Abstract
Rapid industrial growth causes considerable environmental havoc, adversely affecting human and aqueous life. It becomes a significant concern to deal with adequate wastewater treatment strategies by converging on water scarcity. This research work explored the synthesis of titanium-substituted Y-type barium hexaferrite (Co2-Y), having a general formula of Ba2Co2Fe12-xTixO22 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5), using a facile nitrate-based sol–gel auto-combustion route and its suitability was investigated as a heterogeneous catalyst within the photo-Fenton-based degradation of methyl orange (MO), one of the significant pollutants generated from textile industries. Developing a thermochemically stable and magnetically separable heterogeneous catalyst for photocatalytic decomposition of nonbiodegradable organic dye from wastewater was also emphasized. The as-prepared nanocrystalline Co2-Y powders were analyzed using XRD, FTIR, DLS, UV–visible spectroscopy, SEM, VSM, and XPS. Furthermore, the photocatalytic degradation performance of pristine and titanium substituted Ba2Co2Fe11.6Ti0.4O22 ferrite, having the lowest bandgap value among all samples, was quantified and compared in terms of apparent rate constant (karc) value and turnover frequency values. The enriched photocatalytic performance was correlated with the existence of multi-valance states of transition metal cations and the availability of oxygen vacancy, confirmed by the surface chemistry using the XPS analysis. The modified (enhanced thermal and chemical stability) hexaferrite catalyst was magnetically separable and reusable without significant losses to its catalytic performance. This promising catalyst may be considered as a replacement for soft ferrite materials to catalyze the degradation of several other nonbiodegradable organic pollutants from wastewater in large-scale industries.
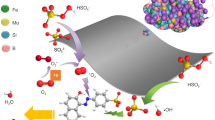
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water quality becomes an essential question in the present scenario due to the increasing rate of pollution. Although plenty of water is available over the earth’s surface, only a fraction (around 0.03% of total water bodies) is convenient for human activities. Water, a vital part of living beings, is deteriorating day by day with rapid industrial growth (Shah et al. 2022). This rapid industrial growth allows the discharge of various pollutants into the water bodies, adversely affecting water quality. Most of these industries, primarily concerned with paper, textile, leather, pharmaceuticals, food, packaging, and cosmetics, consume a huge number of dyes annually. A wide variety of chemicals are being utilized in industrial processing, starting from inorganic compounds to harsh organic and complex polymers. The textile industries occupy the largest share (around 60%) of the total consumption of organic dyes and harmful chemicals during the aqueous processing of clothes (Hai et al. 2007). Figure 1 shows that the water exposed to the untreated discharge of industrial waste contains several toxic, mutagenic, carcinogenic, and threatening compounds that cause esthetical pollution and perturbations to the aquatic (for both microbiological and fishes) along with human life (Hai et al. 2007; Shah et al. 2022).
The dyes are composed of skeleton, chromophore (groups having unsaturated or multiple bonding), auxochrome (enhances the color density), and soluble parts (helps to dissolve within solvent) (Auld 1907). These dyes can be categorized over different applications as direct dyes, vat dyes, acidic dyes, basic dyes, azo dyes, pigment-based dyes, natural dyes, and dispersed dyes (Benkhaya et al. 2017). Among all these dyes, only half (45 to 47%) of these dyes are found to be biodegradable. The remaining (53 to 55%) portion of these dyes is toxic to the ecosystem and has become an issue of concern these days (Muhd Julkapli et al. 2014). Due to above mentioned adverse impact on the ecological system and human life, it has become crucial to develop a facile methodology to degrade these water-polluting agents, i.e., nonbiodegradable dyes.
There are several dyes and organic contaminants (studied for photocatalytic removal from wastewater), such as methylene red, methylene orange, congo red, tetracycline, oxytetracycline, chlortetracycline, Acid Blue 92, Acid Fuchsin, Acid orange 7, Acid Red B, Acid Red 138, Alizarine yellow, Brilliant blue-R, Congo red, Crystal violet, Direct Red 80, Direct Green 6, Erichrome black–T, Fuchsine Red, Indigo carmine, Methylene Blue, Methyl green, Methyl violet, Rhodamine B, Reactive blue 5, Titan yellow (Kefeni et al. 2017; Reddy and Yun 2016; Chen et al. 2020, 2022a, b), which adversely affect the water quality.
Methyl orange (MO) or orange III (dark red crystalline, odorless powder) (deep color, complex pollutant composition, high variation in water quality, high BOD and COD contents, poor biodegradability, highly toxic, carcinogenic, teratogenic, mutagenic, harmful to the environment and organisms) is an anionic azo-based organic nonbiodegradable dye, having a general molecular formula of C14H14N3NaO3S [Molecular weight – 327.34 g.mol−1, density – 1.28 g/cm3, melting point > 300 °C (573 K), solubility in water − 0.5 g/100 mL (20 °C)]. The structure of MO consists of a chromophore, auxochrome, and water-soluble group, as shown in Fig. 2 (PubChem CID 68,617; Mittal et al. 2007; Youssef et al. 2016; Yang et al. 2017; Aroke et al. 2020). It is widely utilized in the textile and printing industries. Approximately 10 to 15% of the initial concentration is disposed of directly into the water bodies or open environment while performing industrial processes (Aroke et al. 2020). The increasing utilization of MO in textiles, along with analytical industries and untreated disposal of this industrial waste within water bodies, have become a primary concern for the researchers.
To date, several strategies, i.e., Fenton-based reaction, flocculation, photocatalysis, precipitation, treating with hydrogen peroxide, adsorption on activated carbon or reduced graphene oxide sheets, treating with intense ultrasonic waves, air drag or inverse osmosis, oxidization using ozone, degradation using UV light irradiation within an aqueous solution, electrochemical degradation and electron beam irradiation, have been explored by the researchers to degrade these nonbiodegradable organic dyes (Meidanchi and Akhavan 2014; Valero-Luna et al. 2016; Shah et al. 2022). However, these processes may vary in expenditure, degradation effectiveness, and environmental issues. Among these, photo-Fenton-based degradation has been recognized as an outstanding technique due to its reasonably mild conditions, facile processing, and almost complete degradation of water-soluble organic dyes from water (Valero-Luna et al. 2016).
Traditionally, the homogenous Fenton technology has several drawbacks, i.e., a narrow range of acceptable pH levels, expensive precursors, and producing a considerable amount of iron sludge at the end of the process. To overcome these flaws, heterogeneously catalyzed Fenton technology is developed. To date, heterogeneously catalyzed Fenton technology (inexpensive, utilization of semiconducting soft ferrite materials, a broad range acceptance for pH level) has become the most effective and largely adaptable advanced catalytic oxidation process for treating industrial wastewater. Furthermore, the degradation rate is accelerated by UV–Vis light irradiation (Fallmann et al. 1999; Wang et al. 2014; Valero-Luna et al. 2016). The process involves the generation of exceedingly reactive hydroxyl radicals (OH•) by the catalytic dissociation of hydrogen peroxide (H2O2) within the presence of iron. The as-generated hydroxyl radicals (OH•) have a higher oxidation potential of 2.80 V and can easily oxidize or mineralize most of the organic molecules to produce H2O, CO2, and other small molecules. It attacks most nonbiodegradable organic compounds (mainly unsaturated compounds) with zero selectivity (Liu et al. 2011; Valero-Luna et al. 2016; Singh et al. 2021).
Many researchers are also focused on photo-Fenton-based degradation of complex organic compounds, using iron-containing material systems (as a heterogeneous catalyst) having excellent redox configuration and moderate magnetic properties to facilitate the separation after completion of the reaction. To date, several iron oxide-based materials such as TiO2-coated Fe2O3 nanograin (Akhavan and Azimirad 2009), Polymer-coated Fe2O3 (Zhang et al. 2020a, b), and spinal ferrite-based catalysts such as CuFe2O4, CoFe2O4, FeFe2O4 (Wang et al. 2014), sulfur-modified ZnFe2O4 (Liu et al. 2011), MnFe2O4 with activated carbon (Nguyen et al. 2011), BaFe2O4 (Borse et al. 2011), NiFe2O4 (Sharma et al. 2015), MgFeCrO4 (Moradnia et al. 2020), and CaFe2O4 (Dom et al. 2011) have been evaluated their suitability as a heterogeneous catalyst to decompose nonbiodegradable dyes from wastewater bodies. The poor thermochemical stability of these spinal ferrites restricts the utilization of these catalysts in a wide-scale application (Suthar et al. 2020). Researchers have investigated a few hard ferrite magnetic materials to overcome these stability issues due to their excellent thermochemical stability. The M-type strontium hexaferrite (SrFe12O19) is utilized to degrade the toluidine blue dye with a poor degradation rate (3 × 10–3 min−1) (Farghali et al. 2008). For instance, M-type barium hexaferrite (BaFe12O19) also results in poor degradation (70.8%) of methylene blue (Valero-Luna et al. 2016). So, the interest of the researchers is restricted to the development of hard ferrite materials with enhanced degradation rate, total degradation of organic dyes, facile synthesis, higher diffusion capability, easy separation, and prolonged reusability. The facile extraction of the hard ferrite materials allows it to be categorized as an eco-friendly catalyst within the green chemistry approaches (Sharma et al. 2015; Valero-Luna et al. 2016).
In continuation to developing a stable and robust catalyst for the degradation of these organic wastes from water bodies, the Y-type barium hexaferrite (Co2-Y) may be considered a suitable candidate due to their similar magnetic characteristics and redox configuration. It belongs to a hexagonal ferrite with several advantages over the spinel ferrites, such as better thermo-chemical stability, high Curie temperature (Tc), outstanding mechanical hardness, and corrosion resistance. The Y-type barium hexaferrite (Co2-Y) posses a bandgap energy (around 0.74 to 2.35 eV) (Alrebdi et al. 2022), which is quite lower than the widely used photocatalyst anatase TiO2 (Eg = 3.2 eV) (Yan et al. 2013) and other spinel ferrites such as CoFe2O4 (2.7 eV) (Holinsworth et al. 2013) and NiFe2O4 (2.5 eV) (Dolia et al. 2006). The capability to absorb the solar spectrum is determined by the bandgap (Eg) value of the ferrite being utilized as a photocatalyst. A narrow energy gap between the valance and conduction band provides a wider absorption of the solar spectrum; hence it can utilize visible light more efficiently (Yan et al. 2013). It is also suggested that the bandgap value can be minimized further by introducing titanium to the ferrite system. The incorporation of titanium generates impurity (especially donor-type) energy levels, which causes a narrow bandgap value within the system (Kumar and Kar 2014; Rajivgandhi et al. 2021).
In consideration of the leaching issue, the robust hexaferrite material (having enhanced thermochemical characteristics) may provide superior results. The experimental evidence performed by S. Jacobo et al. confirms the enhanced structural and chemical stability of barium hexaferrite material in comparison to the spinel type barium ferrite (barium monoferrite) (Jacobo et al. 2002). The environmental implications of the heavy metal ions such as barium, cobalt and iron can be prevented by treating the water for further by utilizing several well established techniques such as carbon-based adsorbents (nanoporous adsorbents, especially activated carbons, carbon nanotubes, and graphene), chitosan-based adsorbents (a natural adsorptive polymer), mineral adsorbents (such as zeolite, silica, and clay), biosorbents, metal–organic framework based adsorbents, membrane-based filtration and separation, ultrafiltration (both micellar enhanced ultrafiltration and polymer enhanced ultrafiltration), reverse and forward osmosis, electrodialysis (to separates ions at the basis of electric potential difference), chemical-based separation (precipitation, flotation, coagulation and flocculation), electric-based separation (electrochemical reduction, electroflotation, and electrooxidation method), utilizing natural and synthetic allophane adsorbents (Al-Jlil 2010, 2017; El Sayed 2018; Kaveeshwar et al. 2018; Baldermann et al. 2018; Bilal et al. 2021; Chai et al. 2021; Qasem et al. 2021; Rathi and Kumar 2021).
Considering all the above factors, the present work describes the impact of titanium substitution on the structural, magnetic, and optical properties of the Co2-Y hexaferrite system. The sample having the least value of energy bandgap (Eg) is further characterized as a possible replacement of spinel ferrites for its catalytic degradation efficiency within photo-Fenton conditions (under visible light irradiation using a cool white LED and H2O2) utilizing methyl orange (MO) as a model dye molecule from the wastewater without any hazardous footprint on the environment.
Materials and methods
Materials
The reagents utilized for the synthesis of titanium-substituted Y-type barium hexaferrite (Co2-Y) were barium nitrate [Ba(NO3)2], cobalt nitrate [Co(NO3)3.6H2O], iron nitrate [Fe(NO3)3.9H2O], titanium nitrate [Ti(NO3)4.4H2O], citric acid [C6H8O7·H2O], and ammonium hydroxide solution [NH4OH]. The chemicals utilized for the dye degradation experiment were methyl orange [C14H14N3NaO3S], hydrogen peroxide [H2O2], and hydrochloric acid [HCl]. All chemicals were analytical grade and purchased from Sigma-Aldrich.
Synthesis of titanium substituted barium hexaferrite
Ultrafine nanocrystalline titanium-substituted barium hexaferrite having a general formula of Ba2Co2Fe12-xTixO22 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) were synthesized by nitrate-based auto combustion method using metal nitrates as precursors. Initially, the stoichiometric amounts of each precursor were calculated and dissolved within deionized (DI) water. The equimolar amount of citric acid (1:1:: metal nitrates: citric acid) was added to it and allowed to be homogenized using continuous magnetic stirring. Then the pH level was neutralized by the dropwise addition of ammonium hydroxide (30%) solution. The admixture was maintained at a temperature of 80 °C up to the transformation of the sol into a dried gel. The further heating of this dried gel resulted in the ignition, which allowed the formation of a fluffy powder (burnt ash). The as-obtained fluffy powders were pulverized and calcined at 1200 °C for a holding time of 2 h at a heating rate of 3 °C per minute. Finally, the sample was powdered using mortar-pestle and sieved using a 250-mesh sieve to be characterized as a catalyst. These powdered samples were pelletized with an addition of 3 wt.% polyvinyl alcohol (PVA) and sintered at 1100 °C for 2 h in an oxidizing atmosphere for thermomagnetic analysis.
Characterization of hexaferrite catalyst
The crystallite phase identification was performed by X-ray diffraction pattern (obtained from Rigaku MiniFlex 600 benchtop) using Cu-kα radiation within the 2θ range of 20° to 80° and a step size of 0.02°. The presence of structural bonds was confirmed using FTIR spectra (obtained from Perkin Elmer BXIII FTIR infrared spectrometer), measured in transmittance mode within the range of 4000–400 cm−1. The samples were dispersed within the isopropyl alcohol (IPA) to characterize their particle size distribution using a dynamic light scattering (DLS) based particle size analyzer (Zetasizer Nano ZS by Malvern Panalytical). The optical properties (bandgap energy) of all the samples were analyzed using the UV- absorption spectrum (obtained from Shimadzu, UV 2600 UV–Vis spectrometer). The scanning electron micrographs, along with EDX spectra (obtained from ZEISS, EVO 18–2045), were used to examine the surface morphology and elemental purity of the sample. The room temperature hysteresis loop and thermo-magnetic behavior of the sintered pellets were measured using SQUID MPMS (Quantum design, Model-MPMS 3, EM-QM, USA) under an applied field of ± 6 T (60,000 Oe). Furthermore, the surface elemental identity and their chemical states were investigated using X-ray photoelectron spectra (K-alpha X-ray Photoelectron Spectrometer (XPS) by Thermo Scientific).
Photocatalytic degradation experiment
The photocatalytic degradation efficiency of the hexaferrite samples was evaluated by the degradation of methyl orange (MO). To examine the degradation efficiency, 0.1 mg of the dried hexaferrite sample was added to the 2 ml of aqueous MO solution (concentration 4.6 ppm) within a quartz cuvette (capacity of 4 ml) with a 1 cm of path length. The pH level of this suspension was tuned at three by the dropwise addition of 0.1 M HCl. The admixture was stirred continuously for the next 15 min (in the dark condition) to achieve the equilibrium condition for adsorption–desorption. Later, 100 ul of 1.4 M hydrogen peroxide (H2O2) was introduced to it. Two protocols were implemented in the next step with two identical sets of the above-mentioned reaction mixture.
The aqueous solution of MO gives a unique UV–visible signature in the 200–800 nm range. Therefore, the decomposition of the dye was monitored with the dynamic UV analysis. The UV–Visible spectrum for the reaction mixture was allowed to be measured within an equal interval of 10 min for both Fenton (in dark conditions) and photo-Fenton (in the presence of a visible light source, a cool white LED emitting an irradiance of 720 Watt/m2) in the wavelength range 400–800 nm). The intensities of all MO UV–visible peaks decreased with time, signifying the MO degradation. Moreover, MO degraded to small aliphatic entities because such molecules do not show any UV–visible signature.
Results and discussion
Structural analysis using XRD
The XRD pattern for the calcined powder of titanium substituted Co2-Y is shown in Fig. 3a. The presence of sharp characteristic peaks corresponding to the Ba2Co2Fe12O22 (standard JCPDS card no 44–0206) within the obtained pattern confirms the formation of high purity Y-type barium hexaferrite (Co2-Y) phase along with good crystallinity. Furthermore, several structural parameters such as average crystallite size, lattice parameters, c/a ratio, unit cell volume, X-ray density, and lattice strain are obtained using the following equations, and the values are tabulated in Table 1 (Augustin and Balu 2017; Suthar et al. 2020).
where DAvg. is the average crystallite size, Ksf signifies the Sherrer’s constant (Ksf = 0.89), λ is the wavelength of X-ray utilized for generating the XRD pattern (λ = 1.54056 Å), \({W}_{hkl}^{act.}\) is the actual peak width at half of its maximum intensity (FWHM) in radians obtained by subtracting the instrumental contribution ( \({W}_{hkl}^{instrumental}\)) from the measured value (\({W}_{hkl}^{meas.}\)) and θ is the peak position or Bragg’s angle, dip is the inter-planer spacing (distance between two sequential atomic planes), h k and l are miller indices (group of numbers indicating the orientation of any particular atomic plane), a and c are the lattice parameters representing the minor and major axis of a hexagonal lattice, Vuc signifies the volume of a unit cell, Dx-ray signify the X-ray density. That is, crystal structure-dependent theoretical density, Mw represents the molecular weight, F is the number of effective formula units existing within a solo unit cell (F = 3 for Co2-Y), Na is Avogadro’s constant having a numeric value of 6.023 × 1023, and εl is lattice strain due to the crystal imperfections or lattice dislocations.
The XRD pattern confirms the successful substitution of Ti4+ at the Fe3+ site. It is found that the crystallite size is in the range of 64–72 nm and decreases with substitution. The XRD results agree with the substitution of a larger cation Ti4+ (0.68 Å), at the site of a smaller cation Fe3+ (0.64 Å) by increasing the value of the lattice parameters along with the unit cell volume (Jia et al. 2013). A peak shifting (Fig. 3b) is observed towards the left side. The crystal axis ratio (c/a) is found to be 7.429 for x = 0.0 and 7.421 for x = 0.5, which lies within the permissible range for the formation of the Co2-Y phase (Suthar et al. 2020). The X-ray densities follow the decreasing trend with substitution. It is in good agreement with both the decreasing molecular weight and the increasing unit cell volume. The crystallite distortions that arise while substituting a larger cation at the smaller cation site are quantified using the determination of lattice strain using the Williamson-Hall method to Eq. (6), where the lattice strain (εl) is measured as the slope of the straight line obtained by linear fitting to the curve between Whkl. Cosθ and 4. Sinθ, as depicted in Fig. 4 (Augustin and Balu 2017). The lattice strain is found to be positive (volumetric expansion within the unit cell) for all these samples, which keeps rising continuously with increasing Ti4+ ions substitution.
FTIR analysis
The structural variations within the titanium-substituted Y-type barium hexaferrite (Co2-Y) are analyzed further using FTIR spectra by confirming the presence of various characteristic bands corresponding to their specific absorption peak positions. In Fig. 5, the FTIR spectra for undoped (x = 0.0) calcined powder consists of two major absorption peaks, one at 400.15 cm−1 (corresponding to asymmetric stretching of metal cation at the octahedral site) and the second at 584.32 cm−1 (corresponding to asymmetric stretching of metal cation at the tetrahedral site). It is considered the characteristic peak for the cationic vibrations within the spinel block of the Co2-Y structure (Adeela et al. 2016).
The substitution of Ti4+ ions at the Fe3+ site allows a slight shifting of these characteristic bands towards the higher wavenumber with increasing concentration. The structural variations can be quantified with the help of determining Fe–O bond lengths using the following equation:
where Bl represents the bond length, Fc is for force constant (reflects the bond stiffness), υwn is the corresponding wavenumber for a specific absorption band, cvl is the light velocity measured in a vacuum having a numerical value of 3 × 108 m/s, µa is the effective mass of constituting atoms, Aw1 and Aw2 are the atomic weights of linked atoms. The force constant (Fc) associated with the Fe–O bond and respective bond length (Bl) values are listed in Table 2. The results show that the Fe–O bond length is increased with the substitution of Ti4+ within the hexaferrite (Co2-Y). The bond length variation can be correlated due to the ionic radius mismatch (introducing a larger cation at a smaller cation site), which also favors the volumetric expansion suggested by XRD studies. Apart from these two characteristic peaks, the co-existence of a few minor absorption peaks in the range of 1064.03 to 1626.66 cm−1 is also observed. It may be attributed due to the presence of metal–oxygen-metal bonding, likewise Fe–O–Fe or Co–O–Co (between 1100 and 1500 cm−1), or the presence of residual traces of N–O stretching vibration (between 1475 and 1550 cm−1), or N–H band (between 1600 and 1640 cm−1). It may also be associated with using an ammonia solution while adjusting the pH. The peaks around 1626 cm−1 and 3440 cm−1 may be ascribed to the O–H stretching vibrations due to the adsorbed moisture content at the surface (Lalegani and Nemati 2015; Adeela et al. 2016).
Particle size distribution analysis
All the calcined hexaferrite samples dispersed within the isopropyl alcohol (IPA) are analyzed for their average particle size (APS) along with poly-dispersion index (PdI), using the dynamic light scattering-based technique (as shown in Fig. 6). The results suggest that particle sizes are found to be in decreasing order (from 1.835 µm for x = 0.0 to 1.055 µm for x = 0.5) with Ti4+ substitution within the Co2-Y hexaferrite. The particle size distribution quality can be explained by PdI value, which indicates the non-uniformity of the powder sample on a scale of 0 to 1 (Danaei et al. 2018). The results suggest that all these samples consist of a wide range of size distribution owing to their platelet-like shape and the substitution of Ti4+ leads to the lower PdI value.
Magnetic property analysis
To investigate the influence of Ti4+ ions substitution on the magnetic properties of Y-type barium hexaferrite (Co2-Y), the magnetization curves are recorded at 300 K (as shown in Fig. 7) at a maximum applied field of ± 6 Tesla. The results suggest the ferrimagnetic behavior in all synthesized samples. The sample doesn’t achieve saturation within the applied magnetic field range; the exact saturation magnetization value is estimated at the high-field regime using the Stoner–Wohlfarth law of approach. The law of approach describes the dependency of magnetization over the H−2, where the magnetization value can be described using the following expression (Fattouh et al. 2021);
where Mssignifies the exact value of saturation magnetization. Ip and Ma are inhomogeneity and magneto-crystalline anisotropy-dependent parameters, which describe the magnetic anisotropy and microstructural environment within the system. \({\chi }_{\infty }\)signify materials susceptibility at a high magnetic field (H). The value of the inhomogeneity constant (Ip) and additive term (H.\({\chi }_{\infty }\)), which represent the field-encouraged magnetization, may be ignored within the presence of a high magnetic field and underneath the Curie temperature (Tc) of that material. In consideration of both circumstances, the expression for magnetization can be rearranged as follows:
The exact value for saturation magnetization can be obtained by considering the above-mentioned expression equivalent to a straight-line equation. By plotting the M vs. H−2 graph, the Y-axis intercept (using linear fitting to the data points, as shown in Fig. 8) provides the exact value of saturation magnetization (Lee et al. 2020; Fattouh et al. 2021). To govern the impact of the substitution over the magnetic properties of a ferrite system, the numbers of Bohr magneton (Nbm) and magnetic anisotropy constants (K1) are also determined using the following relations (Almessiere et al. 2019; Tchouank Tekou Carol et al. 2020);
where MW is the molar weight of the hexaferrite sample, Ms is the saturation magnetization, Hc is the coercivity field, and µo is the vacuum permeability having a numerical value of 12.57 × 10–7 H/m).
To evaluate the variation of temperature-dependent magnetization properties with Ti4+ ion substitution, thermomagnetic curves (ZFC and FC) are obtained within the temperature range of 300 to 900 K at an applied field of 300 Oe, as shown in Fig. 9. The Curie temperature (Tc) (Inserted Figures in Fig. 9) is measured at the minima from the temperature-dependent first-order derivative (dM/dT) curve. All the derived parameters such as saturation magnetization (Ms), coercivity field (Hc), remnant magnetization (Mr), number of Bohr magneton (Nbm), squareness ratio (Mr/Ms), magnetic anisotropy constant (K1), and Curie temperature (Tc) are calculated and tabulated in Table 3.
The results suggest that the value of saturation magnetization (Ms) along with the number of Bohr magneton (Nbm) is found to be decreased with the substitution of titanium ions within the hexaferrite. This decreasing behavior can be explained by the replacement of a magnetic (Fe3+) ion with a nonmagnetic (Ti4+) ion within a hexaferrite system (Kumar et al. 2017). The coercivity values are found to be increased linearly up to the x = 0.2 and achieved their maximum value of 222.24 Oe. The improvement of the coercive field may be due to the enhanced value of lattice imperfections with nonmagnetic inclusion within the system, generation of Fe2+-Ti4+ pair by replacement of two Fe3+ ions, having larger magneto-crystalline anisotropy, larger ionic radius, and enhanced lattice strain valueOunnunkad 2006; Rao et al. 2009; Kumar et al. 2018). Further increment of Ti4+ content allows for a decrease of coercive field. It can be explained by lowering the anisotropy constant value or decreasing the average particle size value along with the dominance of single-domain particles (nearly 1.18 µm) (Park et al. 2015; Hosseinkhan Nejad et al. 2017; Kumar et al. 2018).
The retentivity value (magnetization holding capability after removal of the externally applied field) shows a random behavior with substituting Ti4+ ions. This random behavior can be explained by the ferroxplana nature of Co2-Y hexaferrite, where the preferred plane of magnetization is found to be within its basal plane or in a right circular cone at an angle to the major c-axis. Sometimes the magneto-crystalline anisotropy field may reorient this plane of magnetization without affecting the overall saturation magnetization and coercivity of that material (Pullar 2012; Zhang et al. 2014). The squareness ratio values (explain the anisotropy-dependent magnetic hardness of that system) are found to be within 0.2023 to 0.2622 and lie within the range of 0.05 < Mr/Ms < 0.50. It implies that all these magnetic particles are of the pseudo-single domain in nature and coupled magneto-statically to each other (Adeela et al. 2016). The magnetic anisotropy values are found in good agreement with the coercivity (Hc). In consideration of the earlier reported studies, the substitution of Ti4+ ions with Fe3+ ions is expected to the overall decreasing behavior for magnetic anisotropy constant (K1) (Ka̧kol et al. 1994; Rao et al. 2009). The only abnormalities associated with the sample x = 0.2 results may be due to the dependency of the magnetic anisotropy constant over the occupancy site and the discrete contribution of Fe2+ ions (Fletcher and O’Reilly 1974; Ka̧kol et al. 1994).
The thermomagnetic analysis of all these hexaferrite samples confirms the existence of a sharp ferromagnetic (FM) to paramagnetic (PM) phase transition within it. This transition temperature is determined with the help of a temperature-dependent first-order derivative (dM/dT) curve of magnetization. The presence of an intense downward peak is measured for the transition temperatures of each sample. The Curie temperature for the undoped Co2-Y sample is found to be 603.76 K, which is in good agreement with the earlier reported value of 600 K by S.G. Lee et al. (Lee and Kwon 1996). This transition temperature shows a reverse trend with increasing the substitution of Ti4+ within the system. It can be explained based on the exchange interactions. The exchange interaction within the ferrite system decreases both magnetic ion density and overall magnetic moment value; hence, lesser amount of thermal energy is required to surpass the weak exchange interaction of that sample. The substitution of diamagnetic Ti4+ ions replaces a magnetic ion Fe3+, resulting in the lowering of exchange interactions within the system, and the Curie temperature shows a decreasing behavior (Rao et al. 2009).
UV–Visible spectrum analysis
A diffuse reflectance-based-UV–Visible spectrum is studied with all calcined powders to examine the optical characteristics (a crucial factor in designing photocatalysts). The as-obtained absorption spectra within a wavelength range of 200 to 1400 nm (as displayed in Fig. 10) clearly signify the enhanced absorption of photons for titanium-substituted samples as compared to the pure Co2-Y sample. The bandgap energy (Eg) is the distance between the valance band (highest occupied orbit) and conduction band (lowest unoccupied orbit). This energy gap (Eg) can be measured either in terms of direct bandgap (measured vertical energy distance) or indirect bandgap (measured obliquely or inclined energy distance). To determine the energy band gap value (Eg) for all these hexaferrite samples, the Tauc relationship can be utilized as follows (Mohammed et al. 2021):
Here, α is the coefficient of absorption, hp represents the plank constant having a numerical value of 6.626 × 10–34 J.s, v is the incident photon frequency, KT is an energy-independent constant, and Q is dependent on the type of the transition such as 1/2 and 3/2 for allowed and forbidden direct transition, 2 and 3 for allowed and forbidden indirect transition (Naseri and Ghasemi 2016; Carol et al. 2020).
All these hexaferrite samples demonstrate energy bandgap (Eg) value corresponding to the allowed direct transition with Q = 1/2 using linear extrapolation to the graph between (α.hp.v)2 and Eg; the obtained values are mentioned within the inset of Fig. 10. The results suggest that the bandgap energy is found to be decreased linearly with the substitution of Ti4+ ions. The decreasing order can be explained on behalf of structural defects and enhanced lattice distortions associated with substituting a larger cation into the hexaferrite system (Mohammed et al. 2021). It can also be associated with the increasing lattice parameter, which provides enhanced inter-atomic spacing, so the atoms can vibrate with large amplitude by utilizing thermal energy. This atomic vibration allows for lowering the electronic potential and lowering the energy bandgap (Naseri and Ghasemi 2016). The decreasing behavior of bandgap (Eg) can also be explained by the valency of both ions. Ti4+ ions have a higher valency state which substitutes for Fe3+ ions having a lower valency state. It generates impurity energy levels (donor type), which are responsible for the narrow bandgap (Cai et al. 2014). A slight enhancement of the bandgap (observed with x = 0.5) value can be explained by the dominance of the second factor, i.e., quantum confinement with decreasing particle size. The confinement of both electrons and holes within the nanoscale premises allows for the widening of the bandgap within semiconductors (El-Hagary et al. 2019; Singh and Rajput 2020). Due to enhanced photon absorption, along with the lowest bandgap energy (narrow bandgap results in the easy absorption of photon energy), two samples, Ba2Co2Fe12O22 (pristine Co2-Y hexaferrite) and Ba2Co2Fe11.6Ti0.4O22 (titanium substituted Co2-Y hexaferrite), are studied for its comparative photocatalytic efficiency within the degradation of a nonbiodegradable dye, i.e., methyl orange (MO).
Photocatalytic analysis
The photocatalytic efficiency of Ba2Co2Fe12O22 and Ba2Co2Fe11.6Ti0.4O22 ferrites are investigated for the degradation of methyl orange in an aqueous solution at room temperature with a pH level of 3. The MO degradation performance on the Ba2Co2Fe11.6Ti0.4O22 photocatalyst was checked under different pH conditions. Optimum MO degradation was observed at pH ~ 3 conditions (Fig. S1). The suitable selectivity of pH value decides the interaction between the catalyst and pollutant. The pH value of 3 is optimized by an earlier published study for the degradation of methyl orange within similar conditions (De et al. 2020). Figure 11 shows negligible MO degradation occurs in an aqueous solution containing H2O2, either in the dark or under visible light irradiation, even after 50 min of test time. Figure 12a depicts the change in MO absorbance (with time) when mixed with Ba2Co2Fe12O22 particles in an aqueous suspension with H2O2 in the dark. There was virtually no change in MO absorbance even after 90 min under dark conditions.
The photocatalytic reaction mixture is kept in the dark for 15 min to ensure the adsorption–desorption equilibrium on the photocatalyst’s surface. UV–visible data for the dark condition (light off) is incorporated in Fig. S2. Under the light-off condition, the dye gets adsorbed on the photocatalyst’s surface in the first 10 min. Then, the reaction mixture is kept in the dark for another 5 min. During this interval (last 5 min), there is no change in MO concentration (as shown in Fig. S2), confirming the achievement of adsorption–desorption equilibrium of MO on the photocatalyst’s surface. Nevertheless, there is a slight decrease in MO absorbance (in the presence of Ba2Co2Fe12O22 particles) under visible light irradiation. The result shows that the MO degradation on Ba2Co2Fe12O22 has poor efficiency under visible light irradiation.
The role of Ti4+ substitution over the degradation efficiency can be emphasized by analyzing both pristine and Ti4+ substituted samples for their dye degradation efficiency within similar conditions. The results (as shown in Fig. 13) suggest that the Ba2Co2Fe11.6Ti0.4O22 can efficiently degrade (nearly complete) the methyl orange (MO) within the test time of 90 min. The comparative performance of both these catalysts is analyzed based on the degradation rate for methyl orange, as shown in Fig. 14a. It describes the time-dependent variation of MO concentration (C) at the time (t) with respect to the initial concentration (Co), i.e., 4.6 mg/l. To quantify the performance of both these catalysts and determination of heterogeneously catalyzed kinetics of the MO photodegradation, a standard Langmuir–Hinshelwood (L–H) equation is utilized with the following expression (Almahri 2022).
where t is time in minutes, karc is the apparent rate constant or pseudo-first-order rate constant. The rate constants (karc) are determined as the slope of ln(C/Co) vs. time plots (shown in Fig. 14b). The rate constant values for Ba2Co2Fe12O22 and Ba2Co2Fe11.6Ti0.4O22 are found to be 2.7 × 10–3 and 1.9 × 10–2 min−1, respectively.
The error analysis is carried out for photo-Fenton MO degradation kinetics on Ba2Co2Fe12O22 and Ba2Co2Fe11.6Ti0.4O22 nanoparticles. The standard deviation of the collected data points is calculated as the error. Figure 15a displays the error bar diagram, whereas, in a few points, the error bar is not visible because they are minor than the symbol size. Quenching experiments are also carried out in the presence of different radical or active species-trapping agents. A particular quencher can quench a specific reactive species and affect the photocatalytic performance of the catalyst. Isopropyl alcohol (IPA) and parabenzoquinone (PBQ) can specifically quench hydroxyl radical (•OH) and superoxide radical (O2.−), respectively. As shown in Fig. 15b, the addition of IPA and PBQ decreases the photocatalytic activity with respect to without quencher. Hence, the hydroxyl radical (•OH) and superoxide radical (O2.−) are prime reactive species for photocatalytic MO degradation.
Another significant parameter, i.e., the turnover frequency (TOF), is also examined in these hexaferrite catalysts to compare the photocatalytic degradation performances within similar conditions. The turnover frequency is the number of reactants converted into a product on 1 g of heterogeneous catalyst in a unit of time (Shang et al. 2013). The turnover frequency values for Ba2Co2Fe12O22 and Ba2Co2Fe11.6Ti0.4O22 are found to be 7.49 × 10–7 and 3.93 × 10–6 mol g−1 min−1, respectively. In a combined consideration of both the apparent rate constant (karc) and turnover frequency (TOF) values, the titanium substituted sample Ba2Co2Fe11.6Ti0.4O22 shows the best performance with complete degradation of the methyl orange dye within 90 min. This enrichment of catalytic activity can be correlated to various factors such as enhanced absorbance value, lowest band gap value, and fine particle size to facilitate the larger reactive sites.
Morphological analysis
The surface morphological features of titanium-substituted Co2-Y hexaferrite (Ba2Co2Fe11.6Ti0.4O22) are analyzed using scanning electron micrographs. Figure 16 displays the micrographs of calcined powder at 5 K and 20 K magnifications along with corresponding EDX spectra, which confirms the elemental identity of this compound without any traces of impurity elements. The surface morphology reveals that the hexaferrite powder has a wide range of size distribution (from 0.15 to 3.61 µm) with an average particle size of 1.435 µm (standard deviation of 0.718), composed of the larger hexagonal plate-like particle along with small particles (irregular shaped) distributed over the surface of these larger particles.
Surface electronic structures analysis
To validate the successful substitution of Ti4+ ions within the Ba2Co2Fe11.6Ti0.4O22 hexaferrite sample, the electronic structures of all the elements are studied using XPS spectra recorded in the range of 1350 eV to 1 eV region. The relative contribution of each valance state is demonstrated qualitatively by deconvoluting the high-resolution X-ray photoelectron spectrum.
A wide scan of XPS spectra, displayed in Fig. 17a, validates the existence of Ba, Co, Fe, Ti, and O elements within it, without any impurity element except carbon. The atomic percentage of the compound is quantified using a normalized peak area ratio within the overall XPS spectra (utilizing CasaXPS software). The precise measurement of peak areas requires the selection of a proper deconvolution method and background removal, which can resolve the peak broadening mechanisms in XPS (Cabrera‐German et al. 2016). The atomic percentage obtained within the XPS spectra is shown in Fig. 17a. The atomic percentage at the surface XPS spectra is found to be in good agreement with the concerned chemical formula, measured with EDX spectra, describing good homogeneities within the synthesized sample.
The respective C1s peak position (considered as an internal standard to calibrate the XPS data) is adjusted to 284.8 eV to compensate for the binding energies associated with all other peaks (Wu et al. 2015; Suthar et al. 2020). The high-resolution C1s spectra, displayed in Fig. 17b, reveal the existence of three peaks (obtained through deconvolution of spectra) corresponding to the C–C bonding (284.80 eV), C–O–C bond (285.83 eV), O = C–O bond (287.76 eV), correspondingly (Wu et al. 2019). The high-resolution Ba-3d spectra, displayed in Fig. 17c, confirm the existence of spin–orbit doublet for barium ions (Ba2+ state), consistent with Ba-3d5/2 at 779.13 eV and Ba-3d3/2 at 794.45 eV, having an energy interval of 15.32 eV (Suthar et al. 2020). The high-resolution Co-2p spectra, displayed in Fig. 17d, support the existence of spin–orbit doublet for cobalt ions (Co2+ state), consistent with Co-2p3/2 at 779.13 eV and Co-2p1/2 at 794.46 eV having an energy interval of 15.33 eV along with two satellite peaks at 785.06 eV and 803.22 eV, correspondingly (Suthar et al. 2020). The high-resolution Fe-2p spectra, displayed within Fig. 17e, verify the existence of characteristic peaks for iron ions (mixed Fe3+ and Fe2+ states), consistent with Fe-2p3/2 at 709.98 eV and Fe-2p1/2 at 723.48 eV having an energy interval of 13.5 eV along with two satellite peaks at 718.20 eV and 732.47 eV, correspondingly. The further deconvolution of these two major characteristic peaks explains the co-existence of mixed valency states of iron ions within each orbit corresponding to Fe2+-2p3/2 at 709.50 eV, Fe2+-2p1/2 at 723.18 eV, Fe3+-2p3/2 at 711.62 eV and Fe3+-2p1/2 at 725.11 eV (Suthar et al. 2020). The high-resolution Ti-2p spectra, displayed within Fig. 17f, justify the existence of characteristic peaks for titanium ions (mixed Ti3+ and Ti4+ states), consistent with Ti-2p3/2 at 457.48 eV and Ti-2p1/2 at 463.28 eV having an energy interval of 5.8 eV. The further deconvolution of these two major characteristic peaks explains the co-existence of mixed valency states of titanium ions within each orbit corresponding to Ti3+-2p3/2 at 457.50 eV, Ti3+-2p1/2 at 463.13 eV, Ti4+-2p3/2 at 458.70 eV, and Ti4+-2p1/2 at 464.92 eV (Woo et al. 2020). The high-resolution O1s spectra, displayed within Fig. 17g, reveal the existing two peaks (obtained through deconvolution of spectra) corresponding to M–O bonds at 528.98 eV and oxygen vacancy (O1−) at 530.62 eV, correspondingly (Suthar et al. 2020). The XPS study suggests the mixed ionic states for iron ions (Fe2+ and Fe3+) and titanium ions (Ti3+ and Ti4+) within the sample. It may facilitate electron transportation and enhance redox configuration to the sample Ba2Co2Fe11.6Ti0.4O22 (Woo et al. 2020). The existence of oxygen vacancy also helps to understand the enhanced photocatalytic activity. The presence of oxygen vacancy improves visible light absorption. It leads to narrowing the bandgap of the sample by generating an extra impurity level adjacent to the valence band (Wang et al. 2012; Fernández-Climent et al. 2020).
Plausible pathway for photocatalytic degradation of MO
The Ba2Co2Fe11.6Ti0.4O22 hexaferrite has a narrow bandgap value (Eg = 1.47 eV) as compared to the Ba2Co2Fe12O22 (Eg = 1.58 eV). It shows excellency within the photocatalytic degradation of methyl orange (MO). The degradation process (termed as the Photo-Fenton type process) is performed with H2O2 and under visible light irradiation, which degrades the MO from the wastewater by generating the active hydroxyl radicals (OH•). It appears that Ti doping of Ba2Co2Fe12O22 significantly improves the photocatalytic reductive cleavage of H2O2 for enhanced hydroxyl radical generation. These radicals are strong oxidants (2.84 V with respect to a standard hydrogen electrode (SHE)) (Chahar et al. 2021). Ti-doping of Ba2Co2Fe12O22 could also improve its interaction with MO molecules in an acidic medium (Kumar et al. 2022). The visible light plays a crucial role in this degradation process by generating photogenerated electron–hole pairs by absorption of photon energy, and the electron moves from the valance band to the conduction band. These electrons (e−) and holes (h+) are responsible for conducting redox reactions, resulting in the generation of hydroxyl radicals (OH•), as shown in Fig. 18a. The generation of hydroxyl radicals (OH•) can also be understood with the electronic transition during the oxidation and reduction of transition metal cations, as shown in Fig. 18b. The releasing electron with the oxidation of Ti3+ and Fe2+ is utilized to generate active hydroxyl radicals (OH•). The generation of these hydroxyl radicals (OH•) can be explained using the following steps (Sharma et al. 2015):
-
Step 1. Absorption of photon energy and generation of electron–hole pair
$${Ba}_{2}{Co}_{2}{Fe}_{11.6}{Ti}_{0.4}{O}_{22}+ hv\to {Ba}_{2}{Co}_{2}{Fe}_{11.6}{Ti}_{0.4}{O}_{22}({e}_{C.B.}^{-}+{h}_{V.B.}^{+} )$$(15) -
Step 2. Utilization of photogenerated electron (\({e}_{C.B.}^{-}\)) by H2O2
$${H}_{2}{O}_{2}+ {e}_{C.B.}^{-} \to {OH}^{-}+ {OH}$$(16) -
Step 3. Utilization of photogenerated hole (\({h}_{V.B.}^{+}\)) by hydroxyl ion (OH−)
$${OH}^{-} + {h}_{V.B.}^{+} \to {OH}$$(17) -
Step 4. The reaction of H2O2 with transition metal cations situated over the catalytic surface.
$${H}_{2}{O}_{2}+ {Fe}^{3+} \to {Fe}^{2+}+{H}^{+} +O{OH}$$(18)$${H}_{2}{O}_{2}+ {Fe}^{2+} \to {Fe}^{3+}+ {OH}^{-}+{OH}$$(19)$${H}_{2}{O}_{2}+ {Ti}^{4+} \to {Ti}^{3+}+{H}^{+} +O{OH}$$(20)$${H}_{2}{O}_{2}+ {Ti}^{3+} \to {Ti}^{4+}+ {OH}^{-}+{OH}$$(21)
The hydroxyl radicals generated through these steps within the process degrade the methyl orange dye, as follows.
The superiority of the proposed titanium-substituted Co2-Y hexaferrite can be understood with a comparative analysis (as shown in Table 4) of various catalytic systems studied earlier for photocatalytic degradation of nonbiodegradable pollutants. The analysis suggests that the Ba2Co2Fe11.6Ti0.4O22 hexaferrite possesses a higher apparent rate constant value among all studied systems. It shows a faster degradation response and provides an advantageous step toward developing a magnetically separable heterogeneous photocatalyst. The overall degradation efficiency of Ba2Co2Fe11.6Ti0.4O22 can be correlated with the facile generation of hydroxyl radicals (OH•) along with small crystallite size, presence of active Ti3+ species (promote the interaction of catalyst with reactants, retard the recombination of electron and hole) and Fe3+ and Fe2+ (responsible for trapping of electrons and holes, alter the recombination rate) (Khan and Swati 2016).
Reusability analysis
A better recyclability and chemical stability of any photocatalyst define its suitability for possible adoption in large-scale processes. The re-processibility of the proposed photocatalyst (Ba2Co2Fe11.6Ti0.4O22) was monitored for five consecutive runs after a facile magnetic separation, followed by washing with DI water and drying at 100 °C. The recovered catalyst is again utilized for the same process to degrade the methyl orange from its aqueous solution, keeping all parameters similar to earlier. The results suggest no remarkable degradation efficiency loss (%), as shown in Fig. 19.
Conclusions
The single-phase pristine and Ti4+ substituted Y-type barium hexaferrite (Co2-Y) having a general formula of {Ba2Co2Fe12-xTixO22 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5)} are successfully prepared using the nitrate-based sol–gel auto-combustion route. The as-prepared Co2-Y hexaferrite powders have robust thermo-chemical stability, which can overcome the problem associated with spinel-based ferrites. The structural investigations using XRD and FTIR confirm the successful substitution of titanium ions within the hexaferrite system. The magnetic studies confirm the presence of ferrimagnetic nature within all samples. The UV–visible spectrum analysis supports the enhancement in visible light absorption and decreasing behavior for bandgap value by increasing the substitution level of Ti4+ ions within the sample. The photocatalytic degradation study of aqueous MO solution in the presence of H2O2 suggests the superiority of Ba2Co2Fe11.6Ti0.4O22 compared to pristine Co2-Y barium hexaferrite with an apparent rate constant of 1.9 × 10–2 min−1 and turn over frequency of 3.93 × 10–6 mol g−1 min−1. This enhanced photocatalytic activity for sample Ba2Co2Fe11.6Ti0.4O22 can be correlated to the fine crystallite size; the presence of active Ti3+ species to promote the reactivity of catalyst surface; the presence of mixed Fe3+ and Fe2+ to slow down the recombination process by trapping the photogenerated electrons and holes; the existence of oxygen vacancy to enhance the visible light absorption and leads to narrowing the bandgap by creating an extra impurity level adjacent to the valence band. The results suggest that the Ba2Co2Fe11.6Ti0.4O22 ferrite can be utilized as a promising material (facile synthesis, enhanced thermal and chemical stability, and enhanced apparent rate constant value) to catalyze the degradation of several nonbiodegradable organic compounds from wastewater in large-scale industries, without any hazardous footprint on the environment.
Data availability
On reasonable request, the corresponding author can provide the datasets used and/or analyzed during the current study.
References
Adeela N, Khan U, Iqbal M, Riaz S, Irfan M, Ali H, Javed K, Bukhtiar I, Maaz K, Naseem S (2016) Structural and magnetic response of Mn substituted Co2Y-type barium hexaferrites. J Alloy Compd 686:1017–1024. https://doi.org/10.1016/j.jallcom.2016.06.239
Akhavan O, Azimirad R (2009) Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl Catal A 369(1–2):77–82. https://doi.org/10.1016/j.apcata.2009.09.001
Al-Jlil SA (2010) Equilibrium study of adsorption of cobalt ions from wastewater using Saudi roasted date pits. Res J Environ Toxicol 4(1):1–12. https://doi.org/10.3923/rjet.2010.1.12
Al-Jlil SA (2017) Adsorption of cobalt ions from wastewater on activated Saudi clays. Appl Water Sci 7(1):383–391. https://doi.org/10.1007/s13201-014-0253-z
Almahri A (2022) The solid-state synthetic performance of bentonite stacked manganese ferrite nanoparticles: adsorption and photo-fenton degradation of MB dye and antibacterial applications. J Market Res 17:2935–2949. https://doi.org/10.1016/j.jmrt.2022.02.052
Almessiere MA, Slimani Y, Gungunes H, Manikandan A, Baykal A (2019) Investigation of the effects of Tm3+ on the structural, microstructural, optical, and magnetic properties of Sr hexaferrites. Results Physics 13:102166. https://doi.org/10.1016/j.rinp.2019.102166
Alrebdi TA, Yahaya IK, Mohammed J, Wudil YS, Paray A, Carol TTT, Hafeez HY, Srivastava AK (2022) Phase structure refinement, electric modulus spectroscopy, Urbach energy analysis, and magnetic properties of Ce3+–Ni2+-substituted Y-type barium hexaferrites. Mater Scid Eng: B 280:115682. https://doi.org/10.1016/j.mseb.2022.115682
Aroke UO, Momoh RO, Hamidu LAJ, Buhari U (2020) Removal of Azo Dye Methyl Orange in aqueous solution by kaolinite clay: equilibrium isotherms, kinetics and error analyses. J Eng Technol 5:422–433. https://doi.org/10.36348/sjet.2020.v05i11.005
Augustin M, Balu T (2017) Estimation of lattice stress and strain in zinc and manganese ferrite nanoparticles by Williamson-Hall and size-strain plot methods. Int J Nanosci 16(03):1650035. https://doi.org/10.1142/S0219581X16500356
Auld SM (1907) The relationship between the colour and constitution of organic compounds. Sci Progress Twentieth Century (1906-1916) 1(4):650–667. https://www.jstor.org/stable/43769120. Accesses 14 Dec 2022
Baldermann A, Grießbacher AC, Baldermann C, Purgstaller B, Letofsky-Papst I, Kaufhold S, Dietzel M (2018) Removal of barium, cobalt, strontium, and zinc from solution by natural and synthetic allophane adsorbents. Geosciences 8(9):309. https://doi.org/10.3390/geosciences8090309
Bilal M, Ihsanullah I, Younas M, Shah MUH (2021) Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: a critical review. Separ Purif Technol 278:119510. https://doi.org/10.1016/j.seppur.2021.119510
Borse PH, Cho CR, Lim KT, Lee YJ, Hong TE, Bae JS, Jeong ED, Kim HJ, Kim HG (2011) Synthesis of barium ferrite for visible light photocatalysis applications. J Korean Phys Soc 58(6):1672–1676. https://doi.org/10.3938/jkps.58.1672
Cabrera-German D, Gomez-Sosa G, Herrera-Gomez A (2016) Accurate peak fitting and subsequent quantitative composition analysis of the spectrum of Co 2p obtained with Al Kα radiation: I: Cobalt spinel. Surf Interface Anal 48(5):252–256. https://doi.org/10.1002/sia.5933
Cai W, Fu C, Gao R, Jiang W, Deng X, Chen G (2014) Photovoltaic enhancement based on improvement of ferroelectric property and band gap in Ti-doped bismuth ferrite thin films. J Alloy Compd 617:240–246. https://doi.org/10.1016/j.jallcom.2014.08.011
Carol TTT, Mohammed J, Basandrai D, Godara SK, Bhadu GR, Mishra S, Aggarwal N, Narang SB, Srivastava AK (2020) X-band shielding of electromagnetic interference (EMI) by Co2Y barium hexaferrite, bismuth copper titanate (BCTO), and polyaniline (PANI) composite. J Magnet Magnet Mater 501:166433. https://doi.org/10.1016/j.jmmm.2020.166433
Chahar D, Taneja S, Bisht S, Kesarwani S, Thakur P, Thakur A, Sharma PB (2021) Photocatalytic activity of cobalt substituted zinc ferrite for the degradation of methylene blue dye under visible light irradiation. J Alloys Comp 851:156878. https://doi.org/10.1016/j.jallcom.2020.156878
Chai WS, Cheun JY, Kumar PS, Mubashir M, Majeed Z, Banat F, Ho SH, Show PL (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296:126589. https://doi.org/10.1016/j.jclepro.2021.126589
Chen L, Chuang Y, Nguyen TB, Chang JH, Lam SS, Chen CW, Dong CD (2020) Novel molybdenum disulfide heterostructure nanohybrids with enhanced visible-light-induced photocatalytic activity towards organic dyes. J Alloys Comp 848:156448. https://doi.org/10.1016/j.jallcom.2020.156448
Chen L, Chen CW, Huang CP, Chuang Y, Nguyen TB, Dong CD (2022a) A) A visible-light sensitive MoSSe nanohybrid for the photocatalytic degradation of tetracycline, oxytetracycline, and chlortetracycline. J Colloid Interface Sci 616:67–80. https://doi.org/10.1016/j.jcis.2022.01.035
Chen L, Tsai ML, Chuang Y, Chen CW, Dong CD (2022b) B) Construction of carbon nanotubes bridged MoS2/ZnO Z-scheme nanohybrid towards enhanced visible light driven photocatalytic water disinfection and antibacterial activity. Carbon 196:877–889. https://doi.org/10.1016/j.carbon.2022.05.055
Danaei M, Dehghankhold M, Ataei S, Davarani FH, Javanmard R, Dokhani A, Khorasani S, Mozafari MR (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10(2):57. https://doi.org/10.3390/pharmaceutics10020057
De AK, Majumdar S, Pal S, Kumar S, Sinha I (2020) Zn doping induced band gap widening of Ag2O nanoparticles. J Alloys Comp 832:154127. https://doi.org/10.1016/j.jallcom.2020.154127
Dolia SN, Sharma R, Sharma RP, Saxena NS (2006) Synthesis, X-ray diffraction and optical band gap study of nanoparticles of NiFe2O4. Indian J Pure Appl Physics 44(10):774–776 (http://nopr.niscpr.res.in/handle/123456789/8367. Accessed 12 Dec 2022
Dom R, Subasri R, Radha K, Borse PH (2011) Synthesis of solar active nanocrystalline ferrite, MFe2O4 (M: Ca, Zn, Mg) photocatalyst by microwave irradiation. Solid State Commun 151(6):470–473. https://doi.org/10.1016/j.ssc.2010.12.034
Benkhaya Said, El Harfi S, Harfi AE (2017) Classifications, properties and applications of textile dyes: a review. Appl J Environ Eng Sci 3(3):311–320. https://doi.org/10.48422/IMIST.PRSM/ajees-v3i3.9681
El-Hagary M, Shaaban ER, Moustafa SH, Gad GMA (2019) The particle size-dependent optical band gap and magnetic properties of Fe-doped CeO2 nanoparticles. Solid State Sci 91:15–22. https://doi.org/10.1016/j.solidstatesciences.2019.03.005
ElSayed EE (2018) Natural diatomite as an effective adsorbent for heavy metals in water and wastewater treatment (a batch study). Water Sci 32(1):32–43. https://doi.org/10.1016/j.wsj.2018.02.001
Fallmann H, Krutzler T, Bauer R, Malato S, Blanco J (1999) Applicability of the Photo-Fenton method for treating water containing pesticides. Catal Today 54(2–3):309–319. https://doi.org/10.1016/S0920-5861(99)00192-3
Farghali AA, Khedr MH, Moustafa AF (2008) Photocatalytic activity and magnetic properties of nanocrystallite strontium hexaferrite prepared by self-flash combustion. Mater Technol 23(2):104–109. https://doi.org/10.1179/175355508X310089
Fattouh F, Rifai L, Habanjar K, Abdallah AM, Hassan RS, Yaacoub N, Awad R (2021) Structural and magnetic properties of hard-soft BaFe12O19/(Zn0.5Co0.5)Fe2O4 ferrites. J Physics: Condensed Matter 33(23):235803. https://doi.org/10.1088/1361-648X/abf478
Fernández-Climent R, Giménez S, García-Tecedor M (2020) The role of oxygen vacancies in water splitting photoanodes. Sustain Energy Fuels 4(12):5916–5926. https://doi.org/10.1039/D0SE01305F
Fletcher EJ, O’reilly W (1974) Contribution of Fe2+ ions to the magnetocrystalline anisotropy constant K1 of Fe3-xTixO4 (0< x< 0.1). J Physics C: Solid State Physics 7(1):171. https://doi.org/10.1088/0022-3719/7/1/024
Arti, Gupta R, Singh SP, Walia R, Kumar V, Verma V (2022) Modification in photovoltaic and photocatalytic properties of bismuth ferrites by tailoring band-gap and ferroelectric properties. J Alloys Comp 908:164602. https://doi.org/10.1016/j.jallcom.2022.164602
Hai FI, Yamamoto K, Fukushi K (2007) Hybrid treatment systems for dye wastewater. Crit Rev Environ Sci Technol 37(4):315–377. https://doi.org/10.1080/10643380601174723
Holinsworth BS, Mazumdar D, Sims H, Sun QC, Yurtisigi MK, Sarker SK, Gupta A, Butler WH, Musfeldt JL (2013) Chemical tuning of the optical band gap in spinel ferrites: CoFe2O4 vs NiFe2O4. Appl Physics Lett 103(8):082406. https://doi.org/10.1063/1.4818315
Jacobo S, Regazzoni AE, Blesa MA (2002) Interfacial chemistry of powdered barium ferrites immersed in aqueous solutions: leaching in acidic chloride media. J Mater Sci 37(14):3053–3057. https://doi.org/10.1023/A:1016089502968
Jia J, Liu C, Ma N, Han G, Weng W, Du P (2013) Exchange coupling controlled ferrite with dual magnetic resonance and broad frequency bandwidth in microwave absorption. Sci Technol Adv Mater 14(4):045002. https://doi.org/10.1088/1468-6996/14/4/045002
Kakol Z, Sabol J, Stickler J, Kozl A, Honig JM (1994) Influence of titanium doping on the magnetocrystalline anisotropy of magnetite. Phys Rev B 49(18):12767. https://doi.org/10.1103/PhysRevB.49.12767
Kaveeshwar AR, Kumar PS, Revellame ED, Gang DD, Zappi ME, Subramaniam R (2018) Adsorption properties and mechanism of barium (II) and strontium (II) removal from fracking wastewater using pecan shell based activated carbon. J Clean Prod 193:1–13. https://doi.org/10.1016/j.jclepro.2018.05.041
Keerthana SP, Yuvakkumar R, Ravi G, Al-Sehemi AG, Velauthapillai D (2022) Synthesis of pure and lanthanum-doped barium ferrite nanoparticles for efficient removal of toxic pollutants. J Hazard Mater 424:127604. https://doi.org/10.1016/j.jhazmat.2021.127604
Kefeni KK, Mamba BB, Msagati TA (2017) Application of spinel ferrite nanoparticles in water and wastewater treatment: a review. Sep Purif Technol 188:399–422. https://doi.org/10.1016/j.seppur.2017.07.015
Khan H, Swati IK (2016) Fe3+-doped anatase TiO2 with d–d transition, oxygen vacancies and Ti3+ centers: synthesis, characterization, UV–Vis photocatalytic and mechanistic studies. Ind Eng Chem Res 55(23):6619–6633. https://doi.org/10.1021/acs.iecr.6b01104
Kim KN, Jung HR, Lee WJ (2016) Hollow cobalt ferrite–polyaniline nanofibers as magnetically separable visible-light photocatalyst for photodegradation of methyl orange. J Photochem Photobiol, A 321:257–265. https://doi.org/10.1016/j.jphotochem.2016.02.007
Kumar P, Kar M (2014) Effect of structural transition on magnetic and optical properties of Ca and Ti co-substituted BiFeO3 ceramics. J Alloy Compd 584:566–572. https://doi.org/10.1016/j.jallcom.2013.09.107
Kumar A, Kumar V, Ghumman SS (2017) Synthesis and characterization of titanium doped barium ferrite and effects of doping on electrical and magnetic properties of the compound. Ferroelectrics 519(1):82–89. https://doi.org/10.1080/00150193.2017.1361215
Kumar S, Supriya S, Pandey R, Pradhan LK, Singh RK, Kar M (2018) Effect of lattice strain on structural and magnetic properties of Ca substituted barium hexaferrite. J Magn Magn Mater 458:30–38. https://doi.org/10.1016/j.jmmm.2018.02.093
Kumar U, Kuntail J, Kumar A, Prakash R, Pai MR, Sinha I (2022) In-situ H2O2 production for tetracycline degradation on Ag/s-(Co3O4/NiFe2O4) visible light magnetically recyclable photocatalyst. Appl Surf Sci 589:153013. https://doi.org/10.1016/j.apsusc.2022.153013
Lalegani Z, Nemati A (2015) Effects of Ce–Co substitution on structural, magnetic and dielectric properties of M-type barium hexaferrite nanoparticles synthetized by sol–gel auto-combustion route. J Mater Sci: Mater Electron 26(4):2134–2144. https://doi.org/10.1007/s10854-014-2658-8
Lee SG, Kwon SJ (1996) Saturation magnetizations and Curie temperatures of Co-Zn Y-type ferrites. J Magn Magn Mater 153(3):279–284. https://doi.org/10.1016/0304-8853(95)00559-5
Lee J, Lee EJ, Hwang TY, Kim J, Choa YH (2020) Anisotropic characteristics and improved magnetic performance of Ca–La–Co-substituted strontium hexaferrite nanomagnets. Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-72608-0
Liu L, Zhang G, Wang L, Huang T, Qin L (2011) Highly active S-modified ZnFe2O4 heterogeneous catalyst and its photo-Fenton behavior under UV–visible irradiation. Ind Eng Chem Res 50(12):7219–7227. https://doi.org/10.1021/ie2005516
Meidanchi A, Akhavan O (2014) Superparamagnetic zinc ferrite spinel–graphene nanostructures for fast wastewater purification. Carbon 69:230–238. https://doi.org/10.1016/j.carbon.2013.12.019
Mittal A, Malviya A, Kaur D, Mittal J, Kurup L (2007) Studies on the adsorption kinetics and isotherms for the removal and recovery of Methyl Orange from wastewater using waste materials. J Hazard Mater 148(1–2):229–240. https://doi.org/10.1016/j.jhazmat.2007.02.028
Mohammed J, Batoo KM, Abdulaziz AS, Safana AS, Hafeez HY, Raslan EH, Hadi M, Assiafan AAK, Imran A, Srivastava AK (2021) Crystal structure refinement and the magnetic and electro-optical properties of Er3+–Mn2+-substituted Y-type barium hexaferrites. Ceram Int 47(13):18455–18465. https://doi.org/10.1016/j.ceramint.2021.03.169
Mohammed J, Batoo KM, Mukhtar G, Labis JP, Raslan EH, Hadi M, Srivastava AK (2022) Crystal-structure analysis, Raman spectroscopy, dielectric measurements, magnetic and optical properties of Cr3+–Ni2+-substituted Co2Y-type barium hexaferrites. Mater Res Bullet 145:111564. https://doi.org/10.1016/j.materresbull.2021.111564
Mohan H, Ramalingam V, Adithan A, Natesan K, Seralathan KK, Shin T (2021) Highly efficient visible light driven photocatalytic activity of zinc/ferrite: carbamazepine degradation, mechanism and toxicity assessment. J Hazard Mater 416:126209. https://doi.org/10.1016/j.jhazmat.2021.126209
Moradnia F, Fardood ST, Ramazani A, Gupta VK (2020) Green synthesis of recyclable MgFeCrO4 spinel nanoparticles for rapid photodegradation of direct black 122 dye. J Photochem Photobiol A: Chem 392:112433. https://doi.org/10.1016/j.jphotochem.2020.112433
MuhdJulkapli N, Bagheri S, Hamid SBA (2014) Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. Sci World J 2014:692307. https://doi.org/10.1155/2014/692307
Naseri M, Ghasemi R (2016) Structure and physical properties of Fe6O8/ba Fe6O11 nanostructure. J Magn Magn Mater 406:200–206. https://doi.org/10.1016/j.jmmm.2016.01.019
National Centre for Biotechnology Information (2022) PubChem Compound Summary for CID 23673835, Methyl orange. [cited 2023 Jan 21. Availablr from https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-orange
Nejad EH, Farzin YA, Heydari MA (2017) Enhancement of soft magnetic properties of La–Zn co-doped nanocrystalline Ni2Y hexaferrite. J Magn Magn Mater 423:226–231. https://doi.org/10.1016/j.jmmm.2016.09.097
Nguyen TD, Phan NH, Do MH, Ngo KT (2011) Magnetic Fe2MO4 (M: Fe, Mn) activated carbons: fabrication, characterization and heterogeneous Fenton oxidation of methyl orange. J Hazard Mater 185(2–3):653–661. https://doi.org/10.1016/j.jhazmat.2010.09.068
Ounnunkad S (2006) Improving magnetic properties of barium hexaferrites by La or Pr substitution. Solid State Commun 138(9):472–475. https://doi.org/10.1016/j.ssc.2006.03.020
Park J, Hong YK, Lee W, An SY, Seo JW, Hur KH (2015) Coercivity of SrFe12 O19 hexaferrite platelets near single domain size. IEEE Magn Lett 6:1–3. https://doi.org/10.1109/LMAG.2015.2460215
Pullar RC (2012) Hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrite ceramics. Prog Mater Sci 57(7):1191–1334. https://doi.org/10.1016/j.pmatsci.2012.04.001
Qasem NA, Mohammed RH, Lawal DU (2021) Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 4(1):1–15. https://doi.org/10.1038/s41545-021-00127-0
Qin Q, Liu Y, Li X, Sun T, Xu Y (2018) Enhanced heterogeneous Fenton-like degradation of methylene blue by reduced CuFe2O4. RSC Adv 8(2):1071–1077. https://doi.org/10.1039/C7RA12488K
Rajivgandhi GN, Ramachandran G, Kanisha CC, Alharbi NS, Kadaikunnan S, Khaled JM, Alanzi KF, Li WJ (2021) Effect of Ti and Cu doping on the structural, optical, morphological and anti-bacterial properties of nickel ferrite nanoparticles. Results Physics 23:104065. https://doi.org/10.1016/j.rinp.2021.104065
Rao KS, Kumar AM, Varma MC, Choudary GSVRK, Rao KH (2009) Cation distribution of titanium substituted cobalt ferrites. J Alloy Compd 488(1):L6–L9. https://doi.org/10.1016/j.jallcom.2009.08.086
Rathi BS, Kumar PS (2021) Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ Pollut 280:116995
Reddy DHK, Yun YS (2016) Spinel ferrite magnetic adsorbents: alternative future materials for water purification? Coord Chem Rev 315:90–111. https://doi.org/10.1016/j.ccr.2016.01.012
Ruby S, Rosaline DR, Inbanathan SSR, Anand K, Kavitha G, Srinivasan R, Umar A, Hegazy HH, Algarni H (2020) Sunlight-driven photocatalytic degradation of methyl orange based on bismuth ferrite (BiFeO3) heterostructures composed of interconnected nanosheets. J Nanosci Nanotechnol 20(3):1851–1858. https://doi.org/10.1166/jnn.2020.17174
Shah P, Unnarkat A, Patel F, Shah M, Shah P (2022) A comprehensive review on spinel-based novel catalysts for visible light assisted dye degradation. Process Saf Environ Prot 161:703–722. https://doi.org/10.1016/j.psep.2022.03.030
Shang X, Li B, Zhang T, Li C, Wang X (2013) Photocatalytic degradation of methyl orange with commercial organic pigment sensitized TiO2. Proc Environ Sci 18:478–485. https://doi.org/10.1016/j.proenv.2013.04.064
Sharma R, Bansal S, Singhal S (2015) Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M= Cu, Zn, Ni and Co) in the structure. RSC Adv 5(8):6006–6018. https://doi.org/10.1039/C4RA13692F
Singh H, Rajput JK (2020) Effect of calcination temperature on magnetic, structural, thermal and optical properties of BFO-T nanoparticles. SN Appl Sci 2(8):1–11. https://doi.org/10.1007/s42452-020-3140-2
Singh AK, Hollmann D, Schwarze M, Panda C, Singh B, Menezes PW, Indra A (2021) Exploring the mechanism of peroxodisulfate activation with silver metavanadate to generate abundant reactive oxygen species. Adv Sustain Syst 5(4):2000288. https://doi.org/10.1002/adsu.202000288
Suthar M, Srivastava AK, Joshi RK, Roy PK (2020) Nanocrystalline cerium-doped Y-type barium hexaferrite; a useful catalyst for selective oxidation of styrene. J Mater Sci: Mater Electron 31(19):16793–16805. https://doi.org/10.1007/s10854-020-04234-5
Tran CV, La DD, Hoai PNT, Ninh HD, Hong PNT, Vu THT, Nadda AK, Nguyen XC, Nguyen DD, Ngo HH (2021) New TiO2-doped Cu–Mg spinel-ferrite-based photocatalyst for degrading highly toxic rhodamine B dye in wastewater. J Hazard Mater 420:126636. https://doi.org/10.1016/j.jhazmat.2021.126636
Valero-Luna C, Palomares-Sanchéz SA, Ruíz F (2016) Catalytic activity of the barium hexaferrite with H2O2/visible light irradiation for degradation of Methylene Blue. Catal Today 266:110–119. https://doi.org/10.1016/j.cattod.2015.08.049
Waheed IF, Al-Janabi OYT, Foot PJS (2022) Novel MgFe2O4-CuO/GO heterojunction magnetic nanocomposite: synthesis, characterization, and batch photocatalytic degradation of methylene blue dye. J Mol Liquids 357:119084. https://doi.org/10.1016/j.molliq.2022.119084
Wang J, Wang Z, Huang B, Ma Y, Liu Y, Qin X, Zhang X, Dai Y (2012) Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl Mater Interfaces 4(8):4024–4030. https://doi.org/10.1021/am300835p
Wang Y, Zhao H, Li M, Fan J, Zhao G (2014) Magnetic ordered mesoporous copper ferrite as a heterogeneous Fenton catalyst for the degradation of imidacloprid. Appl Catal B 147:534–545. https://doi.org/10.1016/j.apcatb.2013.09.017
Woo SH, Park DS, Choi WS, Kang H, Baek SW, Kim HS, Shin TH, Park JY, Schlegl H, Kim JH (2020) X-ray photoelectron spectroscopic study of impregnated La0.4Sr0.6Ti0.8Mn0.2O3±d anode material for high temperature-operating solid oxide fuel cell. Solid State Ionics 345:115175. https://doi.org/10.1016/j.ssi.2019.115175
Wu LQ, Li YC, Li SQ, Li ZZ, Tang GD, Qi WH, Xue LC, Ge XS, Ding LL (2015) Method for estimating ionicities of oxides using O1s photoelectron spectra. AIP Adv 5(9):097210. https://doi.org/10.1063/1.4931996
Wu Y, Lin Y, Xu J (2019) Synthesis of Ag–Ho, Ag–Sm, Ag–Zn, Ag–Cu, Ag–Cs, Ag–Zr, Ag–Er, Ag–Y and Ag–Co metal organic nanoparticles for UV-Vis-NIR wide-range bio-tissue imaging. Photochem Photobiol Sci 18(5):1081–1091. https://doi.org/10.1039/C8PP00493E
Yan H, Wang X, Yao M, Yao X (2013) Band structure design of semiconductors for enhanced photocatalytic activity: the case of TiO2. Progress Nat Sci: Mater Int 23(4):402–407. https://doi.org/10.1016/j.pnsc.2013.06.002
Yang S, Jin R, He Z, Qiao Y, Shi S, Kong W, Wang Y, Liu X (2017) An experimental study on the degradation of methyl orange by combining hydrodynamic cavitation and chlorine dioxide treatments. Chem Eng Trans 59:289–294. https://doi.org/10.3303/CET1759049
Youssef NA, Shaban SA, Ibrahim FA, Mahmoud AS (2016) Degradation of methyl orange using Fenton catalytic reaction. Egypt J Pet 25(3):317–321. https://doi.org/10.1016/j.ejpe.2015.07.017
Zhang X, Yue Z, Meng S, Yuan L (2014) Magnetic properties of in-plane oriented barium hexaferrite thin films prepared by direct current magnetron sputtering. J Appl Physics 116(24):243909. https://doi.org/10.1063/1.4905028
Zhang T, Liu J, Zhou F, Zhou S, Wu J, Chen D, Xu Q, Lu J (2020a) Polymer-coated Fe2O3 nanoparticles for photocatalytic degradation of organic materials and antibiotics in water. ACS Applied Nano Materials 3(9):9200–9208. https://doi.org/10.1021/acsanm.0c01829
Zhang X, Geng Z, Jian J, He Y, Lv Z, Liu X, Yuan H (2020b) Potassium ferrite as heterogeneous photo-fenton catalyst for highly efficient dye degradation. Catalysts 10(3):293. https://doi.org/10.3390/catal10030293
Acknowledgements
The authors are thankful to the IMPacting Research, INnovation and Technology (IMPRINT) (SERB) (File No: IMP/2019/000165), New Delhi, for the financial support and also grateful to the IIT (BHU) for providing facilities.
Author information
Authors and Affiliations
Contributions
• Mukesh Suthar: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft
• Arup Kumar De: conceptualization, methodology, resources, data curation.
• Arindam Indra: formal analysis, writing—review and editing
• Indrajit Sinha: data curation, writing—review and editing
• Pradip Kumar Roy: resources, supervision, project administration, funding acquisition, writing—review and editing
Corresponding author
Ethics declarations
Ethical approval
Not applicable to this work.
Consent to participate
Not applicable.
Consent to publication
The final manuscript was reviewed and approved by all authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• An attempt to use Ba2Co2Fe12-xTixO22 ferrite for photocatalytic degradation of MO.
• Visualize the role of Ti+4 over the bandgap energy for catalytic activity.
• Ba2Co2Fe11.6Ti0.4O22 exhibits its excellency in the presence of H2O2/visible light.
• Complete degradation of MO from wastewater (WW) occurs within 90 min.
• The boosted catalytic activity is elucidated with surface chemistry.
• A plausible pathway for MO degradation correlates with the creation of active OH•.
• Catalyst is magnetically separable and reusable.
• Promising large-scale WW treatment without a hazardous footprint on the environment.
Novelty
• The photocatalytic degradation efficiencies of nanocrystalline Ba2Co2Fe12-xTixO22 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) hexaferrites are evaluated to decompose methyl orange from wastewater in presence of H2O2/visible light. The results suggest that the Ba2Co2Fe11.6Ti0.4O22 hexaferrite may offer its candidacy (improved thermochemical stability and enhanced apparent rate constant value) as a replacement of spinel ferrites to catalyze the degradation of several nonbiodegradable organic compounds from wastewater in large-scale industries without any hazardous footprint on the environment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suthar, M., De, A.K., Indra, A. et al. Synthesis and characterization of titanium-substituted nanocrystalline Co2-Y hexaferrite: magnetically retrievable photocatalyst for treatment of methyl orange contaminated wastewater. Environ Sci Pollut Res 30, 44457–44479 (2023). https://doi.org/10.1007/s11356-023-25432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25432-8