Abstract
The present study focuses on the preparation and electrochemical characterization of the calcium ferrite (Ca2Fe2O5+δ), a semiconductor crystallizing in the brownmillerite structure. The oxygen over-stoichiometry δ is extended to 0.16. It was synthesized by sol–gel auto combustion using nitrates as precursors and citric acid as chelating agent. The X-ray diffraction and specific surface area analysis showed a single phase with a specific surface area of ~ 6 m2 g−1. The optical gap of Ca2Fe2O5 (Eg = 2.31 eV), determined by the diffuse reflectance spectroscopy, is assigned to d-d transition of Fe3+: 3d orbital in octahedral coordination. It enables the photocatalytic exploitation of a large part of the solar spectrum. The p-type behavior of the oxide was demonstrated by photo-electrochemistry with an electro affinity of 3.69 eV (± 0.20 eV). A flat band potential (Efb = 0.93 VSCE) was evaluated from the capacitance-potential (C−2—E) characteristic while the holes density (NA = 2.63 × 1016 cm−3) was determined from the thermo-power S300K (= 1.1 mV K−1). The electrochemical oxygen intercalation is evidenced from the intensity-potential (J—E) profile. The semicircle in the Nyquist diagram is assigned to the bulk contribution whose diameter (233 kΩ cm2) decreases down to 203 kΩ cm2 under illumination, thus confirming the semi conductivity of the brownmillerite. The cathodic potential of the conduction band (CB) showed the feasibility of the water reduction. Indeed, Ca2Fe2O5 with a good chemical stability over a large pH range (5–14) was positively tested as photocatalyst for the H2 production upon visible light. The best activity was observed in alkaline medium (NaOH, 10−3 M) with an average evolution rate of 0.07 cm3 h−1 (mg catalyst)−1 under a light flux of 29 mW cm−2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ternary oxides are widely employed in the solar energy conversion and continue to attract a growing interest because of their low cost, chemical stability, and facile preparation. Therefore, the search of new photocatalysts has been particularly focused on inorganic semiconductors (SC) for the photo-electrochemical (PEC) conversion of light to useful energies [1] and the perovskite-derived compounds remain privileged as electro-catalysts [2, 3] and photocatalysts [4, 5]. Among the oxides, the brownmillerites with the general formula A2B2O5 (A = Ca, Sr, and B = Fe, Al, Co) display a high ionic conductivity owing to its two-dimensional character and found applications in Li-ion batteries [6] and photocatalysis [7]. They are related to the perovskite (ABO3) structure by introduction of an ordered array of oxygen vacancies with alternate layers of octahedrally and tetrahedrally coordinated metal [8]. This structure allows the accommodation of 3d cations of different valences with the capacity to support various types of defects [9], and the origin of the photocurrent comes from the electronic excitation from the valence band to acceptor levels. Most ferrite oxides exhibit a semiconductor behavior, absorb in the visible region with a band gap of ~ 2 eV, and possess a cathodic conduction band; such characteristics are required in photocatalysis [10, 11].
Indeed, the main hindrance limiting the photo-electrochemical performance of the semiconducting materials is the large optical gap. Ca2Fe2O5 is an anion-deficient perovskite with a narrow gap (Eg) formed from abundant elements where both iron and calcium are available and nontoxic. It exhibits p-type behavior and possesses a cathodic conduction band and a high reducing ability [12, 13]. However, there are fewer reports in the literature on its electrochemical and photocatalytic properties [14]. Ca2Fe2O5 is characterized photo-electrochemically to evaluate its activity as bias-free H2 photocathode since the conduction band (CB) is more cathodic than the H2O/H2 level. However, it does not correspond to the redox level required for water separation due to the high oxygen overvoltage, which exceeds 1 V and therefore a hole scavenger is necessary to close the electrical circuit and prevent photocorrosion [15].
Experimental
The sol–gel auto combustion method was used to prepare the brownmillerite; Ca(NO3)2.4H2O (Panreac, 98.5%) and Fe(NO3)3.9H2O (Scharlau, 99%) were used as precursors with a molar ratio (1/1). They were dissolved in distilled water to which citric acid was added dropwise; then, the solution was evaporated in a Rota vapor under vacuum. The resulting gel was denitrified at 300 °C in a ventilated place until total disappearance of NOx fumes; the powder was ground in an agate mortar and fired at 800 °C (5 °C min−1), naturally cooled to room temperature, and stored in a desiccator.
The phase was identified with a XPERT-PRO diffractometer using a monochromatic Cu Kα line (λ = 0.15406 nm). The crystallite dimension was calculated from the full width at half maximum of the strongest XRD peak. The specific surface area was determined by the BET method on ASAP2010 micromeritics equipment using N2 gas as adsorbent at 77 K. The diffuse reflectance data were collected with a Jasco 650 UV–VIS spectrophotometer fitted to an integration sphere; PTFE was used as reference. The morphology of the oxide was visualized by scanning electron microscopy (SEM) using a JSM Jeol 6360L microscope.
Pellets (thickness ~ 2 mm) were obtained by compacting the powder under a pressure of 5 kbar and annealed at 800 °C, yielding a relative density of 90% with good mechanical properties. The pellets were introduced in glass tubes and isolated with the hardening araldite. For the ohmic contact, a copper wire was fixed on one face of the electrode using silver cement. The front active surface was polished with fine emery papers and thoroughly washed with water before use. The electrochemical characterization was realized at ambient temperature in NaOH solution (Biochem, 98%, 10−3 M). A conventional three-electrode set-up was used with the reference SCE, Pt counter-electrode (0.5 cm2), and the working electrode (0.38 cm2). The interfacial capacitance was measured as a function of potential with a PGZ301 potentiostat/galvanostat, AC voltage signal of 10 kHz in frequency, and 10 mV peak to peak in magnitude were used. An inert atmosphere was ensured by bubbling pure N2 gas through the solution. The complex impedance data were recorded with small amplitude wave signals in the range (10−2–105 s−1).
The incorporation of O2− species in the crystal lattice implies the formation of Fe4+ as required by the electro-neutrality condition: Ca22+Fe3+2-τFe4+τO5+ τ/2. The concentration of Fe4+ ion was analyzed using the Mohr salt according the redox process: \({\mathrm{Fe}}^{2+}+{\mathrm{Fe}}^{4+}\to 2{\mathrm{Fe}}^{3+}\). The remaining Fe2+ is back titrated by KMnO4, and τ is given by:
where M is the molar weight of Ca2Fe2O5, m the mass of dissolved sample, N the normality of KMnO4 (10−2 N), and ∆V the difference between the volume consumed and the blank test. A formulation Ca2+2Fe3+1.68Fe4+0.32O2−5.16 is determined (τ=0.32).
The photoactivity was assessed by measuring the evolved H2 volume under visible light, provided by three tungsten-halogen lamps in a device described elsewhere [16]. Hydrogen was identified by gas chromatography (Agilent Technology 7890A). Because its H2 has weak solubility in water, its volume was collected by liquid displacement in a graduated burette with a small internal diameter for a higher sensitivity. All solutions were prepared from reagents of analytical quality and distilled water (~ 0.8 MΩ cm).
Results and discussion
Structural properties
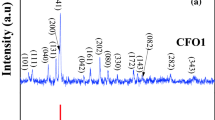
The XRD pattern of Ca2Fe2O5 calcined at 800 °C (Fig. 1) is characteristic of a single-phase crystallizing in the brownmillerite structure. All peaks are indexed in an orthorhombic symmetry (Space Group: Pnma) in agreement with the JCPDS Card No. 47–1744; the lattice constants: a = 5.431(5) Å, b = 14.763(9) Å, and c = 5.601(4) Å were refined by the least square method; a slight discrepancy is noticeable with the literature due to the oxygen over-stoichiometry. The brownmillerite contains oxygen vacancy row which functions as channels for oxygen diffusion between tetrahedral layers. Indeed, the additional oxygen ions, determined by chemical titration through the analysis of Fe4+, are disorderly distributed; this is deduced from the fact that the diffractogram is satisfactory indexed in a non-stoichiometric perovskite since any long-range ordering should produce peaks associated with the super-lattice.
The crystallite size (D ~ 55 nm) was calculated from the broadening of the strongest XRD peak (β, rad.): D = 0.9 λ (β cosϴ)−1. An active surface area of 6 m2 g−1 was determined experimentally by the BET method; the surface area and the total pore volume (0.0113 cm3/g) of Ca2Fe2O5 are relatively small, due to the calcination temperature (800 °C).
The SEM micrograph (Fig. 2) shows a homogeneous microstructure with an agglomeration of irregular and shaped particles. The porous surface of the solid was activated by the gases evolution (CO2 and NOx) escaping from the gel during the combustion and resulting in grain spraying and pore formation [17].
Optical and electrical properties
Most ferrites oxides are colored and absorb over the visible light due to their narrow band gap (< 2.5 eV) [18]. The optical gap (Eg) and the band edges position of Ca2Fe2O5 are crucial in photocatalysis. The diffuse reflectance spectrum (Fig. 3) shows a broad absorption at 536 nm, a value consistent with the brown color of the powder. The optical transition of the semiconductor is accurately obtained from the relation [19]:
The exponent n is equal to 2 or 0.5 respectively for direct or indirect allowed transitions. The intersection of the linear part (αhv)2 plot with the hv-axis gives a direct transition at 2.31 eV.
The oxygen crystal field splits the Fe3+ 3d orbital in sixfold coordination and the gap Eg of Ca2Fe2O5 is correlated to the internal transition t2g → e2g.
Generally, the layered perovskite accommodates oxygenated species with a charge compensation mechanism [20]. Indeed, the positive thermo-power (S300K = 1.1 mV K−1), measured at room temperature, confirms the p-type conduction of Ca2Fe2O5 with holes as majority carriers:
Such character is due to oxygen insertion between the layers of FeO6 octahedra sharing corners. The concentration of available sites No (= 9.1 × 1021 cm−3) is calculated from the experimental density. The S value gives a density of effective free holes NA of 2.63 × 1016 cm−3, and a mobility μh (= 2.78 × 10−7 cm2 V−1 s−1), calculated from the formula (σ = e NA μh). The hole conduction is associated with Fe3+cations, forming small polaron lattice with a fraction of Fe4+ cations resulting from Ca2Fe2O5+δ by the electro-neutrality condition [21, 22]. The mixed Fe4+/3+ valences facilitate the charge transport [23, 24] and the hole-type conduction is explained by the O2− insertion according to the following reaction [20]:
Photo-electrochemistry
Ca2Fe2O5 is an interesting material for the PEC characterization and the appropriate position of the electronic bands makes it important to explore. The voltammogram J(E) recorded in the dark in NaOH solution (pH ~ 11) shows a small current density (< 0.2 mA cm−2) over a wide potential range (Fig. 4) attributed to the thermal excitation from the valence band to acceptor levels, which overcomes the activation energy of electron hopping. The decrease in current below ~ − 0.7 V is attributed to the evolution of H2 as evidenced by the release of gas bubbles. The diffusion plateaus, before the water oxidation corresponds to oxygen insertion in the layered crystal according to the reaction:
The J(E) characteristic plotted upon irradiation shows that the photocurrent Jph augments in the cathodic direction (Fig. 4 inset), supporting the p-type semi-conductivity according to the Gartner model:
The photocurrent onset potential (Eon = −0.08 V) for which Jph appears is deduced from the cross point of the line Jph2 (E) with the potential axis (Fig. 5 inset). The chrono-amperometric profile of the electrode polarized at −0.5 V, i.e., the variation of the current density (J) against the time under light (SM 1) supports the p-type conduction of Ca2Fe2O5 with the oxidation of Fe3+ to Fe4+; the quantity of Fe4+ (τ = 0.32) can be quantified by coulometry.
The charge involved in the cyclic voltammetry averages ~ 1020 carriers cm−3 assuming a homogeneous oxygen distribution. The electro-kinetic parameters are derived from the semi-logarithmic plot (SM 2): the low exchange current density (24 µA cm−2) and the high polarization resistance (4.09 kΩ cm2) confirm the electrochemical stability of the oxide in the working solution (pH ~ 11).
In the absence of sub-band gap states, the potential Eon can be reasonably assimilated to the flat band potential (Efb). However, the latter is reliably obtained from the capacitance dependence (Fig. 5) on the applied potential (C−2–E) according to the well-known relation [25]:
where e and εo are the electronic charge and dielectric constant of vacuum respectively. The data were collected at 10 kHz to evaluate the potential Efb; such frequency is high enough to eliminate the parasite capacitances. The dielectric constant of Ca2Fe2O5 (ε ~ 45) was determined experimentally from the capacitance measurement on sintered pellet. The line (C−2–E) is negatively sloped and corroborates the p-type character of Ca2Fe2O5+δ. The intersection with the potential axis gives a potential Efb (= 0.93 V), more positive than the potential Eon (~ −0.08 V) and the large difference indicates the existence of surface states within the gap region.
The PEC characterization enables us to establish an energy diagram (Fig. 7) which predicts from a thermodynamic point of view whether the evolution of H2 is feasible or not. The potential of the couple H2/H2O depends on the pH and is determined by the intersection of the tangent of the cathodic branch with the potential axis in the J-E curve, a value of ~ −0.7 V is determined in NaOH medium. The potential Efb relates the physical and electrochemical scales according to the basic equation in photo-electrochemistry (\(Q= \left|e\right|\left[4.75- {E}_{\mathrm{red}}\right])\). Ca2Fe2O5-CB (ECB = − 1.06 V) is more cathodic than the H2O/H2 level and should lead to H2 evolution under visible light. The position of the valence band (EVB = 1.25 V) is deduced from the relation:
As mentioned above, the brownmillerite is interesting to study electrochemically because of the suitable position of the electronic bands. When the light is on, the holes are no longer ionized and the stability is likely to be improved by the p-type nature of Ca2Fe2O5. The low dark current yields an extended space charge region (W ~ 310 nm) resulting from the density NA; the width W is computed for an optimal band bending (ΔU = 0.5 V) using the formula:
The EIS analysis is a reliable technique for quantifying the electron transfer at the solid/electrolyte junction and allows elucidating the interfacial reactions. The complex diagrams of Ca2Fe2O5 plotted in the obscurity and under visible light at the free potential are illustrated in Fig. 6. As expected, the diameter of the depressive semicircle at high frequency (233 kΩ cm2), assigned to the charge transfer (Rct), decreases down to 203 kΩ cm2 under illumination, corroborating the semi conductivity of the brownmillerite. The absence of inclined line at low frequencies (Warburg diffusion) indicates that the electron transfer within the depletion zone is rate determining. The shift from the origin corresponds to the electrolytic solution (Rel = 430 Ω cm2), due to the ionic electrolyte (Na+,OH−). An electrical equivalent circuit (Fig. 6) models suitably the EIS data. The porous nature, the roughness, and heterogeneity of the electrode surface as well as the sub band states in the gap region of Ca2Fe2O5 require the introduction of a constant phase element (CPE) to explain the deviation of pure capacity [14]. A roughness factor (n = 0.8) is calculated by the relation (10):
Photocatalysis
As mentioned above, one of the best characteristics of colored perovskites is the high energetic position of the conduction band, deriving from 3d metal and a narrow gap sensitive to visible light, two attractive properties in the solar energy conversion. In this respect, hydrogen is an ideal fuel because of its cleanliness, renewability and heat combustion per unit mass and its production from the solar energy remains a challenging alternative [26, 27]. As application, the brownmillerite was successfully experimented for the H2 photo-production; the evolved volume on Ca2Fe2O5 was studied in aqueous solution at two pHs (Fig. 8). The best performance was obtained in basic medium and this can be explained as follows: as pH increases from 7 to 11, the H2O/H2 level which varies by ~ − 0.6 V/pH approaches the conduction band and reaches an band bending of ~ 0.25 V, equal to (EH2O/H2—Efb), close to the optimal value. This enhances considerably the electron transfer, thus producing a higher H2 volume. It is worthwhile to outline that the oxide is chemically instable at high pH as shown by XRD analysis (SM 3). The water oxidation cannot occur by holes through a valence band (VB) process owing to the inadequate position of the couple O2/H2O (~ 1 V). This fact can be explained by the lowest band bending value (EO2/H2O—Efb = ~ 0 V).
The reproducibility of the Ca2Fe2O5.16 was also investigated by conducting the cyclability tests up to two consecutive runs (Fig. 9).
The initial performance was restored with a deactivation of 20% after passing N2 gas through the electrolytic solution, which reactivates the photocatalytic sites.
Generally, the specific surface area of the perovskites is low and the photoactivity can be limited by this parameter [28]. To increase the conversion yield, the brownmillerite was immobilized on different supports, namely the active carbon, zeolite [29], or combined with a wide band gap semiconductor (hetero-junction). Attempts to test our oxide in hetero-junction with ZnO are presently underway; the results are satisfactory and will be reported very soon. In addition, to prevent the photocorrosion of Ca2Fe2O5 and to enhance its photoactivity, the holes scavengers I− and Fe (CN)64− will be also tested.
Conclusion
Ca2Fe2O5 was successfully synthesized by citrate sol–gel auto combustion. The single phase, confirmed by XRD analysis, has been characterized by photo-electrochemistry for evaluation in the hydrogen production under visible light. The brownmillerite is classified as a narrow band gap semiconductor with a good stability over a wide pH range and suitable positions of the conduction band. The thermo-power measurement indicated p-type conduction, a characteristic confirmed by the (capacitance-potential) plot. The exchange current density and the polarization potential in NaOH electrolyte are characteristic of a long-lived material. The electrochemical impedance spectroscopy showed the predominance of the bulk contribution and confirmed the semi conductivity. Ca2Fe2O5 was successfully tested for the H2 evolution upon visible light. Satisfactory results with ZnO will be reported in the future.
References
Hammache Z, Soukeur A, Omeiri S, Bellal B, Trari M (2019) Physical and photo-electrochemical properties of MgFe2O4 prepared by sol gel route: application to the photodegradation of methylene blue. J Mater Sci Mater Electron 30(6):5375–5382
Risch M (2017) Perovskite electrocatalysts for the oxygen reduction reaction in alkaline media. Catalysts 7:154
Dimitrovska-Lazova S, Aleksovska S, Mirceski V, Pecovska- Gjorgjevich M (2019) Correlation between composition, electrical and electrochemical properties of LnCo1-xCrxO3 (Ln = Pr, Gd and x = 0, 0.5 and 1) perovskites. J Solid State Electrochem 23(3):861–870
Kanhere P, Chen Z (2014) A review on visible light active perovskite-based photocatalysts. Molecules 19(12):19995–20022
Boudjellal L, Belhadi A, Brahimi R, Boumaza S, Trari M (2018) Physical and photoelectrochemical properties of the ilmenite NiTiO3 prepared by wet chemical method and its application for O2 evolution under visible light. Mater Sci Semicond Process 75:247–252
Dhankhar S, Bhalerao G, Baskar K, Singh S (2016) Synthesis and characterization of polycrystalline brownmillerite cobalt doped Ca2Fe2O5. AIP Conf Proc 1731:5–8
Boumaza S, Boudjellal L, Brahimi R, Belhadi A, Trari M (2020) Synthesis by citrates sol-gel method and characterization of the perovskite LaFeO3: application to oxygen photo-production. J Sol-Gel Sci Technol 94(2):486–492
Da Silva CC, Sombra ASB (2011) Temperature dependence of the magnetic and electric properties of Ca2Fe2O5. Mater Sci Appl 02(09):1349–1353
Hirabayashi D, Yoshikawa T, Mochizuki K, Suzuki K, Sakai Y (2006) Formation of brownmillerite type calcium ferrite (Ca2Fe2O5) and catalytic properties in propylene combustion. Catal Letters 110(1–2):155–160
Priyanga GS, Thomas T (2018) Direct band gap narrowing and light-harvesting-potential in orthorhombic In-doped-AlFeO3 perovskite: a first principles study. J Alloys Compd 750:312–319
Pailhé N, Wattiaux A, Gaudon M, Demourgues A (2008) Correlation between structural features and vis-NIR spectra of α-Fe2O3 hematite and AFe2O4 spinel oxides (A= Mg, Zn). J Solid State Chem 181(5):1040–1047
De Haart LGJ, Blasse G (1985) Photoelectrochemical properties of ferrites with the spinel structure. J Electrochem Soc 132(12):2933–2938
Song S, Xu L, He Z, Ying H, Chen J, Xiao X, Yan B (2008) Photocatalytic degradation of C.I. Direct Red 23 in aqueous solutions under UV irradiation using SrTiO3/CeO2 composite as the catalyst. J Hazard Mater 152(3):1301–1308
Wheeler GP, Choi KS (2018) Investigation of p-type Ca2Fe2O5 as a photocathode for use in a water splitting photoelectrochemical cell. ASC Appl Energy Mater 1(9):4917–4923
Boumaza S, Boudjemaa A, Bouguelia A, Bouarab R, Trari M (2010) Visible light induced hydrogen evolution on new hetero-system ZnFe2O4/SrTiO3. Appl Energy 87(7):2230–2236
Boumaza S, Brahimi R, Boudjellal L, Belhadi A, Trari M (2020) Photoelectrochemical study of La2NiO4 synthesized using citrate sol gel method- application for hydrogen photo-production. J Solid State Electrochem 24(2):329–337
Gowreesan S, Ruban Kumar A (2017) Structural, magnetic, and electrical property of nanocrystalline perovskite structure of iron manganite. Appl Phys A 123(11):689
Omeiri S, Bellal B, Bouguelia A et al (2009) Electrochemical and photoelectrochemical characterization of CuFeO2 single crystal. J Solid State Electrochem 13:1395–1401
Bagtache R, Sebai I, Abdmeziem K, Trari M (2019) Visible light induced H2 evolution on the hetero-junction Ag/NiO prepared by nitrate route. Sol Energy 177:652–656
Asenath-Smith E, Lokuhewa IN, Misture ST, Edwards DD (2010) p-Type thermoelectric properties of the oxygen-deficient perovskite Ca2Fe2O5 in the brownmillerite structure. J Solid State Chem 183(7):1670–1677
Shaula AL, Pivak YV, Waerenborgh JC, Gaczynski P, Yaremchenko AA, Kharton VV (2006) Solid State Ionics 177(33–34):2923–2930
Zhou HD, Goodenough JB (2005) Electronic behavior of three oxygen non-stoichiometric Fe4+/Fe3+ oxoperovskites. J Solid State Chem 178(12):3679–3685
Tilley RJD (2004) in: Understanding solids: the science of materials John Wiley & Sons. England, Chichester, pp 363–390
West AR (1984) in: Solid state chemistry and its applications. John Wiley & Sons, Chichester, England, pp 553–582
Benamira M, Lahmar H, Messaadia L, Rekhila G, Akika FZ, Himrane M, Trari M (2020) Hydrogen production on the new hetero-system Pr2NiO4/SnO2 under visible light irradiation. Int J Hydrogen Energy 45(3):1719–1728
Boumaza S, Bouguelia A, Bouarab R, Trari M (2009) Physical and photoelectrochemical studies for hydrogen photo-evolution over the spinel ZnCr2O4. Int J Hydrogen Energy 34(11):4963–4967
Boudjemaa A, Rebahi A, Terfassa B, Chebout R, Mokrani T, Bachari K, Coville NJ (2015) Fe2O3/carbon spheres for efficient photo-catalytic hydrogen production from water and under visible light irradiation. Sol Energy Mater Sol Cells 140:405–411
Lam SM, Sin JC, Mohamed AR (2017) A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: a mini review. Mater Res Bull 90:15–30
Jiang G, Lin Z, Chen C, Zhu L, Chang Q, Wang N, Wei W, Tang H (2011) TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon N Y 49(8):2693–2701
Acknowledgements
The authors would like to thank Dr F. Touahra and Dr. A. Boudjemaa for numerous analyses.
Funding
This work was supported by the Faculty of Chemistry (Algiers).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 148 KB)
SM 1 The chrono-amperometry of the brownmillerite Ca2Fe2O5 under visible light in NaOH (0.1 M) solution.
SM 2 The semi-logarithmic profile (log J-E) of Ca2Fe2O5 plotted in the dark. Electrolyte NaOH (0.1 M) solution, scan rate: 5 mV/ s.
SM 3 Powder X-ray diffraction pattern of Ca2Fe2O5 realized after the photo-catalytic test at pH ~ 13.
Rights and permissions
About this article
Cite this article
Benallal, S., Boumaza, S., Brahimi, R. et al. Electrochemical characterization of the brownmillerite Ca2Fe2O5+δ synthesized by citrate sol–gel method. Application to photocatalytic H2-production. J Solid State Electrochem 26, 1541–1547 (2022). https://doi.org/10.1007/s10008-022-05183-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05183-1