Abstract
Biological soil crusts (biocrusts) naturally coexist with vascular plants in many dryland ecosystems. Although most studies of dryland biocrusts have been conducted in warm deserts, dryland biocrusts also exist in the Arctic, where they may be an important source of nitrogen (N) and carbon (C) to nutrient-limited environments. In Kangerlussuaq, Greenland, wind-driven soil erosion has created a heterogeneous landscape where biocrusts dominate distinct patches of soil but are absent from the surrounding shrub and graminoid tundra. Prior to this study, little was known about the physical development and nutrient cycling of west Greenland biocrusts and their role in maintaining landscape heterogeneity. We characterized the physical properties, lichen assemblages, and nutrient concentrations of biocrusts and underlying soils along gradients in biocrust development and age. We found that biocrusts took 180 ± 40 years to fully develop and that biocrusts became thicker and soil penetration resistance increased as they developed. The N-fixing lichen Stereocaulon sp. was found throughout the study region at all stages of biocrust development. Natural 15N abundance suggests that Stereocaulon sp. obtains about half of its N from biological fixation and that some biologically fixed N is incorporated into the underlying soils over time. Although the N and C concentrations of underlying soils increased slightly with biocrust development, nutrient concentrations under the most developed biocrusts remained low compared to the surrounding vegetated tundra. Our results suggest that biocrusts are a persistent feature and play an important role in maintaining the high spatial heterogeneity of the Kangerlussuaq terrestrial landscape.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Biocrusts in Greenland take roughly 200 years to fully develop on eroded soils
-
Biocrust lichens actively fix atmospheric nitrogen
-
Soils underlying biocrusts remain nutrient poor compared to the surrounding tundra
Introduction
Biological soil crusts (biocrusts) are assemblages of cyanobacteria, green algae, lichens, and mosses that can play important roles in ecosystem structure and function (Weber and others 2016b). In many Arctic landscapes, biocrusts are often the first to colonize exposed land after glacier retreat, and they have been shown to facilitate primary succession (Breen and Levesque 2006, 2008; Bowker 2007; Yoshitake and others 2010). Biocrusts aid in soil development by stabilizing the soil surface and protecting fine-grained material from wind and water erosion (Belnap and Büdel 2016). Biocrusts can also increase the nutrient content of developing soils by fixing atmospheric nitrogen (N) and carbon (C) (Evans and Lange 2003; Barger and others 2016; Sancho and others 2016). In many glacier foreland settings, as soils develop and succession proceeds, biocrust assemblages give way to vascular plants (Breen and Levesque 2006; Bowker 2007; Yoshitake and others 2010).
However, in dryland and other high abiotic stress ecosystems, biocrusts naturally coexist with vascular plant communities in a dynamically stable mosaic, in which biocrusts dominate certain areas without ever giving way to plant communities (Bowker 2007). In these dryland settings, where water and nutrients may be severely limited, biocrusts may compete with vascular plants for resources and inhibit plant growth (Bowker 2007). Although dryland biocrust studies have focused mostly on hot deserts (Weber and others 2016a), dryland biocrusts also exist in the Arctic (Hansen 2001).
In the semiarid Kangerlussuaq region of West Greenland, lichen-rich biocrusts have existed for at least 230–800 years alongside shrub and graminoid tundra (Heindel and others 2017a). This spatially heterogeneous mosaic of biocrusts and vascular plants has been established by wind-driven soil deflation, which has removed distinct patches of soil down to the underlying glacial till or bedrock. These deflation patches, which range in size from tens to hundreds of square meters, likely began forming during the ‘Little Ice Age’ (AD 1350–1880) when the climate was colder and more arid (Heindel and others 2017a). Deflation patches continue to expand today along their active margins, or “scarps”, which move across the landscape when blocks of soil and vegetation are undercut and collapse (Heindel and others 2018) (Figure 1).
Deflation patch expansion increases the potential habitat for biocrusts. By colonizing these disturbed areas and potentially limiting the establishment of vascular plants, biocrusts may thus preserve the existence of deflation patches and be critical in maintaining the spatial heterogeneity of the Kangerlussuaq landscape. Vegetation cover within deflation patches ranges from 10 to 20% and is predominately graminoid and herbaceous (Heindel and others 2015). Given their estimated age and plant composition, there are no clear signs of deflation patches giving way to the surrounding tundra, which is dominated by the deciduous shrubs Betula nana and Salix glauca.
Previous studies have addressed biocrust N cycling in glacier forelands and moist Arctic tundra (Breen and Levesque 2006, 2008; Stewart and others 2011a, b). In these often N-limited ecosystems, atmospheric N fixation can account for more than half of total N inputs into the ecosystem, and biocrusts can be responsible for much of that N fixation (Stewart and others 2011a). For instance, in the poorly drained hummock-hollow tundra around Daring Lake, Northwest Territories, N fixation by biocrusts and associated lichens and mosses contributed 50–70% of total ecosystem N inputs (Stewart and others 2011a). In Kangerlussuaq, where precipitation is low and soils are well drained and rarely saturated, biocrust N fixation may also be an important source of N to the ecosystem.
Determination of the natural abundance of stable N isotopes (15N and 14N) in biological materials and soils is a powerful tool to track N sources and to reveal spatial and temporal patterns of N cycling (Craine and others 2009). Different pools of N, such as atmospheric N2, inorganic soil N, and N in plant tissue, may have different 15N/14N ratios (expressed as δ15N, or per mil 15N enrichment relative to the atmosphere) due to biological and kinetic isotopic fractionation. Lichens with active atmospheric N-fixing symbionts have been shown to have a different δ15N than lichens with only green algae symbionts because their N comes from different sources (Kobylinski and Fredeen 2015). As a result, natural 15N abundances have been successfully used to quantify N inputs from biocrusts and N-fixing lichens (Russow and others 2005; Stewart and others 2011a; Kobylinski and Fredeen 2015).
In this paper, we investigate the physical development, lichen diversity, and nutrient cycling of the dryland biocrusts in the Kangerlussuaq region. First, we take advantage of the geomorphology of deflation patches to quantify the rate of biocrust development. Biocrusts close to the actively eroding scarps are young and have only been developing for decades, whereas biocrusts near the centers of deflation patches may have been developing for hundreds of years. Second, we consider the role that biocrusts play in nutrient cycling by analyzing biocrust lichens and underlying soils for N and C concentrations and δ15N. Although the biocrusts studied here contain free-living cyanobacteria that may also fix atmospheric N, we focus on lichens because they dominate the well-developed biocrusts in this region. Characterizing the physical development and nutrient concentrations of biocrusts and underlying soils may help explain why deflation patches in the Kangerlussuaq region have remained largely devoid of vegetation for hundreds of years. In addition, our study of dryland biocrusts adds an important dimension to our understanding of the diversity of Arctic biocrusts.
Methods
Regional Setting
The town of Kangerlussuaq is located at the head of Søndre Strømfjord, roughly 120 km inland from the southwest coast of Greenland. Our study area extended from 2 km west of Kangerlussuaq to the margin of the Greenland Ice Sheet (GrIS; Figure 2). Kangerlussuaq has a continental climate, with a mean summer temperature of 9.5°C, a mean winter temperature of − 18.7°C, and a mean annual precipitation of 250 mm water equivalent, all for the period 1981–2011 (Hanna and others 2012; Mernild and others 2015). A considerable climate gradient exists across the study area, with colder air temperatures and stronger katabatic winds at the margin of the GrIS compared to the town of Kangerlussuaq (Dijkmans and Törnqvist 1991; Bullard and Austin 2011; Urbanowicz and others 2017). For instance, from mid-June to mid-July 2013, the mean air temperature was 11.37°C close to the GrIS and 13.54°C close to the town of Kangerlussuaq (Urbanowicz and others 2017). A vegetation gradient exists as well; areas close to the GrIS are characterized by steppe tundra, while areas close to the town are dominated by the deciduous shrubs Betula nana and Salix glauca (Heindel and others 2015).
Deglaciation from the last glacial maximum occurred in the Kangerlussuaq region about 7000 years ago (Ten Brink and Weidick 1974; Levy and others 2012). Following deglaciation, soil development in the Kangerlussuaq region has occurred on a 30-cm to 1-m thick loess deposit that mantles the region (Dijkmans and Törnqvist 1991). Subsequent soil deflation has removed the loess deposit down to the underlying glacial till or bedrock, creating a patchy mosaic of mixed shrub tundra, bedrock, and biocrust-dominated deflation patches.
Field Methods
To capture the vegetation and climate gradients that exist between Kangerlussuaq and the GrIS, we established 5 sites between the town and the GrIS (Figure 2). Sites are lettered from east to west, with Site A located 1 km from the GrIS, and Site E located 35 km from the GrIS. We selected sites to be evenly spaced and to be relatively accessible by road.
During the summer of 2014, we randomly selected five deflation patches at each site. At each deflation patch, we sampled the biocrust at four locations along a transect perpendicular to the scarp: directly below the scarp edge, at 1 m and 2 m from the scarp edge, and at the center of the patch. Due to the progressive expansion of deflation patches (Figure 1), each transect represents a chronosequence of biocrust development. To sample, we removed an approximately 25 cm2 square of biocrust, including the soil that was naturally attached to the biocrust. Samples were stored in sterile Whirl-pak® sampling bags and kept frozen during transport to Dartmouth College, where we completed all laboratory analyses. Although samples were not kept structurally intact, we were still able to separate the lichen-rich biocrusts from the underlying soil after transport to the laboratory.
At each sampling location, we measured the penetration resistance of the crust using a pocket penetrometer, taking the average of three measurements. On the samples we collected for laboratory analyses, we measured the thickness of the lichen-rich biocrust, which was visually distinct from the underlying soil. In addition, we measured the thickness of the underlying unit of cohesive soil that naturally held together during sampling. For both thicknesses, we took the average of three measurements at each sampling location.
To address the possibility that biocrusts develop at different rates across the five sites, we developed a qualitative level of biocrust development (LOD) independent from distance to the scarp and biocrust age (see below). We could not use the distance to the scarp for this purpose because we know from previous work that deflation patches expand at different rates, introducing a confounding variable (Heindel and others 2017a). Our qualitative biocrust LOD was based on the percentage of the ground surface that was covered in visible biological material (Belnap and others 2008). Biocrust LOD ranged from 1 (no visible biological material) to 5 (100 percent biological material; Figure 3).
Biocrust Age
Where possible (5 deflation patches at Site A, 4 at Sites B and C, 1 at Site D, and 3 at Site E), the biocrust samples were paired with lichenometry measurements. By assuming that Rhizocarpon spp. lichens colonized bedrock and boulders at the same time that the adjacent biocrust began developing, the lichenometry measurements allowed us to assign age estimates to the biocrust samples. Where there were not enough Rhizocarpon spp. lichens growing within deflation patches, we were unable to assign age estimates to the biocrust samples. Lichenometry methods are described in detail in Heindel and others (2017a), and the raw lichen data are available for download from the Dryad Digital Repository (Heindel and others 2017b). In brief, along the transect perpendicular to the scarp at each patch where we collected biocrust samples, we also measured Rhizocarpon spp. lichen diameters on adjacent boulders and bedrock. Using a Rhizocarpon growth curve specific to West Greenland (Forman and others 2007), we converted lichen diameters to exposure ages (the time when the boulder was exposed by deflation). For each patch, we modeled the relationship between the distance from the scarp and exposure age. This linear relationship, which is unique for each patch, corresponds to the rate of deflation patch expansion and was used to assign age estimates to biocrust samples.
Lichen Diversity, Nutrient and δ15N Analyses
To analyze the biocrust samples, we first manually separated the lichen-rich biocrust from the underlying mineral soil and then sorted the biocrust into moss and lichens. For each sample, we counted lichen morphotypes for a rough estimate of lichen diversity. Across all samples, and using some additional specimens collected outside of the sampling areas, we identified as many biocrust lichen genera and species as possible (Brodo and others 2001).
We separated the lichens into known N-fixing species and non-N-fixing species for total N and 15N abundance analyses. We gently washed the lichens with DI water to clean them of all remaining soil and dried and ground them into a fine powder for analyses.
We sieved the underlying soil samples to less than 2 mm, removing by hand any remaining organic material. We ground the soil samples and analyzed them at Dartmouth College for C and N concentrations on a Carlo Erba NA 1500 elemental analyzer (Carlo Erba Instruments, Milan, Italy). A subset of the soil samples and all lichen samples were sent to the University of California Davis Stable Isotope Facility, where the natural abundance of 15N was determined on a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Seron Ltd., Cheshire, UK). Variations in 15N abundance are reported in δ notation:
where Rsample is the 15N/14N ratio of the sample and Rstandard is the 15N/14N ratio of atmospheric N2.
Statistical Analyses
To test whether the rate of biocrust development varied by site, we ran an analysis of covariance (ANCOVA) with LOD as the response variable and age, site, and the interaction between the two as explanatory variables. To analyze the physical development of the biocrusts, we used ANCOVAs with penetration resistance, biocrust thickness, and the thickness of the underlying cohesive soil as response variables and site and LOD as explanatory variables.
After separating lichens into N-fixing species and non-N-fixing species, we used two tests to characterize lichen diversity. To look at spatial patterns in the presence of N-fixing species, we used a Cochran Armitage Trend Test. This test is used when the response variable has two levels (presence and absence of N-fixing lichens), and the explanatory variable (site) is ordinal (ordered by proximity to the GrIS). In addition, we used an ANCOVA to test whether site, the presence of N-fixing lichens, and LOD were important factors in determining lichen diversity.
For our analyses of lichen and soil nutrients, we used ANCOVAs to explain variation in %C, %N, C:N, and δ15N. For lichen samples, the explanatory variables were site, LOD, and lichen type (N-fixing vs. non-N-fixing). Similarly, for soil samples, the explanatory variables were site, LOD, and the presence of N-fixing lichens.
Results
Biocrust Age and Rate of Development
At all sites, biocrust level of development (LOD) increased with distance from the scarp and with estimated biocrust age (Figure 4). To estimate the time for biocrust development across the region, we averaged the age of fully developed (LOD 5) biocrusts at all 5 sites, giving us an estimate of 180 ± 40 (mean ± standard error) years for biocrusts to fully develop. The increase in visible biological material (our metric for biocrust development) did not have a linear relationship with biocrust age. Although we estimate that it takes 47 ± 7 years for biocrusts to cover 50 percent of the soil surface (LOD 3), it takes another 135 years for full biocrust coverage (LOD 5).
For a given distance from the scarp and age, biocrust LOD varied by site, suggesting differences in the rate of biocrust development (Figure 4). The ANCOVA with LOD as the response variable and age, site, and the interaction between the two as explanatory variables showed that all three were significant (P <0.05). Using a Tukey HSD test, we found that biocrusts at Site A (closest to the GrIS) took significantly longer to develop than biocrusts at Sites B and E, whereas crusts at Sites C and D had intermediate rates of development.
Biocrust Physical Characteristics
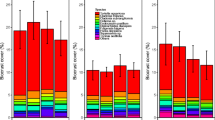
Penetration resistance, biocrust thickness, and the thickness of cohesive soil attached to the biocrust all tended to increase with biocrust development (Figure 5). Penetration resistance ranged from 0 to 4 kg cm−2, and LOD and site were both significant in explaining the variance using an ANCOVA. Penetration resistance increased with increasing LOD (Figure 5A), and using a Tukey HSD test, Site E (farthest from the GrIS) had significantly higher penetration resistance (1.6 ± 0.1 kg cm−2) than Sites A and B (0.8 ± 0.1, 1.0 ± 0.1 kg cm−2, respectively). Biocrust thickness ranged from 0.1 to 2 cm and varied significantly by site but not by LOD (Figure 5B). Site B had significantly thicker biocrusts (1.2 ± 0.1 cm) than Sites D and E (0.6 ± 0.1, 0.6 ± 0.2 cm, respectively). The thickness of cohesive soil attached to the biocrust ranged from 1 to 5 cm and varied significantly by LOD. LOD 2 had a significantly smaller thickness of attached soil than LODs 4 and 5 (Figure 5C).
Lichen Diversity
Lichen diversity ranged from 0 to 10 morphotypes in our 25 cm2 surface samples. Across all sites, we identified 13 biocrust lichens to the species level, as well as four lichen genera that were too immature to identify further (Table 1). The only lichen we identified with a known N-fixing symbiont was the tripartite Stereocaulon sp., which was present at all sites. There was a significant trend in the presence of Stereocaulon sp. across sites (Cochran Armitage Trend Test, P = 0.006), with more samples containing Stereocaulon sp. at Site A, proximal to the GrIS (81% of samples), than at Site E (50%).
Lichen diversity increased with biocrust LOD and with the presence of N-fixing lichens (Stereocaulon sp.) but did not vary by site. On average, biocrusts with LOD 1 had 1.6 ± 0.7 lichen morphotypes, whereas biocrusts with LOD 5 had 5.1 ± 0.4 morphotypes. Results of the ANCOVA (R2 = 0.71) showed that variance in lichen diversity was explained (P < 0.0001) by biocrust LOD (36% of variance) and the presence of N-fixing lichens (30% of variance), with higher lichen diversity where N-fixing lichens were present.
Lichen N Fixation
All lichen samples, including both N-fixing and non-N-fixing lichens, were depleted in 15N relative to atmospheric N2 (δ15N = 0‰), with individual samples ranging from − 0.38 to − 6.87‰. There was a significant difference between the δ15N of presumed N-fixing lichens and non-N-fixing lichens (P < 0.0001), with N-fixing lichens having a δ15N closer to the atmosphere (− 1.56 ± 0.09‰) than non-N-fixing lichens (− 2.93 ± 0.19‰, Figure 6A). The ANCOVA (R2 = 0.35) with δ15N as the response variable showed that lichen type was the only significant effect, explaining 31% of the total variance in lichen δ15N. Site and biocrust LOD were not significant explanatory variables.
A Nitrogen isotope results for lichens, separated into lichens that are known to fix N and those that do not fix N. B Nitrogen isotope results for soils, separated into soil samples with N-fixing lichens present and soil samples with no N-fixing lichens present. Error bars are plus and minus one standard error.
Although the ANCOVA for lichen N concentration was not significant, N-fixing lichens generally had higher % N (1.23 ± 0.04%) than non-N-fixing lichens (1.00 ± 0.08%).
Soil Nutrients
Carbon and N concentrations (% dry weight) in soils underlying biocrusts showed increasing trends with increasing LOD (Figure 7A, B). However, the ANCOVA (R2 = 0.58) with %C as the response variable revealed that site was the only significant explanatory variable (P < 0.0001), explaining 55% of the total variance. Using a Tukey HSD test, biocrust samples at Site D had significantly higher %C than samples from all other sites. Sites B, C, and E had intermediate soil %C, and Site A had the lowest %C (Table 2). Soil N increased with LOD (ANCOVA R2 = 0.63) with marginal significance (P = 0.06; Figure 7B). Site was also a significant effect for soil N; a Tukey HSD test showed that Site D had higher %N than all other sites (Table 2). The presence of N-fixing lichens was not a significant explanatory variable for either %C or %N in underlying soils.
As biocrusts developed, %N increased more significantly than %C, resulting in a decrease in the C:N ratio. The C:N ratio of soils underlying biocrusts showed a decreasing trend with increasing LOD (Figure 7C), with LOD 1 averaging 15.6 ± 0.8 and LOD 5 averaging 12.1 ± 0.5. The ANCOVA (R2 = 0.36) for soil C:N ratio confirmed that LOD was a significant explanatory variable (P < 0.001). Site was also a significant explanatory variable but showed no pattern with distance from the GrIS (Table 2).
Soils underlying the biocrusts were enriched in 15N relative to the atmosphere, with individual soil samples ranging from 0.75 to 7.09‰. The δ15N of underlying soils became less enriched as LOD increased with marginal significance (P = 0.08; Figure 7D). Soil δ15N and the presence of N-fixing lichens were also related (ANCOVA R2 = 0.52), with the presence of N-fixing lichens explaining 9% of the variance in soil δ15N. Soils with N-fixing lichens had lower δ15N values (2.95 ± 0.23‰) than soils without N-fixing lichens (4.34 ± 0.25‰, Figure 6B). In addition, the δ15N of underlying soils varied significantly by site. Accounting for the effects of the other explanatory variables, the ANCOVA and Tukey HSD tests showed that Site D had significantly lower δ15N than Site E, with all other sites intermediate (Table 2).
Discussion
Biocrust Development
The estimate of 180 ± 40 years for the development of a lichen-rich biocrust falls within the wide published range of 4–15 to 100–2000 years (Weber and others 2016a). This estimate refers roughly to the amount of time it takes for visible biological material to completely cover the ground surface; it does not refer to the age of the deflation patch itself. Deflation patches in the Kangerlussuaq region are approximately 230–800 years old (Heindel and others 2017a). The biocrusts farthest from the scarp, which we did not sample for this study, may be as old as the deflation patch itself.
Our results support previous findings that biocrust development and recovery tend to be slower at sites with low precipitation, coarse-grained substrates, and high disturbance intensity (Belnap and Eldridge 2003; Weber and others 2016a). We found the lowest rate of biocrust development at the site closest to the GrIS (Site A), where air temperatures are coldest (Urbanowicz and others 2017), katabatic winds are strongest (Bullard and Austin 2011), relative humidity is low (Kopec and others 2014), and we have observed more coarse-grained sediment within deflation patches. Slow biocrust development may allow more soil deflation to occur before biocrusts are able to trap fine-grained sediment. At sites far from the GrIS, biocrusts may be able to develop more quickly on the loess deposit, preventing deflation from completely removing the fine-grained sediment down to the underlying glacial till or bedrock.
We found that biocrust thickness, soil cohesion, and penetration resistance increased as the biocrust developed. Over time, biocrusts provide greater soil stability (Thomas and Dougill 2007; Belnap and Büdel 2016), limiting additional wind erosion. Active soil deflation is thus mainly confined to the scarp (Heindel and others 2018), where biocrusts have yet to develop and loess is still directly exposed to the wind. At the same time that biocrusts stabilize the soil surface from erosion, they may also limit water infiltration and seedling germination and growth (Deines and others 2007; Langhans and others 2009; Zhang and Nie 2011). To consider the possible impact of biocrust development on water infiltration, during the summer of 2016 we opportunistically measured volumetric soil moisture at a depth of 10 cm using a ThetaProbe (ML2X, Delta-T Devices, Cambridge, England) at Sites B–E. We found that although soil moisture was low everywhere, soil moisture tended to decrease with biocrust development (3.1 ± 0.2% under LOD 1 crusts, 1.8 ± 0.2% under LOD 5 crusts). Although these are preliminary data, they suggest that biocrust development may limit the infiltration of water into the underlying soils, lessening the quality of this habitat for plant establishment.
Although we do not have direct data on the interaction between biocrusts and vascular plants, we do know from remote sensing and ground surveys that deflation patches are only 10–20% vegetated, compared to the fully vegetated surrounding tundra (Heindel and others 2015). Deflation patches close to the GrIS tend to have lower vegetation cover than deflation patches far from the GrIS (Heindel and others 2015). It is unclear whether this pattern in vegetation cover is due to an interaction with biocrusts, or whether the conditions that promote biocrust development also promote the establishment of vascular plants. Plant communities within deflation patches are not the same as the plant communities outside of deflation patches. Although herbaceous species make up 10% of the vegetation cover outside of deflation patches, they account for 25–40% of the vegetation cover within deflation patches, even on deflated surfaces that are hundreds of years old (Heindel, unpublished data). Further work is needed to determine the direct effect of biocrusts on vascular plants, but it is clear that biocrusted areas are persistently lower in biomass and different in plant community than the surrounding tundra.
Lichen Diversity and N Fixation
Four of the 13 biocrust lichens that we identified to the species level (C. pyxidata, L. wulfenii, P. pubescens, and S. fragilis) are lacking from the most recent published list of lichens from the Kangerlussuaq area (Hansen 2000). The crustose L. wulfenii likely plays an important role in soil stabilization, due to the branching morphology of its dense rhizines, which anchor the lichen to the soil substrate and bind the soil together. The biocrust lichen assemblage that we identified is most similar to the assemblage identified by Hansen (2001) as growing within steppe-like vegetation dominated by sedges and grasses. However, Hansen (2001) did not differentiate between lichens growing together with graminoid species and those growing as part of biocrusts on bare ground. The biocrust lichen assemblages that we identified did not vary greatly within or among sites, other than the result that Stereocaulon sp. is more common closer to the GrIS.
Our finding of increased lichen diversity with biocrust development has been reported in other systems where biocrust development follows a successional pattern with cyanobacterial crusts giving way to lichen or moss crusts over time (Weber and others 2016a). For instance, on dune surfaces in Germany, cyanobacterial crusts dominate along the tops of dunes where disturbances are frequent, while moss crusts dominate only at the bases of dunes where there has been sufficient time for crust development (Brankatschk and others 2013). Similarly, in Northern China, along a gradient from mobile to stabilized dunes, moss crusts exist only on the most stabilized dune systems, whereas algae crusts are found on the mobile dunes (Guo and others 2008). Because deflation patches persist for hundreds of years, they may become hotspots of lichen diversity as biocrusts develop without disturbance or competition from vascular plants. The fact that deflation patches remain part of a dynamically stable mosaic allows the development of a rich assemblage of lichen species that does not exist in the surrounding mixed shrub tundra.
Of the species we identified Stereocaulon sp. is the only lichen with the capacity to fix atmospheric N2. Stereocaulon spp. are tripartite lichens, with green algae as the main photobiont and N-fixing cyanobacteria housed in cephalodia (Brodo and others 2001). In many Arctic and Antarctic deglaciated forelands and recently disturbed areas, Stereocaulon spp. are frequently found as part of early successional communities (Line 1992; Stewart and others 2011a). For instance, on the sub-Antarctic Macquarie Island, Stereocaulon sp. was commonly found on dry rocky habitats and was shown to have a significant role in N cycling on the plateau regions of the island (Line 1992).
We found consistently depleted δ15N values in lichens, likely due to the depleted values of N compounds in rainfall and dry deposition (Ellis and others 2003; Beck and Mayr 2012; Kobylinski and Fredeen 2015). The clear separation that we observed between the δ15N values of non-N-fixing lichens and underlying soils suggests that these lichens obtain N predominantly from atmospheric N deposition rather than from the soil substrate (Ellis and others 2003; Oulehle and others 2016). N-fixing lichens, on the other hand, can obtain N through both biological N fixation and atmospheric N deposition, shifting their δ15N value closer to the atmospheric value of 0‰.
If we assume that non-N-fixing lichens rely solely on atmospheric deposition for their N supply, we can estimate the percentage of N that the N-fixing lichen Stereocaulon sp. obtains through biological N fixation (Russow and others 2005). Following the natural 15N abundance method (see Russow and others 2005), we compared the δ15N of Stereocaulon sp. with that of non-N-fixing lichens to estimate that Stereocaulon sp. obtains roughly half of its N from biological N fixation. This estimate assumes no significant fractionation during biological N fixation (Shearer and Kohl 1986; Evans and Ehleringer 1993). Although this estimate highlights the importance of biological N fixation for this species, additional work is needed to quantify ecosystem N inputs through biocrust N fixation.
Soil Nutrients
Soils underlying highly developed biocrusts had slightly higher C and N concentrations compared to soils underlying poorly developed biocrusts, a pattern that has been found in prior studies (Evans and Lange 2003; Barger and others 2016; Sancho and others 2016). The increase was more pronounced with N than C, creating lower soil C:N ratios. Our soil δ15N results suggest that a possible source of this additional soil N is from biological N fixation, either released from N-fixing lichens or from free-living cyanobacteria found in these soils. Although free-living cyanobacteria are known to readily transfer fixed N to soils, lichens may only release fixed N during rainfall or freeze–thaw events (Rousk and others 2016). Our results suggest that in the Kangerlussuaq biocrusts, Stereocaulon sp. is able to retain most of its fixed N and C, as we found no difference in the %N and %C of soils underlying biocrusts with and without Stereocaulon sp. Instead, free-living cyanobacteria may contribute to the enrichment of soil nutrients as the biocrust develops, but additional work is needed to understand how N is transferred from the biocrusts into the underlying soils.
Soil C and N concentrations also varied significantly by site, but the differences did not follow a clear trend with distance from the GrIS. Instead, Site D was consistently different from the other sites, with higher C and N concentrations and lower δ15N. Site D is located at an intermediate distance from the GrIS and is not markedly different from neighboring sites in aspect, elevation, or exposure to winds. However, many deflation patches at Site D looked inactive, with highly developed biocrusts growing up the scarps. It could be that biocrusts at Site D are on average older than biocrusts at the other sites. Due to the lack of Rhizocarpon spp., we were only able to estimate biocrust age at one deflation patch at Site D.
Despite evidence of N fixation by lichens, the nutrient concentrations of soils underlying biocrusts are still very low compared to soils outside of deflation patches where the soil organic horizon remains intact. In surface soils underlying undisturbed vegetated tundra, the average C concentration is roughly 10% (Bradley-Cook and Virginia 2016). Deflation patch soils underlying highly developed biocrusts are an order of magnitude lower in C concentration, with only 1.2% C on average. Any future increase in soil nutrient concentrations is likely to be a very slow process and one that could be set back or interrupted by changes in climate or disturbance by animals and humans.
The slow accumulation of nutrients in soils underlying biocrusts may be one factor that contributes to the persistence of deflation patches on the Kangerlussuaq landscape for hundreds of years. These biocrust-dominated landforms, originally created by wind-driven soil erosion, provide habitat for rich lichen assemblages and become part of the dynamically stable mosaic of bedrock, biocrusts, and plant communities that characterizes the Kangerlussuaq landscape. In the future, climate change may have the potential to disrupt this stability. Although temperatures are clearly increasing in the Kangerlussuaq region (Hanna and others 2012), increasing precipitation trends are currently insignificant due to high interannual variability in precipitation amount (Mernild and others 2015). Elsewhere, experimental warming and rainfall manipulations have resulted in significant changes to biocrust communities, often reducing the lichen coverage and diversity (Ladrón de Guevara and others 2014; Ferrenberg and others 2015). In the Kangerlussuaq region, increased soil moisture may favor the revegetation of deflation patches by vascular plants (Anderson and others 2017), but the future balance between precipitation and evapotranspiration, and the effect of a changing climate on biocrust function and diversity are poorly constrained.
Conclusions
The lichen-rich biocrusts of Kangerlussuaq, Greenland, play an important role in maintaining landscape heterogeneity. First, wind erosion disturbs the soil and plant cover, exposing nutrient-poor loess and glacial till. After this wind-driven soil deflation occurs, biocrusts progressively colonize the exposed surface, taking nearly 200 years to fully develop. As the biocrust develops, the thickness and penetration resistance increase, potentially impeding the germination and establishment of seedlings. Although soil C and N concentrations increase as biocrusts develop, the rate of N enrichment below biocrusts is very slow, and soil C and N concentrations within deflation patches remain an order of magnitude lower than nutrient concentrations outside of deflation patches. Our results suggest that deflation patches, triggered by long-term variations in climate, may remain a stable part of the Kangerlussuaq landscape for hundreds to thousands of years, maintaining a dynamic mosaic of vegetated tundra, bedrock, and biocrusts. However, if future climate change in the Arctic favors greater primary production, and the rate of soil deflation declines, then the productivity of the Kangerlussuaq tundra could increase, favoring a transition to a more vegetated and shrub-dominated landscape.
References
Anderson NJ, Saros JE, Bullard JE, Cahoon SMP, Mcgowan S, Bagshaw EA, Barry CD, Bindler R, Burpee BT, Carrivick JL, Fowler RA, Fox AD, Fritz SC, Giles ME, Hamerlik L, Ingeman-Nielsen T, Law AC, Mernild SH, Northington RM, Osburn CL, Pla-Rabes S, Post E, Telling J, Stroud DA, Whiteford EJ, Yallop ML, Yde JC. 2017. The arctic in the twenty-first century: changing biogeochemical linkages across a paraglacial landscape of Greenland. Bioscience 67:118–33.
Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J. 2016. Patterns and controls on nitrogen cycling of biological soil crusts. In: Weber B, Büdel B, Belnap J, Eds. Biological soil crusts: an organizing principle in drylands. Berlin: Springer. p 257–85.
Beck A, Mayr C. 2012. Nitrogen and carbon isotope variability in the green-algal lichen Xanthoria parietina and their implications on mycobiont–photobiont interactions. Ecol Evol 2:3132–44.
Belnap J, Büdel B. 2016. Biological soil crusts as soil stabilizers. In: Weber B, Büdel B, Belnap J, Eds. Biological soil crusts: an organizing principle in Drylands. Berlin: Springer. p 305–20.
Belnap J, Eldridge DJ. 2003. Disturbance and recovery of biological soil crusts. In: Belnap J, Lange O, Eds. Biological soil crusts: structure, function, and management. Berlin: Springer. p 363–84.
Belnap J, Phillips SL, Witwicki DL, Miller ME. 2008. Visually assessing the level of development and soil surface stability of cyanobacterially dominated biological soil crusts. J Arid Environ 72:1257–64.
Bowker MA. 2007. Biological soil crust rehabilitation in theory and practice: an underexploited opportunity. Restor Ecol 15:13–23.
Bradley-Cook JI, Virginia RA. 2016. Soil carbon storage, respiration potential, and organic matter quality across an age and climate gradient in southwestern Greenland. Polar Biol 39:1283–95.
Brankatschk R, Fischer T, Veste M, Zeyer J. 2013. Succession of N cycling processes in biological soil crusts on a Central European inland dune. FEMS Microbiol Ecol 83:149–60.
Breen K, Levesque E. 2006. Proglacial succession of biological soil crusts and vascular plants: biotic interactions in the High Arctic. Can J Bot Rev Can Bot 84:1714–31.
Breen K, Levesque E. 2008. The influence of biological soil crusts on soil characteristics along a High Arctic glacier foreland, Nunavut, Canada. Arct Antarct Alp Res 40:287–97.
Brodo IM, Sharnoff S, Sharnoff SD, Canadian Museum of Nature. 2001. Lichens of North America. New Haven: Yale University Press.
Bullard JE, Austin MJ. 2011. Dust generation on a proglacial floodplain, West Greenland. Aeolian Res 3:43–54.
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A, Nardoto GB, Pardo LH, Penuelas J, Reich PB, Schuur EAG, Stock WD, Templer PH, Virginia RA, Welker JM, Wright IJ. 2009. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–92.
Deines L, Rosentreter R, Eldridge DJ, Serpe MD. 2007. Germination and seedling establishment of two annual grasses on lichen-dominated biological soil crusts. Plant Soil 295:23–35.
Dijkmans JWA, Törnqvist TE. 1991. Modern periglacial eolian deposits and landforms in the Sondre Stromfjord area, West Greenland and their palaeoenvironmental implications. Meddelelser Om Groenl Geosci 25:1–39.
Ellis C, Crittenden P, Scrimgeour C, Ashcroft C. 2003. The natural abundance of N-15 in mat-forming lichens. Oecologia 136:115–23.
Evans R, Ehleringer J. 1993. A break in the nitrogen-cycle in aridlands: evidence from delta-N-15 of soils. Oecologia 94:314–17.
Evans R, Lange O. 2003. Biological soil crusts and ecosystem carbon and nitrogen dynamics. In: Belnap J, Lange O, Eds. Biological soil crusts: structure, function, and management. Berlin: Springer. p 263–80.
Ferrenberg S, Reed SC, Belnap J. 2015. Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc Natl Acad Sci 112:12116–21.
Forman SL, Marín L, Van DV, Tremper C, Csatho B. 2007. Little ice age and neoglacial landforms at the Inland Ice margin, Isunguata Sermia, Kangerlussuaq, west Greenland. Boreas 36:341–51.
Guo Y, Zhao H, Zuo X, Drake S, Zhao X. 2008. Biological soil crust development and its topsoil properties in the process of dune stabilization, Inner Mongolia, China. Environ Geol 54:653–62.
Hanna E, Mernild SH, Cappelen J, Steffen K. 2012. Recent warming in Greenland in a long-term instrumental (1881–2012) climatic context: I. Evaluation of surface air temperature records. Environ Res Lett 7:045404.
Hansen E. 2000. A contribution to the lichen flora of the Kangerlussuaq area, West Greenland. Cryptogam Mycol 21:53–9.
Hansen ES. 2001. Lichen-rich soil crusts of Arctic Greenland. In: Belnap J, Lange OL, Eds. Biological Soil Crusts: Structure, Function, and Management. Berlin: Springer. pp 57–65.
Heindel RC, Chipman JW, Dietrich JT, Virginia RA. 2018. Quantifying rates of soil deflation with Structure-from-Motion photogrammetry in west Greenland. Arct Antarct Alp Res 50:SI00012.
Heindel RC, Chipman JW, Virginia RA. 2015. The spatial distribution and ecological impacts of Aeolian soil Erosion in Kangerlussuaq, West Greenland. Ann Assoc Am Geogr 105:875–90.
Heindel RC, Culler LE, Virginia RA. 2017a. Rates and processes of aeolian soil erosion in West Greenland. The Holocene 27:1281–90.
Heindel RC, Culler LE, Virginia RA. 2017b. Data from: rates and processes of aeolian soil erosion in West Greenland. Dryad Digital Repository. https://doi.org/10.5061/dryad.v82g6.
Kobylinski A, Fredeen AL. 2015. Importance of arboreal cyanolichen abundance to nitrogen cycling in sub-boreal spruce and fir forests of Central British Columbia, Canada. Forests 6:2588–607.
Kopec BG, Lauder AM, Posmentier ES, Feng X. 2014. The diel cycle of water vapor in west Greenland. J Geophys Res-Atmospheres 119:9386–99.
Ladrón de Guevara M, Lázaro R, Quero JL, Ochoa V, Gozalo B, Berdugo M, Uclés O, Escolar C, Maestre FT. 2014. Simulated climate change reduced the capacity of lichen-dominated biocrusts to act as carbon sinks in two semi-arid Mediterranean ecosystems. Biodivers Conserv 23:1787–807.
Langhans TM, Storm C, Schwabe A. 2009. Biological soil crusts and their microenvironment: impact on emergence, survival and establishment of seedlings. Flora 204:157–68.
Levy LB, Kelly MA, Howley JA, Virginia RA. 2012. Age of the Ørkendalen moraines, Kangerlussuaq, Greenland: constraints on the extent of the southwestern margin of the Greenland Ice Sheet during the Holocene. Quat Sci Rev 52:1–5.
Line M. 1992. Nitrogen-fixation in the sub-Antarctic Macquarie Island. Polar Biol 11:601–6.
Mernild SH, Hanna E, McConnell JR, Sigl M, Beckerman AP, Yde JC, Cappelen J, Malmros JK, Steffen K. 2015. Greenland precipitation trends in a long-term instrumental climate context (1890–2012): evaluation of coastal and ice core records. Int J Climatol 35:303–20.
Oulehle F, Rowe EC, Myska O, Chuman T, Evans CD. 2016. Plant functional type affects nitrogen use efficiency in high-Arctic tundra. Soil Biol Biochem 94:19–28.
Rousk K, Sorensen PL, Michelsen A. 2016. Nitrogen transfer from four nitrogen-fixer associations to plants and soils. Ecosystems 19:1491–504.
Russow R, Veste M, Bohme F. 2005. A natural N-15 approach to determine the biological fixation of atmospheric nitrogen by biological soil crusts of the Negev Desert. Rapid Commun Mass Spectrom 19:3451–6.
Sancho LG, Belnap J, Colesie C, Raggio J, Weber B. 2016. Carbon budgets of biological soil crusts at micro-, meso-, and global scales. In: Weber B, Büdel B, Belnap J, Eds. Biological soil crusts: an organizing principle in drylands. Berlin: Springer. p 287–304.
Shearer G, Kohl D. 1986. N-2-fixation in field settings—estimations based on natural N-15 abundance. Aust J Plant Physiol 13:699–756.
Stewart KJ, Coxson D, Grogan P. 2011a. Nitrogen inputs by associative cyanobacteria across a low Arctic Tundra landscape. Arct Antarct Alp Res 43:267–78.
Stewart KJ, Lamb EG, Coxson DS, Siciliano SD. 2011b. Bryophyte-cyanobacterial associations as a key factor in N-2-fixation across the Canadian Arctic. Plant Soil 344:335–46.
Ten Brink NW, Weidick A. 1974. Greenland ice sheet history since the last glaciation. Quat Res 4:429–40.
Thomas AD, Dougill AJ. 2007. Spatial and temporal distribution of cyanobacterial soil crusts in the Kalahari: implications for soil surface properties. Geomorphology 85:17–29.
Urbanowicz C, Virginia RA, Irwin RE. 2017. The response of pollen-transport networks to landscape-scale climate variation. Polar Biol 40:2253–63.
Weber B, Bowker MA, Zhang Y, Belnap J. 2016a. Natural recovery of biological soil crusts after disturbance. In: Weber B, Büdel B, Belnap J, Eds. Biological soil crusts: an organizing principle in drylands. Berlin: Springer. p 479–98.
Weber B, Büdel B, Belnap J. 2016b. Biological soil crusts: an organizing principle in Drylands. Berlin: Springer.
Yoshitake S, Uchida M, Koizumi H, Kanda H, Nakatsubo T. 2010. Production of biological soil crusts in the early stage of primary succession on a High Arctic glacier foreland. New Phytol 186:451–60.
Zhang Y, Nie H. 2011. Effects of biological soil crusts on seedling growth and element uptake in five desert plants in Junggar Basin, western China. Chin J Plant Ecol 35:380–8.
Acknowledgements
We thank Robbie Score and the staff of CH2M Hill Polar Field Services for their logistics and field support in Kangerlussuaq, Greenland. Becca Novello and Phoebe Racine helped collect samples, Kristin Winkle processed soil samples with funding through the Dartmouth Women in Science Project (WISP), Paul Zietz (Dartmouth Environmental Measurements Lab) conducted the total C and N analyses, and Lee McDavid (Dartmouth Institute of Arctic Studies) provided administrative support. A special thanks to Troy McMullin and Irwin Brodo at the Canadian Museum of Nature for lichen species identification. This work was supported by the National Science Foundation Office of Polar Programs [grant numbers 0801490 and 1506155 to RAV]. FCG was funded by a Sophomore Science Scholarship through Dartmouth Undergraduate Advising & Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
RCH, AMS, and RAV designed the study. FCG and AMS developed methods. RCH, FCG, and AMS collected data. RCH analyzed data and wrote early drafts of the manuscript. All authors contributed significantly to manuscript revisions.
Rights and permissions
About this article
Cite this article
Heindel, R.C., Governali, F.C., Spickard, A.M. et al. The Role of Biological Soil Crusts in Nitrogen Cycling and Soil Stabilization in Kangerlussuaq, West Greenland. Ecosystems 22, 243–256 (2019). https://doi.org/10.1007/s10021-018-0267-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-018-0267-8