Abstract
Bevacizumab (BV), a monoclonal antibody against vascular endothelial growth factor (VEGF), is currently used in the treatment of malignant glioma. To understand mechanisms of resistance to BV, we investigated morphological changes in tumor vessels and expression of angiogenic factors, such as VEGF, Flt-1, basic fibroblast growth factor (bFGF), and platelet-derived growth factor-BB (PDGF-BB), in four autopsied tumors after BV treatment. Three patients had glioblastomas; the fourth had a secondary glioblastoma that developed from a diffuse astrocytoma. BV was administered because of recurrence following the use of the Stupp regimen in these four patients. We compared the initial surgical specimen with that obtained after death following BV treatment. Immunohistochemical staining of the autopsied tumors showed that Flt-1 expression increased while VEGF expression was significantly reduced. Additionally, other angiogenic factors, particularly bFGF, were enhanced. Interestingly, the proliferation of endothelial cells was reduced, but remarkable proliferation of pericytes was observed. These results suggest that following BV treatment, glioblastomas can grow tumor vessels by expressing various angiogenic factors. These mechanisms might be important for rapid regrowth and blood brain barrier repair after BV treatment. Inhibition of multiple angiogenic factors will be required to control tumor vessels in glioblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular endothelial growth factor (VEGF) is highly expressed in many types of cancer cells including high-grade glioma. VEGF binds to its receptor (VEGFR) on vascular endothelial cells, inducing proliferation of these cells and increasing the permeability of tumor vessels [1, 2]. Bevacizumab (BV) is a recombinant anti-VEGF monoclonal antibody that blocks VEGF signal transduction through VEGFR-1 (Flt-1), VEGFR-2, and Neuropilin-1 (NP-1)/NP-2. BV is believed to have an anti-tumor effect by inhibiting neoangiogenesis, and is also believed to suppress edema by reducing the permeability of the tumor vessels [3, 4].
There is no established treatment for recurrent malignant glioma, and its prognosis is poor. In Japan, a phase II study of BV treatment for recurrent malignant gliomas was conducted in 2011 [5]. According to this study, median progression-free survival (PFS) and overall survival (OS) after BV treatment in patients with recurrent glioblastoma were 3.3 and 10.5 months, respectively. These results suggest that BV may extend OS but not PFS. In 2013, BV was approved for both initial and recurrent malignant gliomas in Japan and is now used by many patients with recurrent malignant gliomas. It is known that transient but dramatic reduction of both enhanced lesions and peripheral edemas is achieved by BV treatment, but rapid and uncontrollable tumor recurrence and invasion occur several months after the administration of BV. However, the mechanism of resistance against BV treatment is largely unknown. Actual vascular changes after the administration of BV have rarely been reported in humans or even in mice [6]. Understanding the mechanism of tumor resistance against anti-angiogenic therapy is crucial for the development of new treatment.
In this study, we investigated changes in the vascular structures and expression of angiogenic factors in four autopsied patients with recurrent malignant gliomas treated with BV to clarify the mechanism of BV resistance in such cases.
Materials and methods
Tumor samples
We chose four glioblastoma patients who received BV treatment after recurrence from among those treated at the Department of Neurosurgery, Tokyo Women’s Medical University, between 2005 and 2011. Tumor tissue was obtained by autopsy. We considered the tumor tissue obtained at the initial surgery as “before” BV treatment, while the autopsied tumor sample was deemed “after” BV treatment. Patients 1, 3, and 4 were diagnosed with primary glioblastoma; patient 2 was diagnosed with diffuse astrocytoma (World Health Organization [WHO] grade II) at initial surgery. Patient 2 was then diagnosed with a malignant transformation owing to the appearance of an enhanced lesion, and glioblastoma was confirmed by autopsy. The patients’ characteristics are shown in Table 1. We obtained informed consent and a written agreement from the patients’ families at the times of their deaths, and the autopsies was performed immediately after death.
Immunohistochemistry
Hematoxylin and eosin (H&E) staining and immunohistochemical analysis were performed for each surgical and autopsied specimen. Immunohistochemical staining was performed on glioma markers including glial fibrillary acidic protein (GFAP) (1:200; Cat. #M0761; Dako, Glostrup, Denmark), isocitrate dehydrogenase 1 (IDH1) (1:200; Cat. #D299-3; Medical & Biological Laboratories, Nagoya, Japan), and p53 (1:100; Cat. #M7001; Dako). To secure the quality of autopsied specimens, we compared the intermediate filament such as GFAP between autopsied and surgical specimens. The following primary antibodies were also used: CD31 (1:50; Cat. # MO823; Dako), α-smooth muscle actin (α-SMA) (1:2000; Cat. #M0851; Dako), VEGF (1:1000; Cat. #sc-152; Santa Cruz, Texas, United States), Flt-1 (1:100; Cat. #PA5-32,408; NeoMarkers, California, United States), basic fibroblast growth factor (bFGF) (1:50; Cat. #sc-1884; Santa Cruz), and platelet-derived growth factor-BB (PDGF-BB) (1:1000; Cat. #ab125268; Abcam). In this study we selected Flt-1 from VEGFRs because VEGFR2 has ability to combine with not only VEGF-A but also VEGF-C, VEGF-D and VEGF-E. We thought the changes of Flt-1 expression directly reflected the effect of BV treatment in comparison to VEGFR2. Histofine simple stain MAX PO (Nichirei, Tokyo, Japan) was used as a secondary antibody for GFAP, IDH1 and p53; ENVISION (Dako) was used for all others. Finally, we developed the tissue using 3, 3′-diaminobenzidine. Immunohistochemical studies of VEGF, Flt-1, bFGF, and PDGF-BB were evaluated in a 4-tiered semiquantitative scale as follows: (−), no staining; (1+), 0–30 %; (2+), 30–60 %; and (3+), >60 %. In order to analyze the vascular morphology, endothelial cells and pericytes were identified by a combination of CD31 and α-SMA in addition to H&E staining. The SMA(+) cells are often defined as pericytes [7–9], but it could also include endothelial cells [10]. For their differentiation we additionally used staining with CD31 antibody, which is specific for endothelial cells. Thus in the present study CD31(+)/SMA(±) and CD31(−)/SMA(+) cells were defined as endothelial cells and pericytes, respectively.
Vessel staining and counting
We counted the blood vessels in which a lumen and continuity of endothelium could be identified. CD31 was used as a marker of endothelial cells. Four different fields (100× magnification) in the tumor bulk were chosen blindly for each tumor tissue. The average number of vessels per field was calculated by counting the 4 micro fields. Quantitative data are presented as mean ± standard deviation and were compared with a paired Student’s t test. P < 0.05 was considered statistically significant.
Results
Clinical course of four patients
Case 1: A 62-year-old man presented with sensory aphasia. Brain magnetic resonance imaging (MRI) showed a tumor in the left temporo-parietal lobe. The tumor was gross-totally removed, and the pathological diagnosis was glioblastoma (MIB-1 index, 27.7 %; IDH1, wild type per immunohistochemistry). He received radiotherapy (60 Gy total) and immunotherapy using the autologous formalin-fixed tumor vaccine (AFTV) [11]. The tumor recurred in 14 months, and surgical removal was performed. The patient then received temozolomide (TMZ), yet the tumor recurred in the left tail of the hippocampus. BV was administered at a dose of 10 mg/kg every 2 weeks for a total of six times. The size of the enhanced lesion transiently decreased after BV treatment, but enlarged 4 months after BV administration; the patient died 6 months after initiating BV treatment. The overall survival time was 38 months from the initial surgery.
Case 2: A 31-year-old man presented with weakness of the right upper limb. MRI showed a mass lesion without enhancement in the left frontal lobe. Subtotal resection of the T2 high lesion was performed. The pathological diagnosis was diffuse astrocytoma (MIB-1 index, 8 %; IDH1, mutant). The patient then received radiotherapy (60 Gy total) and chemotherapy using procarbazine, nimustine (ACNU), and vincristine (PAV). A gadolinium (Gd)-enhanced lesion appeared 58 months after the initial surgery, and TMZ chemotherapy was administered. However, the recurrent tumor grew larger after the chemotherapy, and the patient was treated with BV for a total of eight cycles. BV was ineffective and he died 5 months after BV administration; the overall survival time was 69 months from onset.
Case 3: A 62-year-old man suffered from motor aphasia and right hemiparesis. Brain MRI showed a Gd-enhanced tumor in the left temporal lobe (Fig. 1a). The tumor was gross-totally removed (Fig. 1b), and the pathological diagnosis was glioblastoma (MIB-1 index, 43 %; IDH1, wild type). After the surgery, the patient was treated with radiotherapy (60 Gy) and concomitant TMZ chemotherapy followed by AFTV immunotherapy and maintenance TMZ chemotherapy for 12 cycles. 12 months after the initial surgery, the tumor relapsed, as determined by the appearance of an enhanced lesion on MRI (Fig. 1c). He was treated with interferon-β in addition to TMZ for three cycles, but to no effect. The patient was treated with BV at a dose of 10 mg/kg every 2 weeks for a total of eight cycles. After BV treatment, the enhanced lesion was remarkably decreased (Fig. 1d), but the tumor rapidly relapsed 2 months later (Fig. 1e) and the patient died 7 months after initial BV treatment. The overall survival time was 22 months.
The series of magnetic resonance imaging (MRI; T2WI and Gadolinium (Gd)-enhanced T1WI) of Case 3. a Images taken at first presentation. The tumor is located at the basal ganglion and frontal robe. b Images after the surgery. The Gd-enhanced lesion was gross-totally removed. c Tumor recurrence appeared on the medial side of the removed cavity. d Images after bevacizumab treatment. The recurred tumor was decreased remarkably. e Final image of the patient
Case 4: A 51-year-old man presented with left hemiparesis. MRI demonstrated a Gd-enhanced mass in the right frontal lobe. The tumor was gross-totally removed, and the pathological diagnosis was glioblastoma (MIB-1 index, 17.0 %; IDH1, wild type). The patient received radiotherapy (60 Gy) and TMZ chemotherapy. However, the tumor recurred 3 months after the initial surgery, and he was treated with BV for a total of 11 cycles. He died 11 months after initial BV administration; overall survival from the initial surgery was 21 months.
The patients’ overall characteristics are described in Table 1. Radiological changes after BV treatment were similar in all four cases. The Gd-enhanced lesion was transiently reduced and rapidly and uncontrollably recurred within several months.
Changes in the vascular structures and histological findings after BV treatment
First, we observed the histological findings in the surgical and autopsied specimens and assessed any histological differences between before and after BV treatment. In all the autopsied tumor samples, nuclear atypia and the concentration of tumor cells were stronger than those in the initial surgical specimens (Fig. 2a–d). Pseudopalisading necrosis was also increased. The pathological diagnosis was consistent with glioblastoma in all 4 autopsied cases. In the autopsied tumors, the glomerular structure was remarkably reduced, and most of the CD31(+)/SMA(± ) endothelial cells formed a single layer (Fig. 2e–l). On the other hand, the CD31(−)/SMA(+) pericytes were remarkably proliferated around the vessels after BV in all the autopsied tumors (Fig. 2e–l).
Hematoxylin and eosin, alpha-smooth muscle actin (αSMA), and CD31 immunohistochemical staining of the surgical and autopsied samples (scale bar 50 µm). Cases 1 and 3 are shown as representatives. In autopsied samples after the BV treatment, CD31(-)/SMA(+) pericytes were remarkably proliferated around the tumor vessels, and CD31 positive endothelial cells formed a thin single layer. The patency of the vessels was preserved. The blood vessels are indicated by arrows (d, h, l)
Next, to investigate whether BV treatment truly reduces angiogenesis, we compared the density of the tumor vessels between surgical and autopsied samples by counting the number of tumor vessels in each microscopic field. There was no significant change in the number of tumor vessels between the surgical and autopsied specimens (Fig. 3). Supplementary Fig. 1 demonstrate H&E staining and immunohistochemichal staining in cases 2 and 4.
Tumor vessel densities in the surgical and autopsied samples. The mean number of blood vessels per microscopic field (four fields each) was as follows; surgical and autopsied, respectively: Case 1, 26.5 and 29.0; Case 2, 15 and 15.5; Case 3, 24.7 and 28; and Case 4, 26.3 and 27.3. There was no significant difference between the surgical and autopsied samples in any of the cases (P < 0.05)
Expression of angiogenic factors
To assess whether the expression of angiogenic factors changed after BV treatment, we evaluated various angiogenic markers including VEGF-A, Flt-1, bFGF, and PDGF-BB by immunohistochemistry. VEGF-A was present in all tumors at initial surgery but its expression was reduced in all the autopsied tissues except case2 (Fig. 4a–d), whereas the expressions of Flt-1, bFGF, and PDGF-BB were increased in the endothelial cells, pericytes, and tumor cells of the BV treated tumors (Fig. 4e–p). For example, in case 1, VEGF-A expression in the endothelial cells was reduced (Fig. 4a, b), and a strong expression of bFGF was observed in the pericytes and tumor cells (Fig. 4i, j). In case 3, although VEGF-A was highly expressed in endothelial cells in the initial surgery specimen (Fig. 4c), its expression was remarkably reduced in the autopsied tumor (Fig. 4d). Other angiogenic factors, especially Flt-1, were highly expressed in the autopsied sample (Fig. 4g, h). Supplementary Fig. 2 demonstrate immunohistochemichal staining in cases 2 and 4. The results of immunohistochemistry of VEGF-A, Flt-1, bFGF, and PDGF-BB are summarized in the lower column of Fig. 4. GFAP expression was consistent well between autopsied and surgical specimens.
Upper column staining for vascular endothelial growth factor A (VEGF-A), Flt-1, basic fibroblast growth factor (bFGF), and platelet-derived growth factor-BB (PDGF-BB) Scale bar 100 µm. Compared with the surgical samples, the expression of VEGF-A was suppressed with the involution of the endothelial cells (a–d). On the other hand, Flt-1 (e–h) and bFGF (i–l) were highly expressed in the autopsied samples. Lower column the summary of immunohistochemical staining of angiogenic factors. Immunohistochemical studies of VEGF, Flt-1, bFGF, and PDGF-BB were graded as follows: (−), no staining; (1+), 0–30 %; (2+), 30–60 %; and (3+), >60 %. In all cases, VEGF-A was suppressed while the other factors tended to be enhanced
Discussion
This study provided some insight into the mechanism by which recurrent malignant gliomas develop resistance to BV treatment by revealing changes in the vasculature and expression of angiogenic factors post BV treatment.
Interestingly, the density of tumor vessels was unchanged following BV treatment. This result strongly suggests that inhibition of VEGF is not enough to maintain inhibition of neovascularization. Careful examination of the tumor vessels in the autopsied tissues revealed that the vascular endothelium formed a monolayer enveloped by multiple layers of CD31(−)/SMA(+) cells (presumed to be pericytes). Most of the tumor vessels were not collapsed and contained red blood cells, suggesting they were patent. Generally these changes of vascular morphology are not observed in cases without BV treatment. Pathological investigation of the autopsied material was done in one case of anaplastic astrocytoma, which underwent surgical resection, irradiation and chemotherapy, but not antiangiogenic therapy with Bevacizumab. The tumor at autopsy was diagnosed as glioblastoma and microvascular proliferation was evident. CD31 and αSMA staining were performed for both surgical and autopsied specimens of the neoplasm. Proliferation of pericytes around the vessels was not observed, in difference with tumor specimens obtained after Bevacizumab administration (Supplementary Fig. 3). It corresponds to data of di Tomaso et al., who reported that in patients who did not undergo therapy with cediranib (pan-VEGF receptor tyrosine kinase inhibitor) tumor specimens showed microvascular proliferation [12]. Vascular proliferation in glioblastoma had long been considered to involve only endothelial cells, and was often referred to as “endothelial proliferation.” However, recent reports have suggested that vascular proliferation mainly involves the proliferation of pericytes [13, 14]. Thus, it is currently understood that vascular proliferation in glioblastoma consists of proliferated pericytes surrounding an epithelial monolayer, although this is still controversial because of the absence of pericyte-specific immunohistochemical markers. The term “microvascular proliferation” is used in the latest WHO classification [15] in consideration of these details. Taking cellular morphology and localization into account, CD31(+)/SMA(±) cells and CD31(−)/SMA(+) cells are thought to immunohistochemically correspond to endothelial cells and pericytes, respectively.
It is still largely unknown whether pericytes play a role in neovascularization in glioblastoma; however, the various roles of pericytes have recently become clearer. For example, pericytes appear to contribute to stabilization and maturation of microvessels and to the maintenance of the blood–brain barrier [16]. It was also reported that, in response to brain ischemia, pericytes migrate to the peri-infarct area and produce various growth factors [17]. Cheng et al. reported that glioma stem cells differentiate to pericytes with the help of transforming growth factor-β (TGF-β), and that the pericytes are associated with vascular stability and tumor progression [18]. Since TGF-β induces expression of VEGF [19], it is possible that VEGF inhibition may upregulate TGF-β via negative feedback and stimulate glioma stem cell differentiation into pericytes. Thus, blocking the proliferation of pericytes may be a therapeutic strategy to overcome resistance to anti-angiogenic agents such as BV.
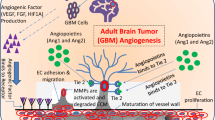
Our results suggest that expression of VEGF-A is suppressed persistently by BV, and that the upregulation of other angiogenic factors compensates for its loss in pericytes and tumor cells, leading to BV resistance. Batchelor et al. have reported that serum b-FGF and stromal cell-derived factor-1-α were elevated in glioblastoma patients treated with another VEGFR inhibitor, cediranib [20]. The molecular mechanism of the upregulation of other angiogenic factors after BV treatment is unclear; it may be a result of negative feedback owing to the continuous inhibition of the VEGF-driven angiogenic pathway (Fig. 5). Since the proliferated pericytes highly express other the angiogenic factors Flt-1 and/or bFGF, they may play an important role in tumor neovascularization after BV treatment. Flt-1 was thought expressed in endothelial cells specifically, but recently reported that the expression was observed also glioblastoma cells [21, 22]. Inhibition of other angiogenic factors following BV treatment may be effective for long-term tumor control.
A possible mechanism of resistance to bevacizumab (BV). After administration of BV, alternate pathways that promote angiogenesis and tumor regrowth can be activated. The basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF) pathways play an important role not only in tumor recurrence and invasion but also in BV resistance
There are limitations in this study. First, the patients were treated not only with BV but also with other therapies, including chemotherapy, radiation therapy, and immunotherapy. Therefore, unlike in vitro or animal experiments, it is difficult to observe the pure effect of BV treatment in human clinical subjects. Second, the time period from BV treatment to autopsy was different in each case, and we examined the tissues at different time periods after BV treatment. While it was previously suggested that activation of macrophages and changes of expression of IL-6, IL-9, MMP2 and MMP9 are playing an important role in resistance to Bevacizumab [23], evaluation of these factors was beyond the objective of our study. Despite these limitations, this study using human clinical samples provided us with important information about the cellular responses to BV treatment.
References
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Presta LG, Chen H, O’Connor SJ et al (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 57:4593–4599
Willett CG, Boucher Y, di Tomaso E et al (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10:145–147
Nagane M, Nishikawa R, Narita Y et al (2012) Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol 42:887–895
Furuta T, Nakada M, Misaki K et al (2014) Molecular analysis of a recurrent glioblastoma treated with bevacizumab. Brain Tumor Pathol 31:32–39
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 7:452–464
Takeuchi H, Hashimoto N, Kitai R et al (2010) Proliferation of vascular smooth muscle cells in glioblastoma multiforme. J Neurosurg 113:218–224
Sato S, Sato Y, Hatakeyama K et al (2011) Quantitative analysis of vessels with smooth muscle layer in astrocytic tumors: correlation with histological grade and prognostic significance. Histol Histopathol 26:497–504
Lu X, Dunn J, Dickinson AM et al (2004) Smooth muscle alpha-actin expression in endothelial cells derived from CD34+ human cord blood cells. Stem Cells Dev 13:521–527
Muragaki Y, Maruyama T, Iseki H et al (2011) Phase I/IIa trial of autologous formalin-fixed tumor vaccine concomitant with fractionated radiotherapy for newly diagnosed glioblastoma. Clinical article. J Neurosurg 115:248–255
di Tomaso E, Snuderl M, Kamoun WS et al (2011) Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res 71:19–28
Sun H, Guo D, Su Y et al (2014) Hyperplasia of pericytes is one of the main characteristics of microvascular architecture in malignant glioma. PLoS One 9:e114246
Takeuchi H, Hashimoto N, Kitai R et al (2010) Proliferation of vascular smooth muscle cells in glioblastoma multiforme. J Neurosurg 113:218–224
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Shepro D, Morel NM (1993) Pericyte physiology. FASEB J 7:1031–1038
Arimura K, Ago T, Kamouchi M et al (2012) PDGF receptor β signaling in pericytes following ischemic brain injury. Curr Neurovasc Res 9:1–9
Cheng L, Huang Z, Zhou W et al (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153:139–152
Seystahl K, Tritschler I, Szabo E et al (2015) Differential regulation of TGF-β-induced, ALK-5-mediated VEGF release by SMAD2/3 versus SMAD1/5/8 signaling in glioblastoma. Neuro Oncol 17:254–265
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Parliament E, Allalunis-Turner MJ, Franko AJ et al (2000) Vascular endothelial growth factor expression is independent of hypoxia in human malignant glioma spheroids and tumours. Br J Cancer 82:635–641
Stefanik DF, Fellows WK, Rizkalla LR et al (2001) Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor; FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol 55:91–100
Lu-Emerson C, Snuderl M, Kirkpatrick ND et al (2013) Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 15:1079–1087
Acknowledgments
We thank Yoichiro Kato, Hideyuki Takeiri, Noriko Sakayori, and Takashi Sakayori for their help with immunohistochemistry and their scientific suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10014_2016_248_MOESM1_ESM.pdf
Hematoxylin and eosin, alpha-smooth muscle actin (αSMA), and CD31 immunohistochemical staining of the surgical and autopsied samples (scale bar, 50 µm) in Case2 and Case4. (PDF 290 kb)
10014_2016_248_MOESM2_ESM.pdf
Staining for vascular endothelial growth factor A (VEGF-A), Flt-1, basic fibroblast growth factor (bFGF), and platelet-derived growth factor-BB (PDGF-BB) Scale bar, 100 µm. Compared with the surgical samples, the expression of bFGF (I–L) were especially highly expressed in the autopsied samples (PDF 388 kb)
10014_2016_248_MOESM3_ESM.pdf
Hematoxylin and eosin, alpha-smooth muscle actin (αSMA), and CD31 immunohistochemical staining of the surgical and autopsied samples (scale bar, 50 µm) in case without BV treatment. Proliferation of pericytes around the vessels was not observed, in difference with tumor specimens obtained after Bevacizumab administration. (PDF 283 kb)
Rights and permissions
About this article
Cite this article
Okamoto, S., Nitta, M., Maruyama, T. et al. Bevacizumab changes vascular structure and modulates the expression of angiogenic factors in recurrent malignant gliomas. Brain Tumor Pathol 33, 129–136 (2016). https://doi.org/10.1007/s10014-016-0248-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-016-0248-6