Abstract
The importance of biocrusts in the ecology of arid lands across all continents is widely recognized. In spite of this broad distribution, contributions of biocrusts to the global biogeochemical cycles have only recently been considered. While these studies opened a new view on the global role of biocrusts, they also clearly revealed the lack of data for many habitats and of overall standards for measurements and analysis. In order to understand carbon cycling in biocrusts and the progress which has been made during the last 15 years, we offer a multiscale approach covering different climatic regions. We also include a discussion on available measurement techniques at each scale: A microscale section focuses on the individual organism level, including modeling based on the combination of field and lab data. The mesoscale section addresses the CO2 exchange of a complete ecosystem or at the community level. Finally, we consider the contribution of biocrusts at a global scale, giving a general perspective of the most relevant findings regarding the role of biological soil crusts in the global terrestrial carbon cycle.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Biological soil crusts (biocrusts) inhabit extensive parts of different terrestrial ecosystems and are especially abundant under conditions where vascular plants encounter natural limits of growth (Belnap and Lange 2003). Due to their poikilohydric character, biocrusts show resilience under extreme conditions, as well as a remarkable adaptation to the various combinations of different climatic factors found throughout all latitudes from the tropics to the poles (Grote et al. 2010; Colesie et al. 2014). Because biocrusts can fix carbon (C) through photosynthetic activity, they are important in local scale C cycles. In addition, due to their broad distribution, they can also be relevant on ecosystem and global scales (Elbert et al. 2012; Porada et al. 2013, 2014).
In this chapter, several different terms are used when considering CO2 balance. Net photosynthesis (NP) and dark respiration (DR), respectively, refer to the uptake and release of CO2 measured with CO2 gas exchange systems. CO2 gas exchange measurements under controlled conditions allow the identification of cardinal points for photosynthesis (e.g., temperature optimum, light saturation, optimum water content). In long-term observations, an important value is the net primary production (NPP), which is the difference between the gross primary production (GPP) and the autotrophic respiration (AR; Chapin et al. 2006). A further highly relevant variable is net ecosystem production (NEP), which is the difference between gross primary production (GPP) and ecosystem respiration (ER; Randerson et al. 2002). Similar values, net ecosystem carbon balance (NECB) and net ecosystem exchange (NEE), are the net rate of carbon accumulation in (or loss from) ecosystems. NECB can be determined by measuring changes in carbon stocks in vegetation and soil. NECB and NEE differ from NEP if inorganic carbon enters or leaves the system in dissolved form or if fluxes other than C fixation and respiration occur. In addition, we use the term net soil exchange (NSE) for chambers that include only bare or biocrusted soil surfaces and exclude vascular plants.
It must also be remembered that in arid soils, biological activity is not the only driver of CO2 exchange. It has been recently reported that inorganic CO2 fluxes in alkaline and saline substrates can exceed those caused by organic activity (Xie et al 2009; Shanhun et al. 2012; Ma et al. 2013). Before producing an accurate approach to the CO2 balance in arid lands, it is therefore necessary to determine whether inorganic activity in calcareous soils needs to be considered as a sink or a source of carbon, and the abiotic conditions need to be specified. On the other hand, in the most extreme habitats, photosynthetic activity in the soil is mainly restricted to the especially protected microhabitat that occurs under semitransparent pebbles, the so-called hypolithic communities, which are extensively considered in Chap. 11 by Pointing.
In the following sections, we use a multiscale approach to discuss the usefulness of available measuring techniques and methods calculating the CO2 balance of biocrust communities. In the first section, we focus on the microscale, that is, the individual organism such as a single lichen or bryophyte thallus or a well-defined cyanobacteria colony. We then address CO2 exchange of a complete ecosystem or at the community level (mesoscale). Finally, we will look at recent approaches to assess the contribution of biocrusts on a global scale.
2 The Microscale: Individual Organism Performance
2.1 Proposed Methodologies on the Microscale
Direct Measurement of CO2 Exchange
Quantitative measurements of CO2 fluxes in biocrust organisms are feasible using CO2 gas exchange systems. The major advantage of CO2 exchange measurements is that they can provide both accurate response curves of NP to environmental factors, as well as actual exchange rates in the field under quasi-natural conditions. On the other hand, a major disadvantage is that data are not provided for CO2 uptake nor do they discriminate between abiotic and biotic CO2 production.
Lange et al. (1997a) developed an automatically operating cuvette with which they measured quantitative CO2 exchange in lichens. A major limitation to the use of these systems is that they are expensive, only quasi-automatic (i.e., they need regular, often daily checking), and usually only a small number of samples (often only one) can be measured. Many other studies on quantitative CO2 exchange measurements are available, but mainly cover only short time periods. Nevertheless, these are often used for calculations and modeling of biocrust C cycling. Lange (2003a) intensively discusses the differences between different extrapolation methods and also the difficulties in upscaling fixation rates to yearly averages. Bader et al. (2010) use the same dataset to show that lower sampling frequencies both within the day and on less than 365 days of the year cause deviations in modeling results and propose supporting extrapolation approaches.
Indirect Measurements Based on Activity Detection
Fluorometers, which detect fluorescence of chlorophyll a, provide a method to record the activity time of poikilohydric photosynthetic organisms (Schroeter et al. 1992; Schlensog and Schroeter 2001). Recent innovations allow the establishment of long-term chlorophyll fluorescence monitoring systems, which are online and report via telephone or satellites (Büdel et al. 2014; Raggio et al. 2014). The systems not only provide an excellent background on activity periods of the organisms in situ but often also allow determination of the mode of activation (humid air, dew, or rain) to be identified. Such data are necessary for any calculations of productivity over time (Fig. 15.1). Recently, a biocrust wetness sensor was developed, which allows determination of the activity status and water content of biocrusts by means of electrical conductivity measurements (Weber et al. 2016). As these sensors are inexpensive, robust, and easy to use, they can be employed for long-term measurements. Installation of multiple sensors in the field allows assessment of spatiotemporal variability in biocrust C exchange under field conditions. However, these methods do not provide quantitative data on CO2 uptake or loss. Therefore, in order to obtain measures such as NPP, these methods must be combined with CO2 gas exchange measurements under varying environmental conditions that can account for the relationship between chlorophyll fluorescence or water content and CO2 exchange in lichens, algae, and bryophytes. Nevertheless, both techniques have an unquestionable advantage of allowing for unattended long-term noninvasive monitoring of metabolic activity.
Summary of the activity detected as fluorescence of chlorophyll a and microclimate measured for Diploschistes diacapsis assessed in continuous measurements over a period of 2 years, 2006 and 2007, in Almeria (Spain). (a) Lichen activity in number of hours active per month: gray upper part of bar indicates fluorescence activity under photosynthetic active radiation and black lower part indicates fluorescence activity in the dark. (b) Sum of electrons transported per month (mol electrons m−2 month−1). (c) Mean PPFD (μmol photons m−2 s−1) for each month: white bars indicate mean value of the complete month and gray bars indicate the mean value for times when the lichen was active. (d) Mean relative humidity next to the lichen at the soil surface in each month (%): white bars indicate the mean RH for the entire month; the total bar is the mean value of times when the lichen was active. (e) Mean lichen temperature of all measurements in each month (solid line) and for the periods when the lichens were active (dashed line, missing data indicates no activity in those months)
Direct Measurement of Mass Gain
A relatively easy way to relate microscale CO2 measurements to the productivity of lichen- or moss-dominated systems (i.e., the NECB) is to use lichenometric data as a baseline to calculate mass gain over a selected time interval. Lichenometry has been widely used to date surfaces, mainly in studies on glacier retreat in both hemispheres (Innes 1988; Winchester and Harrison 2000; Roberts et al. 2010), or to calculate the growth rate of a thallus by direct measurements at time intervals (Armstrong and Bradwell 2010; Sancho and Pintado 2004; Sancho et al. 2011). Annual thallus enlargement measured in lichenometric studies can be transformed to thallus weight gain after determining the thallus weight per unit area (Raggio et al. 2012). The obtained annual C gains are fully comparable across different ecosystems in the world and improve our understanding of the contribution by lichens, especially epilithic communities, to the global C balance (Table 15.1). Theoretically, a combination of high quality digital photographs, appropriate image analysis programs, and an accurate determination of thallus weight on a surface basis could allow long-term productivity monitoring of crustose lichens without using any expensive and complicated systems.
Modeling Methods
Modeling is used to estimate annual C budgets. Continuous cuvette measurements, providing data over more than a year, as conducted by Lange (2000, 2003a, b), are an exception. Most commonly, a series of field measurements of CO2 gas exchange is connected with the concurrent environmental factors. Different types of algorithms or models are then used to link field and laboratory measurements with long-term records of those same environmental factors. The methodological approach is to combine CO2 gas exchange rates assessed during typical activity days (see Lange and Green 2004) with records of long-term chlorophyll a fluorescence and microclimate data (at least one continuous year). These measurements are conducted for the most abundant biocrust types of each area. This approach minimizes theoretical assumptions and extrapolations in the modeling. Several important ecophysiological phenomena in biocrusts have been shown to influence these modeled estimates of CO2 gas exchange, including depression of photosynthesis at high water contents (Lange et al. 1995; Fig. 15.2) and more frequent than expected biocrust activity at suboptimal light and temperature conditions in the field (Lange and Green 2004). As these phenomena are relevant for productivity calculations, they also need to be analyzed in detail by means of constructing gas exchange estimates at different light and water values in the laboratory. Other factors that have to be taken into account during modeling are the biotic and abiotic CO2 fluxes from the soil (Ma et al. 2013; Rey et al. 2014; Weber et al. 2012). These are potentially a major problem in modeling biocrust-derived C fluxes. The large differences between modeled respiration (see Castillo-Monroy et al. 2011) and NPP for desert biocrusts shows that the calculated NPP will be very sensitive to non-biocrust-derived C. However, the literature indicates that abiotic fluxes from soil carbonates, the obvious potential source of C flux from soils, can be very low (Serna-Perez et al. 2006; although see Rey 2015). A combination of the datasets accumulated in the SCIN project (Büdel et al. 2014) with the current knowledge regarding CO2 fluxes in biocrusts from arid and semiarid environments will allow modeling the behavior of these communities in a global change scenario (Fig. 15.3).
Dependence of net photosynthesis (NP, % of maximum) and thallus water content (WC, % of maximum) in different soil crust lichen species collected in the same locality. Different responses of NP are observed at maximum WC (100 %), from high depression of NP at high WC (c, d) to low (b) or nonexisting depression (a). Modified from Lange et al. (1995)
Some other approaches do not produce models for final C balance, but provide useful datasets for future modeling because they contain empirical measurements of gas exchange at the microscale (field and/or laboratory datasets). Lange (2003a) showed that the annual C balance of the lichen approximates its annual increase in biomass, stating that the potential annual primary production of lichen-dominated systems reaches around 8 % of the global mean for terrestrial productivity. Weber et al. (2012) published relevant CO2 fluxes in mosses from a semidesert in South Africa, underlining in their results the physiological importance of measuring complete biocrusts against measuring isolated components of them (see also Sommerkorn et al. 1999). Finally, some works link CO2 fluxes at the biocrust microscale level with future climate change scenarios (Grote et al. 2010; Maestre et al. 2010, 2013). These works provide large datasets that will help to understand possible consequences of environmental change for C balances in arid or semiarid environments where biocrusts are abundant.
2.2 Available Datasets on the Microscale
Tundra
In tundra habitats, poikilohydric organisms form a major part of the overall vegetation. Here, annual C budgets have been estimated. Maximum photosynthesis rates for lichen species in tundra vegetation range from 0.8 nmol g−1 s−1 for Sphaerophorus globosus to 8.0 nmol g−1 s−1 for Dactylina arctica (Tenhunen et al. 1992; Table 15.2). Lichens dominate biocrust communities in well-drained evergreen shrub vegetation as well as in rocky fell-fields and contribute up to 400 g of photosynthetically active biomass m−2 (Tenhunen et al. 1992). Along a gradient of increasing water availability, there is a transition from lichen-dominated biocrusts to those dominated by mosses. The NP rates of typical tundra mosses are about three times higher than those of lichens [e.g., maximum NP for Pogonatum alpinum is 27.8 nmol g−1 s−1 (Oechel and Collins 1976) and Sphagnum squarrosum is 18.9 nmol g−1 s−1 (Murray et al. 1989)]. The relatively high NP rates and the generally moist conditions lead to substantial estimates for annual C fixation rates in tundra areas, where particularly bryophytes are important in C and N cycling (Turetsky 2003). For example, estimated NPP of feather mosses in the boreal forests reach about 24.80 g C m−2 year−1 (Bisbee et al. 2001), and in wet areas Sphagnum species are also very productive with up to 19 g biomass m−2 year−1. Fenton (1980) estimated that mean productivity of Antarctic Polytrichum alpestre ranges from 213 to 350 g m−2 year−1, while productivity of Chorisodontium aciphyllum averages 162 g m−2 year−1.
Temperate Habitats
In temperate regions , there has only been one study addressing annual C budgets for individual biocrust lichens. Lange (2002, 2003a) measured Lecanora muralis using the Klapp cuvette for 15 months and determined its annual C fixation rate to be 21.494 g C m−2 year−1 (Lange 2002, 2003a). Lange (2000) also used this system on the gelatinous lichen Collema cristatum to show very good agreement between NP responses measured in the laboratory and field.
Deserts
In deserts , due to restricted water availability, organisms are only sporadically active (Lange et al. 2006; Sponseller 2007; Pintado et al. 2010), causing NPP to be generally much lower than in temperate or polar regions, but as higher plants are sparse, biocrust cover can be very high and thus still play an important role in overall C cycles. However, as biocrust organisms are only metabolically active when wet and precipitation in deserts is highly variable, estimates for NPP of biocrust components are highly variable as well, ranging between 0 and 29 g m−2 year−1 (Table 15.2). In general, biocrusts in temperate deserts are more easily damaged, and their carbon fixation reduced, by compressional disturbances than those in tundra habitats. In addition, as temperatures are higher in deserts, moisture evaporates more quickly than in tundra, and thus their overall photosynthetic gain is less. However, biocrusts can access a range of water sources, many of them not being available to higher plants. Some desert lichens (e.g., Acarospora cf. schleicheri , Caloplaca volkii, and Lecidella crystallina ) can mainly use fog to reactivate their photosynthesis (Lange et al. 1994) and reach an estimated photosynthetic carbon gain of 16 g C m−2 year−1. Not only scarcity of water affects C cycles in biocrusts but also surplus water with contents above 60 % generally results in a decline in GP in many biocrust species, probably caused by water-filled pores restricting gas diffusion (Grote et al. 2010; Lange et al. 1997b; Green et al. 1994).
The type of biocrust organism also plays an important role in C fixation at a site. On the Colorado Plateau and in the Chihuahuan Desert, Housman et al. (2006) showed that later successional biocrusts dominated by the cyanobacteria Nostoc and Scytonema and the lichens Placidium and Collema typically had a 1.2–1.3-fold higher daily C fixation than early cyanobacterial crusts dominated by the cyanobacterial genus Microcoleus . Biocrusts dominated by Microcoleus vaginatus growing on dunes are estimated to fix 11.7 g C m−2 year−1 (Brostoff et al. 2005). Early successional biocrusts from highly disturbed areas , dominated by algae or cyanobacteria, show lower carbon fixation values than those from undisturbed sites where lichens and mosses occur (Table 15.2; Zaady et al. 2000), being in agreement with the photosynthetic capacities found for the individual biocrust components.
2.3 Modeling on the Microscale
Castillo-Monroy et al. (2011) proposed that biocrusts in a semiarid ecosystem in Spain were responsible for the greatest proportion of the total soil respiration. Their model was based on the assumption that 85 % of soil respiration could be explained by soil temperature and moisture and predicted a C release between 240.4 and 322.6 g C m−2 year−1. Wilske et al. (2009) used a model based on vascular plant studies but adapted that to work only when there is water available in the ecosystem. The model is sustained by (a) the link between biocrust CO2 fluxes and an indicator of annual metabolic activity and (b) a more simplistic precipitation-driven alternative. The final results showed a large interannual variation in C uptake for biocrust NEE ranging between 0.7 and 5.1 g m−2 year−1. An alternative approach is the algorithm proposed by Brostoff et al. (2005), where the NPP is calculated as the difference between maximum NP and maximum DR measured in the field multiplied by the percentage of cover and meteorological indicators of possible biocrust metabolic activity. Using this method, the NPP of biocrusts in the Mojave Desert was 11.7 g C m−2 year−1 and between 3.46 and 6.05 g C m−2 year−1 for different biocrust types in a Chinese Desert (Feng et al. 2014). Finally, Uchida et al. (2006) developed a model for the dominant terricolous lichen Cetrariella delisei in deglaciated areas of the high Arctic. In their productivity model, they obtained a mean NPP value of 5.1 g dw m−2 year−1 (about 2295 mg C m−2) per snow free season (Table 15.1). They based their model on the interaction between abiotic factors and CO2 exchange, always assuming in their algorithms that NP and DR are firstly dependent on water availability and secondly on temperature and radiation. Water is often available under a suboptimal combination of environmental factors, typically low radiation and low temperature, which usually limits biocrust NP in the field (Lange and Green 2004; Raggio et al. 2014). This is considered to be a reliable approach to model NPP of cryptogams (Lange 2003a, b).
3 The Mesoscale: Net Ecosystem Exchange in Biocrusted Soils
3.1 Available Datasets on the Mesoscale
Continuous long-term measurements of net ecosystem C exchange (NEE) in biocrust-dominated soils are only available for two desert regions.
Colorado Plateau, USA
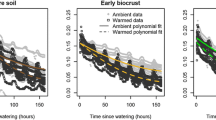
The first study region is the Colorado Plateau Desert, USA, where there have been three studies on two different soil types. These studies, despite using different measurement techniques and on different soils, obtained very similar results. The first study used large auto-chambers with an internal diameter of 0.36 m2 and contain dark, late-successional biocrusts consisting of ~10 % moss (mostly Syntrichia caninervis ), ~5 % lichen (Collema tenax and Collema coccophorum ), and 85 % cyanobacteria (dominated by Microcoleus vaginatus ) (Darrouzet-Nardi et al. 2015). Measurements were made hourly for 21 months (from winter 2006 through summer 2007). Except following large rain events (ideal conditions for photosynthesis), the soil surface acted as a small C source to the atmosphere. The highest C losses were observed in spring (327 mg C m−2 day−1) and the lowest in winter (65 mg C m−2 day−1). Of the 627 measurement days, only 6.4 % showed a positive NSE. These data indicated that only larger or extended events kept soils wet long enough for the biocrust activity to result in uptake outweighing losses, whereas smaller events resulted in net C loss.
The second study on the Colorado Plateau occurred on fine sandy loam soils at a site 120 km from the first site, using the same auto-chamber design as above (Fig. 15.4; Bowling et al. 2011). Measurements were done over the course of 300 days (Julian 0–299), although gaps occurred during that time due to equipment malfunction. Soils were covered with a biocrust dominated by the cyanobacterium Microcoleus vaginatus with some Bryum argenteum moss present as well. Results were very similar to the first study. During the measurement time, only large or almost contiguous smaller rain events resulted in small C uptake (414.7 mg C m−2 day−1) by the biocrusted soils (Fig 15.4). As these events were rare, the soil surfaces were mostly a small C source (up to 985.0 mg C m−2 day−1) over the course of the experiment.
The third study was at the same site as the second study, but measurements were done using eddy covariance (Bowling et al. 2010). This study further corroborated the results obtained in the first two studies: Biocrust soil surfaces at these sites were mostly small sources of C (up to 518 mg C m−2 day−1), with large rain events resulting in small C uptake (up to 933 mg C m−2 day−1). However, there was a sparse cover of vascular plants at this site, and thus these measurements also reflect their influence on C fluxes.
Gurbantunggut Desert
The third desert is the Gurbantunggut Desert in northwestern China. Su et al. (2012, 2013) measured C fluxes of different soils dominated by various types of biocrusts (moss, lichen, and cyanobacteria). As in the Colorado Plateau studies, the results show small C losses from the soil during most of the time, with only short intervals of small C uptake. The similarity in magnitude of the losses and uptake compared to the Colorado Plateau data is striking [−2281 to 1244 mg C m−2 day−1 for the Gurbantunggut Desert (all biocrust and soil types) and −623 to 1660 mg C m−2 day−1 for the Colorado Plateau Desert].
It is unfortunate that we have so few continuous measurements of NEE for biocrusts, as these few studies strongly suggest that spot measures, whether in the laboratory or field, are not sufficient when trying to understand the role of biocrusts in ecosystem C dynamics. Interpretation of the data from any of these studies is complicated by the fact that it is currently not feasible to separate out the role of biocrusts from that of underlying soil factors, such as respiration from roots, deeper biota, and abiotic losses, as well as effects from the somewhat artificial environment created by our measurement techniques. Despite this complication, the similarity of the values obtained from the two studies at geographically distinct sites indicates that the overall photosynthetic contribution of biocrusts in temperate deserts can mostly offset soil abiotic and biotic losses.
4 The Macroscale: Global Carbon Budgets
Two different studies have investigated the contributions of cryptogams to carbon budgets on a global scale (Elbert et al. 2012; Porada et al. 2013). In the first study, Elbert et al. (2012) compiled the available data from more than 200 studies on the carbon fixation rates of cryptogamic organisms occurring on soil, rocks, and leaves of plants. In most cases, short-time measurements with net photosynthesis rates under optimum conditions had been conducted. These values were scaled to annual NPP rates and assigned to major ecosystems (desert, extratropical forest, steppe, tropical forest, and tundra) to obtain mean NPP values, which were then extrapolated to a global scale . Considering only biocrusts occurring under more arid conditions, comparably high flux rates were obtained for steppe ecosystems (16.0 g C m−2 year−1) compared to deserts (5.2 g C m−2 year−1).
Based on their calculations, Elbert et al. (2012) estimated C uptake in these cryptogamic covers to be ~3.9 Pg C year−1, corresponding to ~7 % of the net primary production of terrestrial vegetation. When only cryptogamic ground covers are considered, annual C uptake was estimated at ~2.4 Pg C year−1 (Fig. 15.5). When restricted to biocrusts, uptake values of ~0.6 Pg C year−1 were obtained. This value is ~1 % of the NPP of terrestrial vegetation (~56 Pg year−1, Zhao et al. 2005), but in the desert ecosystem, biocrusts account for about 9 % of the total NPP (~0.07 Pg year−1 compared to a total NPP of ~0.8 Pg year−1; Zhao et al. 2005; Elbert et al. 2012). As mostly measurements of single cryptogamic organisms were used for these calculations with only few data available on complete communities ( e.g., biocrusts), these results may be higher than balances of complete biocrusts with fungi, bacteria, and archaea involved.
Geographic distribution of atmospheric carbon dioxide fixation by cryptogamic ground covers. The color coding indicates the flux intensity in g m−2 year−1. White areas indicate ecosystems for which no data are available; hashed areas were excluded from global budget calculations (annual mean precipitation < 75 mm year−1, and desert areas designated as dune sand/shifting sands and rock outcrops)
In this first upscaling approach, several assumptions were made to facilitate an upscaling from short-term measurements to a long-term global scale. In order to come up with long-term data of higher accuracy, the natural cycles of hydration and dehydration and the resulting productivity have to be followed ideally over several years. This knowledge can greatly improve the understanding of seasonal and interannual variability of CO2 fluxes and allow the development of strong predictive models to assess the behavior of biocrusts in a global change scenario.
In a second approach, Porada et al. (2013) used a process-based model to estimate the global C uptake by lichens and bryophytes across all habitats. In this “Lichen and Bryophyte Simulator,” the organisms are described by a reservoir approach that consists of pools of biomass, sugar reserves, water, and internal CO2 concentration. These pools are changed by input and output flows of C and/or water that are driven by climatic factors and the biotic environment (e.g., location relative to the surrounding vegetation). Utilizing this model, the authors predict a global terrestrial NPP ranging between 0.34 (average method) and 3.3 Gt C year−1 (maximum weighing method) by lichens and bryophytes. This is the first approach analyzing the NPP of cryptogams based on a photosynthesis model. Thus, it still contains uncertainties, as for some physiological parameters, little data are available for lichens and mosses. Also, C losses have not been considered and thus NEE/NSE may be very small.
The global patterns of the ground-based NPP rates calculated by Porada et al. (2013) generally agree with those of Elbert et al. (2012), with the highest NPP values in both studies being reached in extratropical forests and the lowest (zero) in the Sahara Desert, due to the lack of water (Fig. 15.5). The overall values, however, are smaller in the study of Porada et al. (2013) compared to Elbert et al. (2012). This is possibly, in part, because cyanobacteria and algae were not taken into account in the study, despite their extensive global cover. Nevertheless, this approach allows completely new insights into the global relevance of cryptogamic organisms in C cycles. In addition, Porada et al. (2014) also used this model to calculate the potential N fixation, phosphorus uptake, and chemical weathering by lichens and bryophytes. Investigating global estimates, one has to keep in mind that biocrusts may also indirectly enhance CO2 uptake by plants through cyanobacterial nitrogen enrichment of the soil (Belnap 2002; Elbert et al. 2012).
5 General Future Research Needs and Directions
Despite the potential importance of biocrusts in C cycles at all scales, we have little quantitative data by which to understand their role. Using common protocols, we need to better document how biological (e.g., species composition, organism condition) and physical factors (e.g., activity rates and times as determined by climatic factors, soils) influence C fixation and loss. Across all scales, we need to better understand observed NPP/NSE rates: that is, what portion of the measured NSE is due to biocrust photosynthesis and respiration, and what portion of C losses is due to other sources, such as bacteria, fungi, and soil carbonates? At the microscale, we need more studies on how biological and environmental factors interact to determine photosynthesis and respiration. At the mesoscale level, only eddy covariance and flux towers provide real estimation of CO2 fluxes of whole ecosystems. However, these studies are still very rare in biocrust communities, thus seriously limiting our understanding of the role of biocrusts in landscape or larger-scale CO2 balances and budgets. For this reason, we suggest more such installations, with care paid to standardize protocols and measurements. In addition, because eddy covariance offers direct measurements of C fluxes, these studies could be linked to the modeled outputs at the microscale, giving an opportunity for cross-validation. A validated model could allow predicting the influence of environmental change on C cycling across all scales.
References
Armstrong RA, Bradwell T (2010) Growth of crustose lichens: a review. Geogr Ann A 92:3–17
Bader MY, Zotz G, Lange OL (2010) How to minimize the sampling effort for obtaining reliable estimates of diel and annual CO2 budgets in lichens. Lichenologist 42:97–111
Belnap J (2002) Nitrogen fixation in biological soil crust from southeast Utah, USA. Biol Fertil Soils 35:128–135
Belnap J, Lange OL (eds) (2003) Biological soil crusts: structure, function and management, vol 150, 2nd edn, Ecological Studies. Springer, New York
Billings WD (1987) Carbon balance of Alaskan tundra and taiga ecosystems: past, present and future. Quat Sci Rev 6:165–177
Bisbee KE, Gower ST, Norman JM, Nordheim EV (2001) Environmental controls on ground cover species composition and productivity in a boreal black spruce forest. Oecologia 129:261–270
Bowling DR, Bethers-Marchetti S, Lunch CK, Grote EE, Belnap J (2010) Carbon, water, and energy fluxes in a semiarid cold desert grassland during and following multiyear drought. J Geophys Res 115:G04026. doi:10.1029/2010JG001322
Bowling DR, Grote EE, Belnap J (2011) Rain pulse response of soil CO2 exchange by biological soil crusts and grasslands of the semiarid Colorado Plateau, United States. J Geophys Res 116:G04006. doi:10.1029/2011JG001722
Brostoff WN, Sharifi MR, Rundel PW (2005) Photosynthesis of cryptobiotic soil crusts in a seasonally inundated system of pans and dunes in the western Mojave Desert, CA: field studies. Flora 200:592–600
Büdel B, Colesie C, Green TGA, Grube M, Lazaro-Suau R, Loewen-Schneider K, Maier S, Peer T, Pintado A, Raggio J, Ruprecht U, Sancho L, Schroeter B, Türk R, Weber B, Wedin M, Westberg M, Williams L, Zheng L (2014) Improved appreciation of the functioning and importance of biological soil crusts in Europe—the Soil Crust International project (SCIN). Biodivers Conserv 23:1639–1658
Castillo-Monroy AP, Maestre FT, Rey A, Soliveres S, García-Palacios P (2011) Biological soil crust microsites are the main contributor to soil respiration in semiarid ecosystem. Ecosystems 18:835–847
Chapin FS III, Woodwell GM, Randerson JT et al (2006) Reconciling carbon-cycling concepts, terminology and methods. Ecosystems 9:1041–1050
Colesie C, Green TGA, Haferkamp I, Büdel B (2014) Habitat stress initiates changes in composition, CO2 gas exchange and C-allocation as life traits in biological soil crusts. ISME J 8:2104–2115
Coxson DS, Marsh J (2001) Lichen chronosequences (postfire and postharvest) in lodgepole pine (Pinus contorta) forests of northern interior British Columbia. Can J Bot 79:1449–1464
Darrouzet-Nardi A, Reed SC, Grote EE, Belnap J (2015) Observations of net soil exchange of CO2 in a dryland show experimental warming increases carbon losses in biocrust soils. Biogeochemistry 126(3):363–378
Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, Pöschl U (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5:459–462
Evans RD, Lange OL (2003) Biological soil crusts and ecosystem nitrogen and carbon dynamics. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function and management, vol 150, 2nd edn, Ecological Studies. Springer, Heidelberg
Feng W, Zhang Y, Wu B, Qin S, Lai Z (2014) Influence of environmental factors on carbon dioxide exchange in biological soil crusts in desert areas. Arid Land Res Manag 28:186–196
Fenton JHC (1980) The rate of peat accumulation in Antarctic moss banks. J Ecol 68:211–228
García-Pichel F, Belnap J (1996) Microenvironments and microscale productivity of cyanobacteria desert crusts. J Phycol 32:774–782
Green TGA, Lange OL, Cowan IR (1994) Ecophysiology of lichen photosynthesis: the role of water status and thallus diffusion resistances. Cryptogam Bot 4:166–178
Grote EE, Belnap J, Housman DC, Sparks JP (2010) Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: implications for global change. Glob Chang Biol 16:2763–2774
Housman DC, Powers HH, Collins AD, Belnap J (2006) Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan desert. J Arid Environ 66:620–634
Innes JL (1988) The use of lichens in dating. In: Galun M (ed) Handbook of lichenology, vol III. CRC Press, Boca Raton, pp 75–92
Jeffries DL, Link SO, Klopatek JM (1993) CO2 fluxes of a cryptogamic crust. Response to resaturation. New Phytol 125:163–173
Klopatek JM (1992) Cryptogamic crusts as potential indicators of disturbance in semi-arid landscapes. In: McKenzie DE, Wyatt E, McDonald VJ (eds) Ecological indicators. Elsevier, New York, pp 773–786
Lange OL (2000) Photosynthetic performance of gelatinous lichen under temperate habitat conditions: long-term monitoring of CO2 exchange of Collema cristatum. Bibl Lichenol 75:307–332
Lange OL (2002) Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation: dependence of photosynthesis on water content, light, temperature, and CO2 concentration from laboratory measurements. Flora 197:233–249
Lange OL (2003a) Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation: III Diel, seasonal and annual carbon budgets. Flora 198:277–292
Lange OL (2003b) Photosynthetic productivity of the epilithic lichen Lecanora muralis: long term field monitoring of CO2 exchange and its physiological interpretation. II. Diel and seasonal patterns of net photosynthesis and respiration. Flora 198:55–70
Lange OL, Green TGA (2004) Photosynthetic performance of the squamulose-soil crust lichen Squamarina lentigera: laboratory measurements and long term monitoring of CO2 exchange in the field. Bibl Lichenol 88:363–390
Lange OL, Kidron GJ, Büdel B, Meyer A, Kilian E, Abeliovich A (1992) Taxonomic composition and the photosynthetic characteristics of the “biological soilcrusts” covering sand dunes in the western Negev Desert. Funct Ecol 6:519–527
Lange OL, Meyer A, Zellner H, Heber U (1994) Photosynthesis and water relations of lichen soil crusts: field measurements in the coastal fog zone of the Namib Desert. Funct Ecol 8:253–264
Lange OL, Reichenberger H, Meyer A (1995) High thallus water content and photosynthetic CO2 exchange of lichens. Laboratory experiments with soil crust species from local xerothermic steppe formations in Franconia, Germany. In: Daniels F, Schulz M, Peine J (eds) Flechten Follmann: contributions to lichenology in honour of Gerhard Follmann. Geobotanical and Phytotaxonomical Study Group, Universitat Koln, Cologne, pp 139–153
Lange OL, Belnap J, Reichenberger H, Meyer A (1997a) Photosynthesis of green algal soil crust lichens from arid lands in southern Utah, USA: role of water content on light and temperature responses of CO2 exchange. Flora 192:1–15
Lange OL, Reichenberger H, Walz H (1997b) Continuous monitoring of CO2 exchange of lichens in the field: short-term enclosure with an automatically operating cuvette. Lichenologist 29:259–274
Lange OL, Hahn S, Meyer A, Tenhunen JD (1998) Upland tundra in the foothills of the Brooks Range, Alaska, USA: lichen long-term photosynthetic CO2 uptake and net carbon gain. Arct Alp Res 3:232–261
Lange OL, Green TGA, Melzer B, Meyer A, Zellner H (2006) Water relations and CO2 exchange of the terrestrial lichen Teloschistes capensis in the Namib fog Desert: measurements during two seasons in the field and under controlled conditions. Flora 16:268–280
Ma J, Wang ZY, Stevenson BA, Zheng XJ, Li Y (2013) An inorganic CO2 diffusion and dissolution process explain negative CO2 fluxes in saline/alkaline soils. Sci Rep 3:2025
Maestre FT, Bowker MA, Escolar C, Puche MD, Soliveres S, Maltez-Mouro S, García-Palacios P, Castillo-Monroy AP, Martínez I, Escudero A (2010) Do biotic interactions modulate ecosystem functioning along stress gradients? Insights from semi-arid plant and biological soil crust communities. Philos Trans R Soc Lond B Biol Sci 365:2057–2070
Maestre FT, Escolar C, Ladrón De Guevara M, Quero JL, Lázaro R, Delgado-Baquerizo M, Ochoa M, Berdugo M, Gozalo B, Gallardo A (2013) Changes in biocrust cover drive carbon cycle responses to climate change in drylands. Glob Chang Biol 19:3835–3847
Murray KJ, Harley PC, Beyers J, Walz H, Tenhunen JD (1989) Water content effects on photosynthetic response of Sphagnum mosses from the foothills of the Philip Smith Mountains, Alaska. Oecologia 79:224–250
Oechel WC, Collins NJ (1976) Comparative CO2 exchange patterns in mosses from two tundra habitats at Barrow, Alaska. Can J Bot 54:1355–1369
Pintado A, Sancho LG, Blanquer JM, Green TGA, Lázaro R (2010) Microclimatic factors and photosynthetic activity of crustose lichens from the semiarid southeast of Spain: long-term measurements for Diploschistes diacapsis. Bibl Lichenol 105:211–224
Porada P, Weber B, Elbert W, Pöschl U, Kleidon A (2013) Estimating global carbon uptake by lichens and bryophytes with a process-based model. Biogeosciences 10:6989–7033
Porada P, Weber B, Elbert W, Pöschl U, Kleidon A (2014) Estimating impacts of lichens and bryophytes on global biogeochemical cycles. Global biogeochemical cycles early view. Glob Biogeochem Cycles 28:71–85
Raggio J, Green TGA, Crittenden PD, Pintado A, Vivas M, Pérez-Ortega S, De los Ríos A, Sancho LG (2012) Comparative ecophysiology of three Placopsis species, pioneer lichens in recently exposed Chilean glacial forelands. Symbiosis 56:55–66
Raggio J, Pintado A, Vivas M, Sancho LG, Büdel B, Colesie C, Weber B, Schroeter B, Green TGA (2014) Continuous chlorophyll fluorescence, gas exchange and microclimate monitoring in a natural soil crust habitat in Tabernas badlands, Almeria, Spain: progressing towards a model to understand productivity. Biodivers Conserv 23:1809–1826
Randerson JT, Chapin FSIII, Harden JW, Neef JC, Harmon ME (2002) Net ecosystem production: a comprehensive measure of net carbon accumulation by ecosystems. Ecol Appl 12:937–947
Rey A (2015) Mind the gap: non-biological processes contributing to soil CO2 efflux. Glob Chang Biol 21:1752–1761
Rey A, Belelli-Marchesini L, Etiope G, Papale D, Canfora E, Valentini R, Pegoraro E (2014) Partitioning the net ecosystem carbon balance of a semiarid steppe into biological and geological components. Biogeochemistry 118:83–101
Roberts SJ, Hodgson DA, Shelley S, Royles J, Griffiths HS, Deen TJ, Thorne MAS (2010) Establishing lichenometric ages for nineteenth- and twentieth-century glacier fluctuations on South Georgia (South Atlantic). Geogr Ann A 92:125–139
Sancho L, Pintado A (2004) Evidence of high annual growth rate for lichens in the maritime Antarctic. Polar Biol 27:312–319
Sancho LG, Palacios D, Green TGA, Vivas M, Pintado A (2011) Extreme high lichens growth rates detected in recently deglaciated areas in Tierra del Fuego. Polar Biol 34:813–822
Schlensog M, Schroeter B (2001) A new method for the accurate in situ monitoring of chlorophyll a fluorescence in lichen and bryophytes. Lichenologist 33:443–452
Schroeter B, Green TGA, Seppekt RD, Kappen L (1992) Monitoring photosynthetic activity of crustose lichens using a PAM-2000 fluorescence system. Oecologia 92:457–465
Schuur EAG, Crummer KG, Vogel JG, Mack MC (2007) Plant species composition and productivity following permafrost thaw and thermokarst in Alaskan tundra. Ecosytems 10:280–292
Serna-Perez A, Monger HC, Herrick JE, Murray L (2006) Carbon dioxide emissions from exhumed petrocalcic horizons. Soil Sci Soc Am J 70:795–805
Shanhun FL, Almond PC, Clough TJ, Smith CMS (2012) Abiotic processes dominate CO2 fluxes in Antarctic soils. Soil Biol Biochem 53:99–111
Shaver GR, Chapin FS (1991) Production: biomass relationships and element cycling in contrasting arctic vegetation types. Ecol Monogr 61:1–31
Sommerkorn M, Bölter M, Kappen L (1999) Carbon dioxide fluxes of soils and mosses in wet tundra of Taimyr Peninsula, Siberia: controlling factors and contribution to net system fluxes. Polar Res 18:253–260
Sponseller RA (2007) Precipitation pulses and soil CO2 efflux in a Sonoran Desert ecosystem. Glob Chang Biol 13:426–436
Su YG, Wu L, Zhang YM (2012) Characteristics of carbon flux in two biologically crusted soils in the Gurbantunggut Desert, Northwestern China. Catena 96:41–48
Su YG, Wu L, Zhou ZB, Zhang YM (2013) Carbon flux in deserts depends on soil cover type: a case study in the Gurbantunggut Desert, North China. Soil Biol Biochem 58:332–340
Tenhunen JD, Lange OL, Hahn S, Siegwolf R, Oberbauer SF (1992) The ecosystem role of poikilohydric tundra plants. In: Chapin FS, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J (eds) Arctic ecosystems in a changing climate. Academic Press, San Diego
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. Bryologist 106:395–409
Uchida M, Muraoka H, Nakatsubo T, Bekku Y, Ueno T, Kanda H, Koizumi H (2002) Net photosynthesis, respiration and production of the moss Sanionia uncinata on a glacier foreland in the high arctic, Ny-Alesund, Svalbard. Arct Antarct Alp Res 34:287–292
Uchida M, Nakatsubo T, Kanda H, Koizumi H (2006) Estimation of the annual primary production of the lichen Cetrariella delisei in a glacier foreland on the high arctic, Ny-Alesund, Svalbard. Polar Res 25:39–49
Weber B, Graf T, Bass M (2012) Ecophysiological analyses of moss-dominated biological soil-crusts and their separate components from the Succulent Karoo, South Africa. Planta 236:129–139
Weber B, Berkemeier T, Ruckteschler N, Caesar J, Heintz H, Ritter H, Braß H (2016) Development and calibration of a novel sensor to analyze the water content of biological soil crusts and surface soils. Methods Ecol Evol. 7(1):14–22. doi:10.1111/2041-210X.12459
Wilske B, Burgheimer J, Maseyk K, Karnieli A, Zaady E, Andreae MO, Yakir D, Kesselmeier J (2009) Modelling the variability in annual carbon fluxes related to biological soil crusts in a Mediterranean shrubland. Biogeosci Discuss 6:7295–7324
Winchester V, Harrison S (2000) Dendrochronology and lichenometry: colonization, growth rates and dating of geomorphological events on the east side of the North Patagonian Icefield, Chile. Geomorphology 34:181–194
Xie J, Li Y, Zhai C, Li C, Lan Z (2009) CO2 absorption by alkaline soils an its implication to the global carbon cycle. Environ Geol 56:953–961
Zaady E, Kuhn U, Wilske B, Sandoval-Soto L, Kesselmeier J (2000) Patterns of CO2 exchange in biological soil crusts of successional stages. Soil Biol Biochem 32:959–966
Zhao MS, Heinsch FA, Nemani RR et al (2005) Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens Environ 95:164–176
Acknowledgments
L.G. Sancho and J. Raggio were supported by the Ministerio de Economía y Competitividad of Spain (projects CTM2012-38222-C01 and SCIN). Bettina Weber was supported by the Max Planck Society (Nobel Laureate Fellowship) and the German Research Foundation (project WE2393/2). We are especially thankful to Prof. T.G. Allan Green (Universidad Complutense Madrid) for advice and support. JB was supported by USGS Ecosystems program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sancho, L.G., Belnap, J., Colesie, C., Raggio, J., Weber, B. (2016). Carbon Budgets of Biological Soil Crusts at Micro-, Meso-, and Global Scales. In: Weber, B., Büdel, B., Belnap, J. (eds) Biological Soil Crusts: An Organizing Principle in Drylands. Ecological Studies, vol 226. Springer, Cham. https://doi.org/10.1007/978-3-319-30214-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-30214-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30212-6
Online ISBN: 978-3-319-30214-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)