Dixon et al. 2014) and outcompeting smaller corals and other benthic organisms (e.g. Lobel and Ogden 1981). While facilitation can be important for the establishment of macroalgae in temperate systems (e.g. Harris et al. 1984; Pfister and Hay 1988; Wahl and Hay 1995), the emergence of macroalgae in coral reef ecosystems is usually studied as a direct effect of either nutrification and/or reduced herbivory (but see Littler et al. 1986). Here, we examine the role of positive interactions in facilitating phase shifts from coral- to algal-dominated states.
Following catastrophic damage to reefs of Palau (Micronesia) from super-typhoon Bopha in December 2012, we documented a near complete loss of coral cover, with monospecific blooms of the foliose macroalga Liagora sp. observed across the exposed eastern reefs in the weeks following the typhoon. Such a transition from one phase (coral dominated) to another (coral depleted and/or algal dominated) in response to acute disturbance represents a phase shift (sensu Done 1992). Following establishment, Liagora blooms are able to proliferate and persist because of effective anti-herbivory defences, including the presence of secondary metabolites that actively deter herbivorous fish (Paul and Fenical 1980; Wylie and Paul 1988), and incorporation of calcium carbonate directly into their thalli which may act as a chemical defence against acid-mediated digestion for grazing herbivores (Hay et al. 1994). Consistent with previous studies of Liagora blooms in the Caribbean region (e.g. Hughes 1994; Woodley et al. 1981), the post-disturbance bloom was ephemeral, lasting ~6 months. At the peak of the bloom, we observed the proliferation of a second macroalgal species, Lobophora variegata, which was first observed in an encrusting growth form underneath the Liagora canopy. In contrast to the ephemeral foliose rhodophyte Liagora, Lobophora represents an entirely different functional group of algae (Steneck and Dethier 1994), being a perennial phaeophyte with a morphology that is less susceptible to most herbivores (Coen and Tanner 1989). Critically, Lobophora did not exhibit any such colonisation at sites that lacked the Liagora bloom. Such a significant expansion of Lobophora across forereef habitats in Palau is unprecedented in at least the past decade (Y. Golbuu, P. J Mumby, personal observation).
To determine the roles of positive and negative interactions in successional processes, we first tracked the temporal dynamics of the macroalgal assemblages in the months following typhoon Bopha. Secondly, we quantified recruitment processes of Lobophora by determining patterns of abundance inside and outside Liagora canopies. Third, to determine the potential for Liagora canopies to act as refugia, we quantified spatial patterns of grazing by two key herbivores (Ctenochaetus striatus and Chlorurus sordidus) inside and outside of canopies. Fourth, to test the effectiveness of herbivores in removing Lobophora, we experimentally removed Liagora canopies. Finally, to explore the role of herbivory in influencing Lobophora cover, we conducted caging experiments to exclude the effects of large herbivorous fish. Collectively, our results show that Liagora canopies provide an important ephemeral refuge to Lobophora recruits from herbivory, and indicate that such positive interactions can be critical in facilitating the establishment of a macroalgal phase shift in ecosystems characterised by high herbivory.
Materials and methods
Temporal trends in benthic cover
This study was conducted at three sites on the eastern side of Palau that experienced a pronounced Liagora bloom (Lighthouse North, Ngederrak South and Ngederrak North; Fig. 1). Surveys were conducted prior to typhoon Bopha in March 2012, and again following the typhoon in February, April, September and October 2013, and again in April and October 2014. Additional surveys (March 2012, February 2013, April 2014) were conducted at adjacent sites that showed no Liagora or Lobophora blooms (Table S1). At each site, three line-intercept transects (30-m length, n = 3) were conducted at 4–6-m depth parallel to the reef slope at each time period to quantify benthic cover. Line-intercept transects were haphazardly placed on hard substrata, separated by approximately 20-m distance, and placed in a similar location at each time point. Live coral (scleractinian coral), coralline algae, turf algae, encrusting and fleshy macroalgae, cyanobacteria, other invertebrates and non-living substrate (rubble, carbonate and sand) were identified to the lowest possible taxonomic resolution every centimetre along each transect. Percent cover for dominant groups was calculated as a mean from the three replicate transects.
a Map of Palau archipelago (dark blue reef crest, blue reef flat, pale blue lagoon, white open ocean), with inset world map with location of Palau in the western Pacific and b main study sites with Liagora blooms marked with red circles (Lighthouse North, Ngedderak South and Ngedderak North) and adjacent study sites with no evidence of Liagora blooms marked with green triangles (Beluu Lukes, East Sheltered, Short Drop Off) (color figure online)
Habitat availability beneath Liagora canopies and distribution of Lobophora on the reef
Individual Liagora were observed to sway with water motion from a single holdfast, creating a ‘swept’ zone underneath the canopy. As such, two possible substrate types were available for colonisation by Lobophora: areas free of Liagora and not swept by canopies (exposed substrate) and area that lay within the swept radius of Liagora canopies (swept substrate). To test whether the distribution of Lobophora was non-random and significantly associated with swept substrates, we quantified: (1) the availability of exposed vs. swept substrates, and (2) the overall distribution of Lobophora across the reef including both categories of substrate.
To quantify the area of exposed vs. swept substrates, point-intercept transects (30-m length) were conducted at all sites in April 2013. At 20-cm intervals along each transect (total number of points per transect = 150), we quantified whether or not the transect tape was swept by Liagora canopies, and the proportion of each transect that was inside or outside of Liagora canopies was determined. To quantify the distribution of Lobophora among swept and exposed substrates, we selected 100 individual Lobophora by swimming a random number of fin kicks in a frequently changing direction at each site and quantified: (1) maximum diameter, (2) distance to the nearest Liagora holdfast, and (3) whether the Lobophora was swept by the Liagora canopy for each individual Lobophora. From this, the proportion of Lobophora inside or outside of Liagora canopies was determined. A χ 2-test was used to determine whether the distribution of Lobophora was non-random and significantly associated with swept substrates at each site, where observed was the proportion of Lobophora inside and outside of Liagora canopies, and expected was the proportion of transect inside and outside of Liagora canopies. To test further for differences in the size structure of individuals of Lobophora between swept and exposed substrates, we used a Kolmogorov–Smirnov test in the stats package [R software (R Core Team 2014)].
Patterns of herbivore grazing on Liagora canopies
To quantify the capacity of Liagora canopies to act as a refuge from grazing herbivores, we collected observational data on fish grazing behaviour at Lighthouse North for two common and abundant herbivores: Ctenochaetus striatus (n = 31) and Chlorurus sordidus (n = 27). Individuals were observed for 5-min intervals, during which we recorded a visual estimate of total body length, and the number of bites inside and outside of Liagora canopies. To determine the feeding preference of the two herbivore species inside and outside of canopies, [(E i = r i − n i /r i + n i ), where r i is the proportion of all bites that were taken on the ith substrate (i.e. inside and outside of canopies), and ni is the proportional abundance of substrate (Ivlev 1961)]. Differences in bite rates (bites per min) and E i inside and outside of Liagora canopies were compared using t-tests in the stats package [R software (R Core Team 2014)].
Experimental manipulation of Liagora canopy and herbivory on Lobophora cover
We identified 50 individuals of Lobophora within Liagora canopies at Lighthouse North in April–May 2013, and measured the canopy heights. We randomly selected half of the Lobophora individuals and removed the overstory canopy of Liagora, while the other half remained intact. Each individual of Lobophora was tagged, photographed and the surface area measured using Image-J software (US National Institutes of Health, Bethesda, MD), with two stainless steel nails on either side of each individual as reference points for size calibration. The surface area of each individual Lobophora was measured at 0-, 5- and 12-day intervals, and differences between treatments tested using a repeated-measures ANOVA in the stats package [R software (R Core Team 2014)].
Herbivore-exclusion experiments
To explore the role of herbivory in influencing Lobophora cover, we conducted additional caging experiments to exclude the effects of large herbivorous fish. Using a mould, we created tiles (10 × 10 cm) with equally spaced exposed and crevice surfaces (1-cm depth) to mimic small-scale micro-complexity observed on the reef (Fig. S1). Tiles were assigned to three treatments: caged, open, and partially caged, with replicate plots (n = 5 per treatment) separated by ≥1 m and interspersed along the benthos at a depth of ~7 m. The cages and partially closed cages measured 20 × 20 × 30 cm, and were constructed from PVC-coated galvanised steel, with a 2.5 × 2.5-cm mesh size. The partially closed cage had part of the roof and two sides removed to create large holes. Un-preconditioned tiles were deployed facing upright ~5 cm above the benthos in April 2013, photographed in July 2013 (100 days following deployment), and percent cover of Lobophora quantified in the exposed and crevice microhabitats among treatments from the photographs. Significant differences in the percent cover of Lobophora among treatment and microhabitats (crevice vs. exposed surfaces) were tested using a two-way ANOVA, and significant differences among treatments tested using a Tukey’s honest significant difference post hoc test using the stats package, R software [R software (R Core Team 2014)].
Results
Temporal trends in macroalgal cover
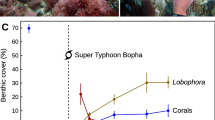
Baseline data recorded prior to typhoon Bopha in February 2012 indicated low macroalgal cover at all three sites (<2.3 %), consisting entirely of Halimeda spp. within the understory of branching acroporid corals. Coral cover ranged from 64 % at Ngederrak South to 77 % at Lighthouse North (Fig. 2a). No Lobophora or Liagora were recorded at any of the three sites (Fig. 2b, c). Following Typhoon Bopha, which struck on 2 December 2012, our surveys revealed a near complete loss of coral at all sites (Fig. 3a). Blooms of Liagora were observed following the typhoon in late December 2012 (G. Mereb, Palau International Coral Reef Center, personal communication). By February 2013, Liagora was the dominant benthic cover, ranging between 7.4 and 38.6 % (Fig. 2b; Table S1). Subsequent surveys in April 2013 revealed a 62.4–95.9 % decline in Liagora cover as the bloom passed its peak (Fig. 2b), coinciding with the appearance of Lobophora, ranging between 3.9 and 10.9 % (Fig. 2c). By September, Liagora was completely absent (Fig. 2b), yet Lobophora continued to increase throughout September and October 2013 (Fig. 2c), reaching a maximum of 41.5 % by April 2014 (12 months after the first sighting). Lobophora persisted at the study sites until October 2014 at the last survey date, 18 months after it was first observed, and 22 months following the typhoon. Repeat surveys conducted at three additional adjacent sites (Fig. 1) that lacked Liagora indicated a complete absence of Lobophora throughout the study period (Table S1).
Habitat availability beneath Liagora canopies and distribution of Lobophora on the reef
The proportion of substrate within the ‘swept’ area of Liagora canopies varied from 59 % at Lighthouse North to 0.1 % at Ngederrak North (Fig. 4a). Despite such variability in Liagora canopies, the distribution of Lobophora was non-random at all sites, with higher abundance of Lobophora occurring within canopies (Fig. 4a) than would have been expected by chance (Lighthouse North χ 2 = 48.2, z = 6.94, p < 0.001; Ngederrak South χ 2 = 109.5, z = 10.47, p < 0.001; Ngederrak North χ 2 = 8.3, z = 2.9, p < 0.01). Measurements at Lighthouse North indicated an average sweep radius of 11.9 ± 0.9 cm per Liagora individual (n = 181). Most Lobophora were found close to the centre of canopies, with an average distance of 2.9 ± 0.8 cm from the holdfast (n = 53). Significant differences were observed in the size structure of Lobophora inside and outside of canopies (Fig. 4b; Kolmogorov–Smirnov test, D = 0.39, p < 0.001), in that a higher proportion of smaller individuals were recorded outside of Liagora canopies. Mean size of Lobophora inside of canopies (n = 93) was significantly higher than outside (19.3 ± 0.7 vs. 14.5 ± 0.8 mm, t-test = 4.16, n = 101, p < 0.001).
a Proportion of substrate within the ‘swept’ area of Liagora canopies vs. outside canopies (n = 3 transects) and the proportion of Lobophora occurring inside and outside of Liagora canopies (n = 100 individuals), and b size frequency distributions of Lobophora individuals inside and outside of Liagora canopies
Patterns of herbivore grazing on Liagora canopies
Bite rates of C. striatus were sixfold higher outside of canopies (21 ± 6.6 bites min−1; Fig. 5a) than within Liagora canopies (3.6 ± 2.5 bites min−1, t-test = 12.72, p < 0.001). Bite rates of C. sordidus were lower than that of C. striatus (Fig. 5b) yet were also significantly higher outside of Liagora canopies (4 ± 2.9 bites min−1) than within canopies (1.6 ± 1.9 bites min−1, t-test = 3.84, p < 0.01). C. striatus exhibited a clear avoidance of Liagora canopies compared with outside of Liagora canopies (Fig. 5c; t-test = 10.45, p < 0.001), and a similar pattern was observed with C. sordidus (Fig. 5d; t-test = 3.57, p < 0.01).
Grazing impact on Lobophora growth
Experimental removal of the Liagora canopy reversed the direction of Lobophora growth from positive to negative. Lobophora continued to grow under the Liagora canopy, with an average increase in surface area of 51.7 ± 8.7 % in 12 days (Fig. 6a). In contrast, Lobophora with the overstory canopy removed were reduced to 53.1 % of their initial size within 12 days of the canopy removal (Fig. 6a), resulting in a clear and significant difference between treatments (repeated-measures ANOVA, F 2,98 = 33.42, p < 0.001). Of the 25 Lobophora individuals in the canopy-removal treatment, reductions of surface area were associated with evidence of continuous scrape marks associated with parrotfish feeding in all individuals (Fig. 6a). The remnant sections of Lobophora appeared visually unaffected, with no signs of pigment loss throughout repeated observations.
a Change in surface area (percent cover) of Lobophora recruits (n = 25 per treatment) through time following experimental removal of Liagora canopies and controls (±SE) and time series (days 0–12) of an individual tagged Lobophora with parrotfish bite marks following removal of Liagora canopy on day 0 and b percent cover of Lobophora after 100 days in exposed and crevice microhabitats of tiles deployed in caged, partially caged and open treatments (±SE, n = 5 per treatment)
Herbivore-exclusion experiments
A significant difference was observed in the cover of Lobophora among herbivory treatments (two-way ANOVA, F 1,2 = 5.774, p < 0.001), but no significant differences were observed among microhabitats (exposed or crevices) or herbivory × microhabitat interactions (Fig. 6b). Post hoc tests revealed no significant difference in the cover of Lobophora among open and partially caged treatments, while a significant difference was observed between caged treatments and open (p < 0.01) and partially caged treatments (p < 0.05), indicating that the cover of Lobophora was higher when herbivores were excluded (Fig. 6b).
Discussion
Temporary and persistent shifts from coral to macroalgal states have been observed following disturbance on coral reefs (e.g. Diaz-Pulido et al. 2009; Done 1992; Hughes 1994). Here, we define a phase shift as a marked change in the community structure of a coral reef as first defined by Done (1992). Importantly, the existence of a phase shift makes no assertion about stability or the presence of alternate attractors (Mumby et al. 2013b; Petraitis and Dudgeon 2004). The mechanisms influencing patterns of succession and persistence of coral—macroalgal phase shifts are largely unknown. Here, we report a case of phase shift facilitation made possible by the response of Liagora to catastrophic typhoon disturbance that removed living coral. In the wake of typhoon Bopha in December 2012, coral cover on the eastern reefs of Palau declined from ~70 to <1 % cover. Consistent with previous studies in the Caribbean region (e.g. Hughes 1994; Woodley et al. 1981), blooms of Liagora were observed across impacted sites. By late February 2013, three months after typhoon Bopha, we documented a phase shift to monospecific canopies of Liagora, reaching a maximum of 38.6 % cover. The extent of the Liagora bloom was primarily determined by the degree of wave exposure, in that highest Liagora cover was observed at highly exposed sites (G. Roff, unpublished data). By April 2013, the Liagora bloom had started to decline. However, a second and more persistent macroalga, Lobophora variegata, had established under the Liagora canopy and continued to increase throughout the study, reaching up to 41.5 % cover, and persisting for over 18 months following the first observations. Through a combination of observation and experiments we conclude that the secondary succession of Lobophora was facilitated by the Liagora canopy, which offered an ephemeral refuge to the understory brown alga from intense herbivory. Previous investigations of associational escapes in macroalgae have been studied on a scale of millimetres (i.e. epiphytic associations) or several centimetres (i.e. neighbouring associations), and focused on explaining patterns of distribution and maintenance of diversity within algal assemblages (e.g. Hay 1986; Littler et al. 1986; Pfister and Hay 1988). Here, we identify a novel associational escape that resulted in a larger community-scale phase shift to Lobophora dominance.
Several lines of evidence led us to conclude that Liagora canopies facilitated the secondary succession of Lobophora. First, the distribution of Lobophora was non-random, in that Lobophora occurred more frequently within than outside of Liagora canopies. Secondly, the size distribution of Lobophora within canopies was consistently larger than those outside of canopies. Thirdly, most Lobophora were found closer to the main holdfast rather than around peripheries of Liagora canopies, where they were more susceptible to herbivory. Fourth, direct measurements of bite rates of two common herbivores were higher outside than inside of canopies, and both actively avoided feeding within Liagora canopies. Fifth, Lobophora is seasonally abundant from November up to and including April in Micronesia (Tsuda 1972), making the timing of the Lobophora increase largely consistent with facilitation and inconsistent with seasonality (Lobophora would usually decrease from April to September). Sixth, removal of Liagora canopies resulted in a significant loss of Lobophora through herbivory whereas Lobophora continued to grow when the Liagora canopy remained intact. Seventh, and perhaps most conclusively, the cover of Lobophora was higher in tiles that were deployed in caged treatments than in open or partially caged treatments, indicating that while Lobophora was present in all treatments after 100 days, exclusion of herbivores resulted in a significantly higher cover of Lobophora. Finally, repeat surveys of exposed Lobophora revealed clear evidence of scrape marks associated with parrotfish feeding within days of removing Liagora canopies (n = 25 individuals). From this, we infer that grazing parrotfish were the primary cause of the reduction in Lobophora following canopy removal. While herbivory has been implicated in the removal of Lobophora, previous studies have implicated browsing species (e.g. Siganus doliatus and Kyphosus vaigiensis) as primary consumers (Bennett et al. 2010). Our results provide an interesting example of grazing species influencing the distribution and abundance of a brown alga on Indo-Pacific reefs.
Importantly, the emergence of Lobophora is not simply a response to mass coral mortality because a previous mass bleaching event at our study sites (Golbuu et al. 2007), that resulted in a similar loss of coral but without a Liagora bloom, did not result in a rise of Lobophora (Y. Golbuu, personal observation). Moreover, only those storm-damaged sites that resulted in a Liagora bloom were found to undergo a phase shift towards Lobophora. Collectively, these results provide strong evidence that Liagora canopies provided a refuge from herbivory, driving patterns of recruit survival and growth in Lobophora. These results are consistent with associational plant refuges against herbivores previously documented in both temperate (Hay 1986; Pfister and Hay 1988; Turner 1983) and tropical environments (Littler et al. 1986).
Through selective herbivory on seedlings and saplings, herbivores are able to alter patterns of ecological succession (Huntly 1991). This influence on community development occurs through several mechanisms. Firstly, the spatial distribution of palatable and unpalatable plants can affect herbivore foraging behaviour, in turn affecting patterns of survival of mixed plant assemblages (e.g. Hay 1986; Hixon and Brostoff 1996; Pfister and Hay 1988). Secondly, plants can influence sapling survival through habitat amelioration from abiotic extremes, which ultimately influences the spatial distribution of communities (e.g. Gomez-Aparicio et al. 2008; Weltzin and McPherson 1999). Thirdly, the structure of plants can provide a physical refuge for new recruits from herbivory (e.g. Fuentes et al. 1986; Harris et al. 1984; Turner 1983). While all three processes could feasibly have contributed to facilitation in our study, our results indicate that physical refuge from herbivory was likely the primary mechanism involved in driving the secondary succession of Lobophora. Similar processes have been documented following storm disturbance in kelp forests, where early successional dominant turf algae provide refuge from herbivores for kelp sporophytes (Harris et al. 1984).
Previous studies of the dynamics of Lobophora on Indo-Pacific reefs have found it to be strongly limited in areas of high herbivory (Diaz-Pulido and McCook 2003; Verges et al. 2011). Indeed, previous surveys of our study sites and extensive surveys around Palau have not reported significant populations of Lobophora in this habitat (though it is found in shallow backreefs). Moreover, Lobophora did not bloom at sites that were unaffected by typhoon Bopha. This raises the question, how did the Lobophora canopy continue to expand even after the Liagora declined, particularly seeing as experimental manipulation of the canopy led to a reduction in Lobophora size? We hypothesize a duality of explanation. First, the density of Liagora was high, reaching ~40 % cover, thereby providing a large, albeit ephemeral, refuge from herbivory. Second, while Lobophora can reproduce sexually and by fragmentation, colonisation tends to occur from vegetative growth along the marginal meristem from points of establishment, in that the rate of expansion increases with the patch size of Lobophora (van Steveninck and Breeman 1987). Thus, we hypothesize that local patches of Lopophora were able to become large enough in the presence of Liagora such that population growth could continue even when subjected to a moderately high herbivore regime once the Liagora died off. Such size-escape thresholds from herbivory have been previously documented in temperate and tropical macroalgae (e.g. Doropoulos et al. 2013; Lubchenco 1983), and both grazing and browsing herbivores avoid high-density patches of macroalgae (Hoey and Bellwood 2011). Had the Liagora bloom died back earlier, when Lobophora patches were smaller, the outcome may have been different as herbivory might plausibly have prevented a net increase in Lobophora population size.
The recovery of kelp forests after storm damage involves a facilitated primary succession (Harris et al. 1984), but to our knowledge, this is the first report of facilitated secondary succession towards a phase shift following acute disturbance. A phase shift in favour of Lobophora is a cause for concern. While Liagora is an ephemeral alga (Hughes 1994; e.g. Woodley et al. 1981), Lobophora is considered to be one of the strongest competitors for space on coral reefs (Nugues and Bak 2006), and is generally unpalatable to herbivores in decumbent growth form (Coen and Tanner 1989). The encrusting growth of Lobophora is competitively dominant (van Steveninck et al. 1988), and through allelopathic mechanisms (Rasher and Hay 2010), Lobophora is able to negatively impact upon multiple coral reef organisms, including corals (Diaz-Pulido et al. 2011; Lobel and Ogden 1981; Rasher and Hay 2010), sponges (Graham et al. 2013) and other macroalgal taxa (Brock 1979). While Lobophora is commonly found on forereefs in the Caribbean (e.g. Renken et al. 2010), blooms of it tend to be confined to inshore and somewhat eutrophic reefs in the Pacific (e.g. Diaz-Pulido et al. 2009). Palauan reefs tend to have relatively high herbivory (Mumby et al. 2013a) so the occurrence of Lobophora is unusual. That Lobophora has now persisted for over 18 months following the initial observations, and 22 months following the typhoon, raises questions as to the potential longer-term impacts of Lobophora in terms of competition with regenerating coral fragments that survived the initial typhoon disturbance, and potential effects on the recruitment of corals to the sites in successive recruitment events.
Many reefs have experienced algal phase shifts in the past few decades, raising questions over how to reverse such communities once established. While phase shifts on coral reefs might be difficult to reverse because of reinforcing biotic feedbacks (Mumby and Steneck 2008), a compelling argument has been made that acute disturbance events, such as typhoons, may lead to a reversal of phase shifts by removing macroalgal cover and promoting successful coral recruitment (Graham et al. 2013). Conversely, our results show that acute disturbance from typhoons may have the reverse effect, in becoming a mechanism by which persistent phase shifts are triggered, rather than reversed. While we do not suggest that this is a general phenomenon on coral reefs, the study of phase shift reversal should begin to consider the roles of algal facilitation.
Author contribution statement
G. R., C. D., and P. J. M. conceived and designed the experiments. G. R., C. D., M. Z., A. R., R. S., Y. G. and P. J. M. performed the experiments. G. R., C. D., and M. Z. analysed the data. G. R. wrote the first draft of the manuscript; all authors provided editorial advice and contributed substantially to the final version of the manuscript.
References
Atsatt PR, O’Dowd DJ (1976) Plant defense guilds. Science 193:24–29
Baumeister D, Callaway RM (2006) Facilitation by Pinus flexilis during succession: a hierarchy of mechanisms benefits other plant species. Ecology 87:1816–1830. doi:10.1890/0012-9658(2006)87[1816:Fbpfds]2.0.Co;2
Bennett S, Verges A, Bellwood DR (2010) Branching coral as a macroalgal refuge in a marginal coral reef system. Coral Reefs 29:471–480
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193. doi:10.1016/0169-5347(94)90088-4
Bertness MD, Leonard GH (1997) The role of positive interactions in communities: lessons from intertidal habitats. Ecology 78:1976–1989 doi:10.1890/0012-9658(1997)078[1976:Tropii]2.0.Co;2
Brock RE (1979) An experimental study on the effects of grazing by parrotfishes and role of refuges in benthic community structure. Mar Biol 51:381–388
Brooker RW et al (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34. doi:10.1111/J.1365-2745.2007.01295.X
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125. doi:10.1016/S0169-5347(02)00045-9
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349. doi:10.1007/Bf02912621
Callaway R, Nadkarni NM, Mahall BE (1991) Facilitation and interference of Quercus douglash on understory productivity in central California. Ecology 72:1484–1499
Coen LD, Tanner CE (1989) Morphological variation and differential susceptibility to herbivory in the tropical brown alga Lobophora variegata. Mar Ecol Prog Ser 54:287–298
Connell JH, Hughes TP, Wallace CC (1997) A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr 67:461–488
Diaz-Pulido G, McCook LJ (2003) Relative roles of herbivory and nutrients in the recruitment of coral-reef seaweeds. Ecology 84:2026–2033
Diaz-Pulido G et al (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS One 4:e5239
Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S, Anthony KRN (2011) High CO2 enhances the competitive strength of seaweeds over corals. Ecol Lett 14:156–162
Dixon DL, Abrego D, Hay ME (2014) Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345:892–897
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132
Doropoulos C, Hyndes G, Abecasis D, Verges A (2013) Herbivores strongly influences algal recruitment in both coral and algal dominated coral reef habitats. Mar Ecol Prog Ser 486:153–164
Duffy JE (2003) Biodiversity loss, trophic skew and ecosystem functioning. Ecol Lett 6:680–687. doi:10.1046/J.1461-0248.2003.00494.X
Edmunds PJ, Carpenter RC (2001) Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc Natl Acad Sci USA 98:5067–5071
Fuentes ER, Hoffman AJ, Poiana A, Alliende MC (1986) Vegetation change in large clearings: patterns in the Chilean matorral. Oecologia 68:358–366
Golbuu Y et al (2007) Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs 26:319–332. doi:10.1007/s00338-007-0200-7
Gomez-Aparicio L, Zamora R, Castro J, Hodar JA (2008) Facilitation of tree saplings by nurse plants: microhabitat amelioration or protection against herbivores? J Veg Sci 19:161–172. doi:10.3170/2007-8-18347
Graham NAJ, Bellwood DR, Cinner JE, Hughes TP, Norstrom AV, Nystrom M (2013) Managing resilience to reverse phase shifts in coral reefs. Front Ecol Environ 11:541–548. doi:10.1890/120305
Harris LG, Ebeling AW, Laur DR, Rowley RJ (1984) Community recovery after storm damage—a case of facilitation in primary succession. Science 224:1336–1338. doi:10.1126/Science.224.4655.1336
Hay ME (1986) Associational plant defenses and the maintenance of species diversity: turning competitors into accomplices. Am Nat 128:617–641
Hay ME, Kappel QE, Fenical W (1994) Synergisms in plant defenses against herbivores: interactions of chemistry, calcification, and plant quality. Ecology 75:1714–1726
Hixon MA, Brostoff WN (1996) Succession and herbivory: effects of differential fish grazing on Hawaiian coral-reef algae. Ecol Monogr 66:67–90
Hoey A, Bellwood D (2011) Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol Lett 14:267–273
Hughes TP (1994) Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hughes TP et al (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365. doi:10.1016/J.Cub.12.049
Huntly N (1991) Herbivores and the dynamics of communities and ecosystems. Annu Rev Ecol Syst 22:477–503. doi:10.1146/Annurev.Ecolsys.22.1.477
Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University Press, New Haven
Littler MM, Littler DS (1984) Models of tropical reef biogenesis: the contribution of algae. In: Round FE, Chapman DJ (eds) Progress in phycological research. Biopress, UK, pp 323–364
Littler MM, Taylor PR, Littler DS (1986) Plant defense associations in the marine environment. Coral Reefs 5:63–71
Lobel LMK, Ogden J (1981) Foraging by the herbivorous parrotfish Sparisoma radians. Mar Biol 64:173–183
Lubchenco J (1983) Littorina and Fucus: effects of herbivores, substratum heterogeneity, and plant escapes during succession. Ecology 64:1116–1123
Mumby PJ, Steneck RS (2008) Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol 23:555–563. doi:10.1016/j.tree.2008.06.011
Mumby PJ et al (2013a) Empirical relationships among resilience indicators on Micronesian coral reefs. Coral Reefs 32:213–226
Mumby PJ, Steneck RS, Hastings A (2013b) Evidence for and against the existence of alternate attractors on coral reefs. Oikos 122:481–491
Nugues MM, Bak RPM (2006) Differential competitive abilities between Caribbean coral species and a brown alga: a year of experiments and a long-term perspective. Mar Ecol Prog Ser 315:75–86
Paul VJ, Fenical W (1980) Toxic acetylene-containing lipids from the red marine alga Liagora farinosa Lamouroux. Tetrahedron Lett 21:3327–3330. doi:10.1016/S0040-4039(00)78680-1
Petraitis PS, Dudgeon SR (2004) Detection of alternative stable states in marine communities. J Exp Mar Biol Ecol 300:343–371. doi:10.1016/j.jembe.2003.12.026
Pfister CA, Hay ME (1988) Associational plant refuges: convergent patterns in marine and terrestrial communities result from differing mechanisms. Oecologia 77:118–129
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci USA 107:9683–9688. doi:10.1073/Pnas.0912095107
Renken H, Mumby PJ, Matsikis I, Edwards HJ (2010) Effects of physical environmental conditions on the patch dynamics of Dictyota pulchella and Lobophora variegata on Caribbean coral reefs. Mar Ecol Prog Ser 403:63–74. doi:10.3354/Meps08441
Stachowicz JJ (2001) Mutualism, facilitation, and the structure of ecological communities. Bioscience 51:235–246. doi:10.1641/0006-3568(2001)051[0235:Mfatso]2.0.Co;2
Steneck RS, Dethier MN (1994) A functional-group approach to the structure of algal-dominated communities. Oikos 69:476–498
Tsuda RT (1972) Marine benthic algae on Guam. 1. Phaeophyta Micronesica 8:87–115
Turner T (1983) Facilitation as a successional mechanism in a rocky intertidal community. Am Nat 121:729–738
van Steveninck EDD, Breeman AM (1987) Deep water vegetations of Lobophora variegata (Pheophycea) in the coral reef of Curacao—population dynamics in relation to the mass mortality of the sea urchin Diadema antillarum. Mar Ecol Prog Ser 36:81–90
van Steveninck ED, Kamermans P, Breeman AM (1988) Importance of physical and biological processes in structuring tropical intertidal populations of Lobophora variegata (Phaeophyceae). Mar Ecol Prog Ser 44:77–84
Verges A, Vanderklift MA, Doropoulos C, Hyndes GA (2011) Spatial patterns in herbivory on a coral reef are influenced by structural complexity but not by algal traits. Plos One 6. doi:10.1371/journal.pone.0017115
Wahl M, Hay ME (1995) Associational resistance and shared doom: effects of epibiosis on herbivory. Oecologia 102:329–340
Weltzin JF, McPherson GR (1999) Facilitation of conspecific seedling recruitment and shifts in temperate savanna ecotones. Ecol Monogr 69:513–534. doi:10.1890/0012-9615(1999)069[0513:Focsra]2.0.Co;2
Williams SL (1990) Experimental studies of Caribbean seagrass bed development. Ecol Monogr 60:449–469. doi:10.2307/1943015
Woodley JD et al (1981) Hurricane Allen’s impact on Jamaican coral reefs. Science 214:749–755
Wulff JL (1997) Mutualisms among species of coral reef sponges. Ecology 78:146–159
Wylie CR, Paul VJ (1988) Feeding preferences of the surgeonfish Zebrasoma flavescens in relation to chemical defenses of tropical algae. Mar Ecol Prog Ser 45:23–32. doi:10.3354/Meps045023
Acknowledgments
This research was funded by a Natural Environment Research Council grant and Australian Research Council Laureate Fellowship to P. J. M.; C. D. was partly supported by an Australian Endeavour Award Postdoctoral Fellowship. We thank five anonymous reviewers for providing comments and insight that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Deron E. Burkepile.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1 Benthic cover among sites for all time periods
Fig. S1 Schematics of tiles used in caging experiments (DOCX 88 kb)
Rights and permissions
About this article
Cite this article
Roff, G., Doropoulos, C., Zupan, M. et al. Phase shift facilitation following cyclone disturbance on coral reefs. Oecologia 178, 1193–1203 (2015). https://doi.org/10.1007/s00442-015-3282-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3282-x